This sample Pharmacotherapy Research Paper is published for educational and informational purposes only. If you need help writing your assignment, please use our research paper writing service and buy a paper on any topic at affordable price. Also check our tips on how to write a research paper, see the lists of psychology research paper topics, and browse research paper examples.
Few would disagree that psychological problems, especially the more severe ones, have biological, psychological, and social (i.e., biopsychosocial) underpinnings. Even staunch behaviorists and psychoanalysts would agree that stressors interact with diatheses (i.e., genetic predispositions) to produce psychological difficulties. As such, it only makes sense then to address each of the contributors so that treatment for these difficulties is successful. Whereas other chapters in this section focus on psychological therapies (e.g., psychotherapy, cognitive-behavioral therapy, family therapy, and therapy with children), this research-paper focuses on the treatment of psychological disorders from a pharmacological perspective (i.e., pharmacotherapy). This focus is not meant to ascribe any more or less importance to the use of drugs or psychotherapeutic interventions in the treatment of difficulties. In contrast, it highlights the necessity of addressing each biopsychosocial underpinning from a variety of perspectives.
This research-paper focuses on the four major classes of pharmacotherapeutic agents: anxiolytic and somnolent medications, antidepressants, mood stabilizers, and antipsychotics. I review the main drugs in each of the four classes in terms of general effects on the neurotransmission process, if known, and both their therapeutic and side effects. It is important to note that information on the neuroscientific bases for drug effectiveness (e.g., pharmacodynamics, pharmacokinetics, sites of action) is beyond the scope of this research-paper but can be found in Chapter 17, Drugs and
Behavior. Only discussion of what these drugs affect will take place in this research-paper (e.g., duloxetine affects serotonin [5HT] and norepinephrine [NE]). Additionally, reference is made throughout this research-paper to particular clinical disorders. Because it is beyond the scope of this research-paper to consider diagnostic and etiological issues of the disorders, the reader is referred to the other chapters in this domain.
Anxiolytic And Somnolent Medications
Anxiolytic medications generally can be broken down into benzodiazepines (BZs), non-benzodiazepines (non-BZs), antipanic medications, and antiobsessional medications, with somnolents relegated to their own category. The mechanisms by which these medications work to reduce the targeted symptoms are varied. As a result, I address each category separately. Given that the majority of the medications used to treat panic symptoms and obsessions are classified as antidepressants, I will cover those medications later in the section on antidepressants.
Benzodiazepines
BZs work via the BZ receptor sites in the central nervous system (CNS). Because the limbic system is highly innervated with BZ receptors, much of the BZs’ effects can be traced to this area (Preston, O’Neal, & Talaga, 2005). The binding of a BZ to a BZ receptor site enhances the effect of gamma-aminobutyric acid (GABA) and increases the flow of chloride ions. This process results in the therapeutic effects of calm, tension release, and lessened worry. Unfortunately, these therapeutic effects are accompanied by a host of side effects. These side effects include mental clouding, sedation, slurred speech, incoordination, prolonged reaction time, and (sometimes) disinhibition (Meyer & Quenzer, 2005; Preston et al., 2005). Additionally, most BZs suppress REM, Stage III, and Stage IV sleep, resulting in less restful sleep (Nishino, Mishima, Mignot, & Dement, 2004). This less restful sleep may lead to daytime sleepiness, mental clouding, and lowered concentration. Of particular concern is the fact that all BZs have an abuse potential. As such, their prescription, therapeutic use, and withdrawal should be monitored closely. Table 89.1 provides a list of the BZs by trade name and generic name. Selection of a medication within this group is typically related to the relative dependence on the liver for metabolism (important when there is liver impairment) and half-life duration (ones with a very short half-life can cause anterograde amnesia).
Non-Benzodiazepines
The drugs that fall into this category are varied. The variety is based not on the relative relief of the type of anxiety targeted but on their mechanisms of action. As a result, some of the main non-BZs are described here. Table 89.1 lists these main non-BZs and some additional ones not covered here due to space limitations.
Buspirone
Buspirone is an antianxiety agent that produces its effects via the 5HT-1A receptor site. Similar to the serotonergic antidepressants, buspirone’s anxiolysis is delayed, with results not being seen for one to two weeks (Eison & Temple, 1986). This delay in onset of therapeutic effect has both advantages and disadvantages. The advantages center around the fact that buspirone does not interact with GABA but rather with 5HT. As a result, it does not have an abuse potential, does not interact with alcohol, and does not cause sleepiness (Marangell & Martinez, 2006). The disadvantages stem mainly from the same piece. Because buspirone does not interact with GABA but rather with 5HT, it cannot be used in the treatment of alcohol/benzodiazepine withdrawal, and, as mentioned earlier, it takes up to two weeks to see the effectiveness.
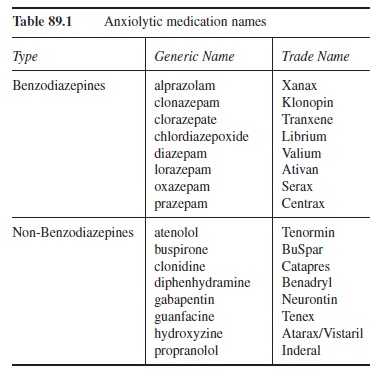 Table 89.1 Anxiolytic medication names
Table 89.1 Anxiolytic medication names
Antihistamines
Antihistamines (i.e., H1 receptor antagonists) block histaminergic receptors in the CNS. This blocking results in drowsiness and sedation, which is the root of its anxiolytic properties (Preston et al., 2005). The antianxiety effects occur in approximately 30 minutes and last 4 to 6 hours (Preston et al., 2005). The two main antihistamines used are hydroxyzine and diphenhydramine. Although these medications are not habit forming, tolerance to their anxiolytic effects can develop (Preston et al., 2005).
Beta Blockers
Beta blockers work by blocking NE at the receptor site. Two of the main drugs in the class are propranolol and atenolol. Beta blockers are effective at reducing the peripheral symptoms of anxiety (e.g., heart rate, sweating, tremor). The cognitive experience of anxiety is not affected by these medications, however. As a result of this differentiation, the beta blockers’ benefits are limited to performance anxiety (Davidson, 2006). Given their typical use as antihypertensives, one disadvantage of these medications is lowered blood pressure. As a result, discontinuation of beta blockers should be monitored closely for rebound hypertension.
Somnolents
Sometimes referred to as hypnotics, somnolents are medications used to treat insomnia (i.e., difficulty initiating sleep, maintaining sleep, or nonrestorative sleep). Drugs used to treat insomnia fall into three broad categories. These categories are benzodiazepine receptor agonist drugs, melatonin receptor drugs, and an “other” drug category.
Benzodiazepine receptor agonists (BZRAs). BZRAs combat insomnia via increasing the activity of GABA. The BZRAs fall into two broad groups: benzodiazepine and non-benzodiazepine BZRAs. The benzodiazepine BZRAs (B-BZRAs) are similar to the benzodiazepine anxiolytic drugs noted earlier and seen in Table 89.1. Generally, B-BZRAs nonselectively bind to the GABA-A complex (Howland, 2005). Although all of the B-BZRAs are effective at initiating sleep, their elimination half-lives vary, which contributes significantly to their ability to help a person maintain sleep and avoid terminal insomnia (i.e., early morning wakening). Because of their similarity to the anxiolytic BZs described earlier, the advantages and disadvantages are also similar. Some notable disadvantages are their abuse potential, residual daytime sleepiness, dizziness, rebound insomnia, and mental clouding (Howland, 2005; National Institutes of Health [NIH], 2005).
The non-benzodiazepine BZRAs (N-BZRAs) are newer and are chemically unrelated to the B-BZRAs. Table 89.2 provides some examples of the N-BZRAs. The N-BZRAs bind more selectively in the GABA-A complex (Howland, 2005). This greater selectively may be the reason for their better tolerability and lessened abuse potential compared to the B-BZRAs (Howland, 2005). However, some residual drowsiness and dizziness can still be present. Notably, among the B-BZRAs and the N-BZRAs, only eszopiclone is approved for use without a specific time limit; all other BZRAs have use limits of approximately a month or less (NIH, 2005).
Melatonin receptor drugs. The hormone melatonin is implicated in the regulation of circadian rhythms, including sleep-wake cycles (Meyer & Quenzer, 2005). Given this implication, it only makes sense that melatonin and melatonin receptor drugs would be effective somnolents. In fact, melatonin is effective with jet lag issues (Howland, 2005), and a new melatonin receptor drug, ramelteon, is effective in treating insomnia (Roth, Stubbs, & Walsh, 2005). Additionally, an advantage of ramelteon is its lack of potential for abuse.
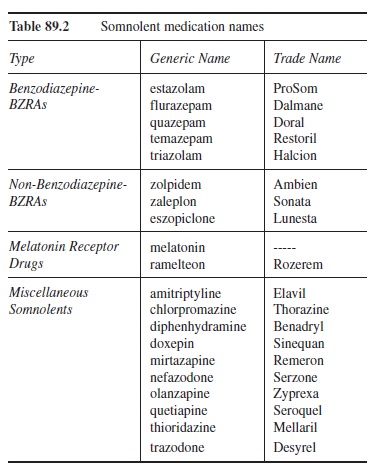 Table 89.2 Somnolent medication names
Table 89.2 Somnolent medication names
Other somnolents. Low doses of some sedating anti-depressant medicines are used sometimes for insomnia (e.g., amitriptyline, doxepin, trazodone, nefazodone, mirtazapine; Krystal, 2004). The decision to use these medicines can be reached because of the patient’s concomitant depression or simply because of the more favorable side-effect profile of some of these medicines (i.e., possible longer term use, lessened/absent potential for abuse). However, the use of these medicines for insomnia (without concomitant depression) is not well researched. Some additional drugs that have been used to treat insomnia include low doses of both the standard (e.g., chlorpromazine and thioridazine) and atypical antipsychotics (e.g., olanzapine and quetiapine) and the histaminergic agent diphenhydramine (Howland, 2005). The side-effect profiles for the antipsychotics (discussed later in this research-paper) can be disadvantageous.
Antidepressant Medications
Antidepressant medications are broken down into seven categories: cyclic antidepressants (CAs), selective serotonin reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors (SNRIs), norepinephrine reuptake inhibitors (NRIs), monoamine oxidase inhibitors (MAOIs), atypical antidepressants, and stimulants. As noted in the category names, these medications produce their effects via a variety of mechanisms. Hence, I address each category separately. Notably though, research does not indicate consistent superiority of one antidepressant relative to another antidepressant (Anderson, Nutt, & Deakin, 2000; Preston et al., 2005). Selection of a particular antidepressant rests in large part on the clinician’s experience with the drug, the patient’s history with the drug, and the patient’s tolerability of the drug’s side effects. Additionally, the therapeutic effects of antidepressants typically are not seen for approximately 4 weeks, a long time for someone who is depressed.
Before describing the categories, it is important to note that the classification name antidepressant is somewhat of a misnomer. Although sometimes an off-label prescription (i.e., not specifically approved by the Food and Drug Administration [FDA]), the drugs that fall into the various categories within this classification are routinely used to treat not only depression but also myriad other difficulties (Marangell & Martinez, 2006). Table 89.3 summarizes the information on the nondepressive disorders thought or found to be effectively treated by the respective antidepressants.
Cyclic Antidepressants
With few exceptions, CAs (inclusive of the tricyclic antidepressants, TCAs) affect both NE and 5HT availability to varying degrees. Their effect on NE and 5HT is not what generally differentiates them from the other types of antidepressants. Instead, it is their activity at receptor sites beyond those that are implicated in their antidepressant/ therapeutic effect.
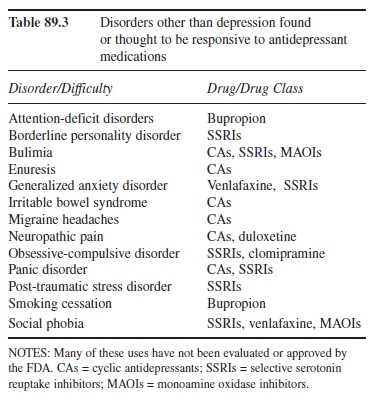 Table 89.3 Disorders other than depression found or thought to be responsive to antidepressant medications
Table 89.3 Disorders other than depression found or thought to be responsive to antidepressant medications
Because of this additional activity at muscarinic, histaminergic, and other sites, they are often referred to as “dirty drugs” (Briley, 1997). These side effects typically fall into four groups (i.e., anticholinergic, adrenergic, antihistaminic, and other), and each of the drugs in this classification varies in the severity of side effects in each of these four groups. The anticholinergic side effects can include difficulties like dry mouth, blurry vision, constipation, intestinal slowing/cessation, and urinary retention. Adrenergic side effects include sweating, sexual dysfunction, and orthostatic hypertension (i.e., lightheadedness due to a drop in blood pressure subsequent to standing, which can lead to falls). Antihistaminic side effects include weight gain and sedation, effects that may be desired if the person suffers from concomitant insomnia and decreased appetite. Other side effects can include hepatitis, anxiety, and cardiac sequelae. As a result of these side effects, CAs are rarely the first treatment choice, and must be carefully monitored. Table 89.4 lists some of the typical CAs.
Selective Serotonin Reuptake Inhibitors
Because they have fewer side effects and are safer in overdose compared to the CAs, the SSRIs are typically the first line of pharmacotherapeutic treatment for depression (Anderson et al., 2000; Preston et al., 2005). The fewer side effects are due specifically to the selective nature of the SSRIs’ actions (i.e., almost exclusive effects on 5HT). Although there can be some mild anticholinergic side effects, the majority of the side effects that are associated with the SSRIs are due to the increased 5HT activity. These side effects can include sweating, nausea, anxiety, gastrointestinal upset, insomnia, and sexual dysfunction (e.g., delayed ejaculation and anorgasmia). Although patients stop using medications for many varied reasons, this last side effect (i.e., sexual dysfunction) appears to be near the top of the list, and special attention should be paid to it because it does not appear to subside during the course of treatment (Marangell & Martinez, 2006). Table 89.4 lists SSRI medications.
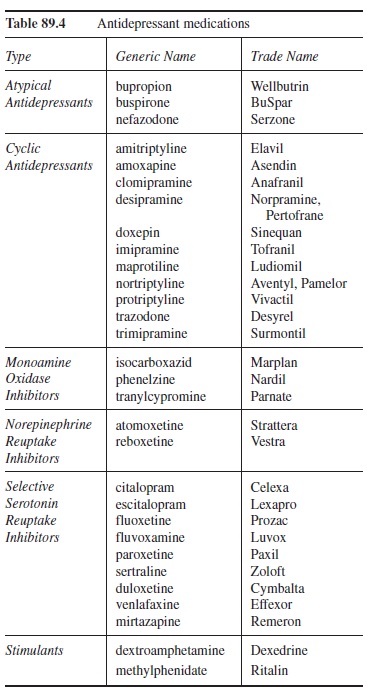 Table 89.4 Antidepressant medications
Table 89.4 Antidepressant medications
Serotonin And Norepinephrine Reuptake Inhibitors
Currently there are two drugs available in this category (i.e., venlafaxine and duloxetine) along with a third that modulates 5HT and NE (i.e., mirtazapine). Table 89.4 lists these drugs along with their trade names. Although venlafaxine and duloxetine prevent the reuptake of 5HT and NE in order to produce the therapeutic effect, mirtazapine blocks alpha2-adrenoreceptors, which increases 5HT and NE activity (Preston et al., 2005). Regardless of the process, all three make 5HT and NE more available. Research on the drugs in this area indicates that they may be more suitable than the SSRIs for severe depression (Wheatley, van Moffaert, Timmerman, & Kremer, 1998). However, due to the additional effect on NE relative to the SSRIs, the SNRIs have more side effects, although not as many as the CAs.
Norepinephrine Reuptake Inhibitors
NRIs include atomoxetine and reboxetine. Unlike atomoxetine, reboxetine is available only in Europe. Both of these drugs produce their therapeutic effects by selectively prohibiting the reuptake of NE. Currently atomoxetine is approved for the treatment of attention deficit disorders and not for depression. However, it has antidepressant effects (Papakostas, Petersen, Burns, & Fava, 2006). NRIs are effective at reducing fatigue (Papakostas et al., 2006) and improving cognition (Preston et al., 2005). The most common side effects of atomoxetine include dry mouth, insomnia, nausea, decreased appetite, constipation, dizziness, sweating, dysuria, and palpitations (Simpson & Plosker, 2004).
Monoamine Oxidase Inhibitors
Shortly before the discovery of the first CA, the first MAOI was discovered (Preston et al., 2005). MAOIs’ therapeutic effects come by blocking monoamine oxi-dase, which breaks down NE, 5HT, and dopamine (DA). The result of the inhibition is that there are more of these neurotransmitters available for binding at the postsynaptic receptor site. Because of the significant side effect profile that includes the risk of hypertensive reaction, MAOIs are a last resort when other antidepressants have failed. A sudden and dramatic increase in blood pressure can occur, which can lead to cerebral hemorrhage and possibly death (Merriman, 1999; Preston et al., 2005). Additional side effects can include dizziness, drowsiness, fatigue, gastrointestinal upset, weight gain, and insomnia (Merriman, 1999). Patients taking MAOIs also have to avoid tyramine-containing foods as well as many over-the-counter medications, especially cold medicines. The list of tyramine-containing foods to avoid is quite long and beyond the scope of this research-paper. However, some of the foods on the list are lumpfish, chicken liver, salami, sauerkraut, beef bouillon, some beers, and most cheeses (Merriman, 1999). If prescribed, great care should be taken. The generic and trade names of the three main MAOIs can be found in Table 89.4.
Atypical Antidepressants
Medications in this category do not fit nicely into the other categories listed. However, research supports the antidepressant effectiveness of the three medications found in this category. The first is bupropion. Bupropion’s anti-depressant effects appear to come via its ability to increase NE and DA activity (Marangell & Martinez, 2006). Due to its DA activity, it does have a small stimulant effect, which may be the reason for the side effects of anxiety and insomnia (Preston et al., 2005). Because of some patients’ intolerability to the sexual side effects of the SSRIs, clinicians sometimes prescribe bupropion instead. Bupropion has significantly fewer sexual side effects (especially for men) relative to many other antidepressants (Kennedy et al., 2006).
The second drug in this category is buspirone. Because it is most often used as an antianxiety agent, I describe it more fully in the anxiolytic section. However, there is evidence to suggest that buspirone may have some anti-depressant features, not surprising given its effect on 5HT (Preston et al., 2005). Interestingly, research shows that both bupropion and buspirone are effective augmentation strategies for patients not responsive to SSRIs alone (Trivedi et al., 2006).
The final drug in this category is nefazodone. Nefazodone is not only a 5HT-2A receptor blocker but also a 5HT and NE reuptake inhibitor. Because of its serotonergic activity, many of its associated side effects are similar to those of the SSRIs. An additional concern is the possibility of liver damage (Fochtmann & Gelenberg, 2005). Hence, liver functioning tests may be ordered prior to and throughout treatment (Hirsch, 2002). Nefazodone may be particularly helpful for those with agitated or anxious depressions (Hirsch, 2002) and those with chronic depression, especially when combined with cognitive-behavioral therapies (Keller et al., 2000).
Stimulants
The two main drugs in this category are methylphenidate and dextroamphetamine. Their antidepressant effects are primarily due to their promotion of availability of DA and NE. Their side effects include anxiety, insomnia, and appetite suppression (Preston et al., 2005). Although they are quite effective as antidepressants, they are usually reserved for depression in medically compromised individuals (e.g., post-stroke; Lingam, Lazarus, Groves, & Oh, 1988).
Mood Stabilizers
Although there is debate about the appropriateness of the term mood stabilizer (Keck & McElroy, 2003), the drugs used to treat bipolar disorder typically fall into two main categories (i.e., lithium and anticonvulsants), with an additional category reserved for other psychotropic medications (e.g., olanzapine and fluoxetine combination). Regardless of the choice of medication and not neglecting the adjuvant benefits of psychotherapeutic intervention, pharmacotherapy is the foundation for treatment for bipolar disorder. This section reviews the categories of mood stabilizers and their side effects.
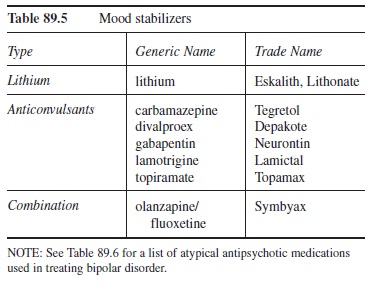 Table 89.5 Mood stabilizers
Table 89.5 Mood stabilizers
NOTE: See Table 89.6 for a list of atypical antipsychotic medications used in treating bipolar disorder.
Lithium
Lithium is the first-line treatment for bipolar disorder. Although it enhances 5HT activity and multiple sites of action have been identified in the CNS, lithium’s specific mechanism of action that pulls in the emotional extremes of the disorder is not established (Meyer & Quenzer, 2005). Interestingly, its ability to rein in the extremes is relatively specific to those with bipolar disorder (i.e., the emotional modulating effects are not present in those who do not have the disorder). Lithium response rates range from 60 to 80 percent (Preston et al., 2005). Additionally, the onset of therapeutic effect is approximately one to two weeks. Stabilization of a therapeutic dose can take much longer (Preston et al., 2005). One of the reasons stabilization can take a long time is because lithium has a very narrow therapeutic window (i.e., the therapeutic dose is very close to the toxic dose), hence dosage levels are slowly adjusted in order to reach a therapeutic level and avoid toxicity. As a result, lithium blood concentration must be measured throughout treatment. Lithium’s side effects run the gamut from benign to fatal; here are some of the more significant ones.
Cardiovascular. When therapeutically prescribed, serious cardiovascular issues are rare. However, alterations in a patient’s electrocardiograms are common (Preston et al.,2005).
Dermatological. Psoriasis and acne are relatively common in lithium treatment (Adis International Limited, 2005). Unless severe, discontinuation is typically not necessary (Oztas, Aksakal, Oztas, & Onder, 2001).
Endocrine. Given that hypothyroidism can be the underlying cause of some depressive disorders, it is imperative to test the functioning of the thyroid. Even when not the cause, thyroid function can be affected when taking lithium. Other implicated thyroid-related problems can include hyperparathyroidism, weight gain, and nephrogenic diabetes insipidus (Livingstone & Rampes, 2006). Monitoring is recommended.
Gastrointestinal. Symptoms in this area include vomiting, diarrhea, and nausea. Although these symptoms can be benign, they can also indicate lithium toxicity, which is why serum levels should be taken.
Hematological. An increase in white blood cell (WBC) count often occurs when lithium is used (Preston et al., 2005). This increase is reversible and rarely requires discontinuation of treatment.
Nervous and neuromuscular systems. Although some symptoms in this category cease over time (e.g., headache and muscle weakness), some other side effects do not remit for some patients (e.g., hand tremor; Preston et al., 2005). Preston et al. note that if these symptoms worsen it could be an indication of lithium toxicity.
Renal. Lithium is eliminated by the kidneys. As a result, if there is any indication to believe that the kidneys are not functioning optimally, the use of lithium should be reconsidered. Side effects that are kidney-related include polyuria (increased urination) and polydipsia (increased thirst; Passavanti et al., 1989).
Teratogenicity. Lithium is contraindicated in women attempting to get pregnant, who are pregnant, or who are nursing.
Weight gain. As noted in the endocrine side effects, weight gain can occur (Atmaca, Kuloglu, Tezcan, & Ustundag, 2002; Vestergaard & Schou, 1989). Consultations with a nutritionist or dietitian can help the patient adjust caloric intake.
Anticonvulsants
Due to the significant side effects and narrow therapeutic window of lithium, other mood-stabilizing medications are often sought. In fact, one of the anticonvulsants is recommended as a first-line treatment like lithium (i.e., divalproex; Preston et al., 2005). Two other anticonvulsants also are effective in the treatment of bipolar disorder (i.e., lamotrigine, Schaffer, Zuker, & Levitt, 2006; and carbamazepine, Nasrallah, Ketter, & Kalali, 2006). Similar to lithium, these drugs’ mechanisms of action that result in mood stabilization are not clear. Also, each of these medications has its own set of side effects.
Carbamazepine
Side effects are prevalent with carbamazepine treatment. CNS effects include dizziness, incoordination, and sedation (Preston et al., 2005). Gastrointestinal effects include nausea and vomiting, and hematological effects include the rare but serious conditions of agranulocytosis and aplastic anemia (Marangell & Martinez, 2006). Dermatological side effects include dermatitis and rash (Marangell & Martinez, 2006).
Divalproex
Due to the possibility of liver damage (especially in young children), liver enzyme levels should be monitored (Marangell & Martinez, 2006). Marangell and Martinez also note the hematological effects of coagulation defects and changes in platelet counts as well as the gastrointestinal symptoms of indigestion, heartburn, and nausea. Taking the medication with food may help with the gastrointestinal symptoms. However, unlike carbamazepine, weight gain is a common side effect (Morrell et al., 2003). Like lithium, hand tremor is common.
Lamotrigine
Common CNS difficulties include dizziness, headache, incoordination, drowsiness, and hand tremor (Preston et al., 2005). Common gastrointestinal side effects include nausea and vomiting (Preston et al., 2005). However, the important side effect to monitor is potential dermatologic reactions (i.e., rashes). Specifically, there is an increased risk of Stevens-Johnson syndrome, which can be potentially life-threatening (Fein & Hamann, 2005; Goldsmith, Wagstaff, Ibbotson, & Perry, 2004).
Other Mood Stabilizers
Research suggests that the atypical antipsychotic medications are effective in treating bipolar disorder (Marangell & Martinez, 2006). Currently, however, only two are FDA approved to treat bipolar disorder (i.e., olanzapine and aripiprazole). These medications are covered in the section devoted to antipsychotic medications. However, special attention should be paid to a medication that is a combination of an atypical antipsychotic (i.e., olanzapine) and an SSRI (i.e., fluoxetine). It is marketed under the name Symbyax and has been approved by the FDA to treat the depressive phase in bipolar disorder. This specific use is important, given the concern of triggering a manic episode when treating the depressive phase with antidepressant monotherapy (Miller, 2004). However, it is still seen by some as a second-line agent in the treatment of bipolar disorder (Miller, 2004). Side effects of the medication combination are consistent with those of the individual drugs involved.
Antipsychotic Medications
Antipsychotic medications are used to treat psychotic disorders (e.g., schizophreniform disorder, schizophrenia) and sometimes other disorders (e.g., bipolar disorder and major depressive disorder with psychotic features). The antipsychotic medications are usually divided into two categories (i.e., first-generation and second-generation antipsychotics). First-generation antipsychotics are older and are sometimes referred to as neuroleptics, conventional antipsychotics, or standard antipsychotics. Second-generation antipsychotics are newer and are sometimes referred to as atypical antipsychotics. To improve clarity, standard and atypical are the terms used throughout this section and chapter. Table 89.6 provides a list of drugs that fall into these two categories.
Standard Antipsychotics
In addition to being the older antipsychotic medications (chlorpromazine being first used in 1952), the drugs in this category share some other similarities. Although the standard antipsychotics can be broken down into high-potency and low-potency groups, in equivalent doses they are equally effective, especially at reducing the positive symptoms of psychosis (e.g., hallucinations and delusions; Preston et al., 2005). This reduction is specifically tied to the site of therapeutic action, blockade of postsynaptic DA receptors (Marangell & Martinez, 2006). Additionally, all of the standard antipsychotic medications produce a host of side effects to varying degrees. As a result, choice of a specific standard antipsychotic is typically tied to the side-effect profile, history of the drug’s effectiveness with the patient, and the patient’s ability to tolerate the associated side effects. Given the significant impact of side effects on this category of drugs, the general categories of side effects are reviewed.
Extrapyramidal Side Effects
Extrapyramidal side effects (EPS) are due to blocking of the DA receptors in the basal ganglia. There are three types of EPS: Parkinsonian side effects, dystonia, and akathisia. Parkinsonian side effects (so named due to the similarity with Parkinson’s disease symptoms) include slowed movements/shuffling gait, decreased facial expression/flat affect, rigidity, and lethargy (Preston et al., 2005). Dystonic symptoms include severe muscle spasms especially of the head and neck, which can be very frightening to patients. Akathisia is intense feelings of restlessness often accompanied by fidgeting, pacing, and rocking (Edgerton & Campbell, 1994). In severe cases, akathisia is associated with increased risk of suicide (Hansen & Kingdom, 2006). EPS appear within the first several hours or days of treatment or adjustment in medications.
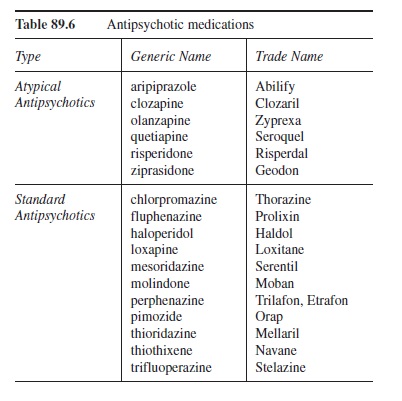 Table 89.6 Antipsychotic medications
Table 89.6 Antipsychotic medications
Anticholinergic Side Effects
Anticholinergic side effects, due to blocking of acetyl-choline receptors, are common for many of the medications already discussed in this research-paper and for antipsychotic medications as well. They include dry mouth and eyes, intestinal slowing and constipation, blurred vision, sedation, dysuria, and sexual dysfunction (Preston et al., 2005). Depending on the antipsychotic medication prescribed, the severity of these side effects ranges from mild to severe. Anticholinergic side effects appear within the first several hours or days of treatment or adjustment in medications.
Antiadrenergic Side Effects
These side effects are produced via blockade of alpha-adrenergic receptors. The most serious of these side effects is orthostatic hypotension (i.e., lightheadedness due to drop in blood pressure subsequent to standing, which can lead to falls). Antiadrenergic side effects appear within the first several hours or days of treatment or adjustment in medications.
Tardive Dyskinesia
Unlike extrapyramidal, anticholinergic, and antiadrenergic side effects, tardive dyskinesia (TD) appears much later in treatment or when treatment is discontinued. TD is a movement disorder that involves choreiform or rhythmic movements of the jaw, tongue, and extremities (Edgerton & Campbell, 1994). Although the symptoms improve for some patients over time, for other patients TD lasts years after discontinuing the medications (Glazer, Morgenstern, Schooler, Berkman, & Moore, 1990).
Atypical Side Effects
Atypical side effects include allergic reactions to the medicine, agranulocytosis (a serious blood disorder that now is believed to have been caused by contaminated medicine), hepatitis (in the past), grand mal seizures, hyperthermia, and lactation (Preston et al., 2005). An additional atypical side effect is neuroleptic malignant syndrome (NMS). NMS is a very serious movement disorder characterized by muscle rigidity and high fever that can result in coma and death if not treated promptly (Edgerton & Campbell, 1994). Lastly, another atypical (but common) side effect is weight gain.
Managing Side Effects
Due to the plethora of side effects, their management is a chief concern for the prescribing clinician. Without adequate attention, treatment compliance deteriorates. Although a person can adjust to some of the side effects noted (e.g., increased liquid intake to address dry membranes and constipation), some side effects, especially EPS, require the prescription of additional medications. As seen in Table 89.7, most of these additional medications focus on EPS (i.e., akathisia, Parkinsonian symptoms, and dystonia). Unfortunately, these medications have side effects of their own. As a result, a careful balancing act of drug choice and dosing is necessary.
Atypical Antipsychotics
Because of the side-effect profiles associated with the standard antipsychotics, the atypical antipsychotics are considered the first line treatment for psychotic disorders (Marangell & Martinez, 2006; Tandon & Jibson, 2005). The more favorable side-effect profiles are most likely due to their sites of action. The atypical antipsychotics have high affinity for blocking 5HT-2 receptors and block DA-2 receptors to varying degrees (Julien, 2005; Lieberman, 2005). The benefits of the atypical antipsychotics go beyond their more favorable side-effect profiles. For example, although standard antipsychotics are effective at treating the positive symptoms of psychosis, the atypical antipsychotics effectively treat not only the positive symptoms but also the cognitive symptoms (e.g., thought disorganization) and negative symptoms (e.g., affective flattening and anhedonia) of psychosis (Stahl, 2006; Tandon & Jibson, 2005). However, side effects are still of concern. As a result, the main atypical antipsychotics are reviewed here in terms of their drug-specific profiles.
Aripiprazole
Introduced in 2003, aripiprazole is one of the newest atypical antipsychotics. Its use is not associated with weight gain or electrocardiographic abnormalities (Julien, 2005). Although there were original reports of no EPS (Julien, 2005), this may not be the case (Ziegenbein, Sieberer, Calliess, & Kropp, 2006). Regardless, the EPS do not appear to be as common as in other antipsychotic medications. Additionally, aripiprazole is less sedating than other antipsychotics but can cause nausea and anxiety when treatment is initiated (Preston et al., 2005).
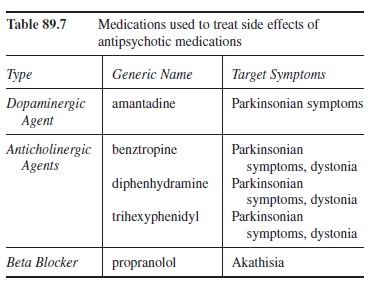 Table 89.7 Medications used to treat side effects of antipsychotic medications
Table 89.7 Medications used to treat side effects of antipsychotic medications
Clozapine
Clozapine has a very low incidence of EPS, and very few cases of TD have been reported (Preston et al., 2005). However, on the down side, clozapine is very sedating and does have some anticholinergic and antiadrenergic side effects (Preston et al., 2005). Although sedation is the most troubling side effect for patients, the most serious side effect is a severe blood disorder, agranulocytosis, which has caused death (Tiihonen, 2006). Because of the life-threatening nature of this side effect, weekly WBC counts must be done, significantly increasing the cost of using this drug. Furthermore, this side effect and resultant cost makes this a much less preferred atypical antipsychotic or first-line treatment.
Olanzapine
Olanzapine is very similar to clozapine but without the toxicity on WBC (Julien, 2005). Although EPS are rarely observed with olanzapine use, weight gain, which may be caused by problems in carbohydrate metabolism associated with the drug (Preston et al., 2005), is quite common (Julien, 2005). Additionally, there is some indication that olanzapine may be helpful in treating aggression in children with conduct disorder (Masi et al., 2006) and Tourette’s syndrome (Stephens, Bassel, & Sandor, 2004).
Quetiapine
Quetiapine is also similar to clozapine without the WBC toxicity (Julien, 2005). Although quite effective in addressing the positive symptoms of psychosis, quetiapine is less consistent in its ability to address the negative symptoms (Julien, 2005). However, there is some research to suggest that it has some benefit with the cognitive symptoms (Sharma, 2001). In addition to being helpful in treating schizophrenia, evidence suggests that it is also helpful in treating psychosis co-occurring with Parkinson’s disease, bipolar disorder, and schizoaffective disorder (Julien, 2005). Quetiapine has also been used adjuvantly with SSRIs in treating obsessive-compulsive disorder (Dell’osso, Mundo, & Altamura, 2006).
Risperidone
Risperidone is a potent inhibitor of both 5HT-2 and DA-2 receptors (Preston et al., 2005). It demonstrates a low occurrence of EPS (except at higher doses; Julien, 2005). Additional side effects include sedation, anxiety, agitation, headache, and nausea (Preston et al., 2005).
Brief hypotension and tachycardia may occur (Marangell & Martinez, 2006). Use of risperidone may be effective in treating disruptive behavior disorders in children (Pandina, Aman, & Findling, 2006).
Ziprasidone
Ziprasidone not only blocks 5HT-2 and DA-2 receptors but also is an agonist at 5HT-1A receptors and inhibits 5HT and NE reuptake (Julien, 2005). These features make it a possible adjuvant drug in treating depression (Papakostas et al., 2004). Ziprasidone has a low occurrence of EPS (Julien, 2005). As opposed to other atypical antipsychotics, weight gain is limited, and ziprasidone may actually precipitate weight loss (Brown & Estoup, 2005). Other side effects that may appear include headache, dyspepsia, nausea, constipation, and sedation (Marangell & Martinez, 2006). According to Julien and Preston et al., the main limitation to ziprasidone is its effect on cardiac conduction, which makes it contraindicated for individuals with cardiovascular disease and those with other possible cardiac issues.
Summary
This research-paper surveyed four of the main categories of pharmacotherapeutic agents (i.e., anxiolytic and somnolent medications, antidepressants, mood stabilizers, and anti-psychotic medications). The overview did not cover other areas of pharmacotherapy, such as medications typically prescribed for childhood disorders (e.g., attention deficit disorder), cognitive enhancers prescribed for dementia (e.g., dementia of the Alzheimer’s type), and pharmacotherapeutic agents used in treating substance dependence. For more in-depth coverage of these medications, the reader is referred to Preston, O’Neal, and Talaga’s (2006) very readable text on child and adolescent psychopharmacology, Marangell and Martinez’s (2006) guide, which covers the cognitive enhancers, and Lingford-Hughes, Welch, and Nutt’s (2004) guidelines on pharmacological management of substance disorders. Regardless, this research-paper gives the reader a reference point not only for an overview of the covered pharmacotherapeutic drugs and their targeted symptoms, side effects, and general sites of action but also a springboard to investigate other medications that may be of interest.
References:
- Adis International Limited. (2005). Long-term lithium therapy is associated with skin reactions—Predominantly acne and psoriasis. Drugs & Therapy Perspectives, 21(3), 22-24.
- Anderson, I. M., Nutt, D. J., & Deakin, J. F. W. (2000). Evidence-based guidelines for treating depressive disorders with antidepressants: A revision of the 1993 British Association for Psychopharmacology guidelines. Journal of
- Psychopharmacology, 14, 3-20. Atmaca, M., Kuloglu, M., Tezcan, E., & Ustundag, B. (2002). Weight gain and serum leptin levels in patients on lithium treatment. Neuropsychobiology, 46, 67-69.
- Briley, M. (1997). From dirty drugs to hyperselectivity and part way back again. Human Psychopharmacology, 12, S121-S125.
- Brown, R. R., & Estoup, M. W. (2005). Comparison of the metabolic effects observed in patients treated with ziprasidone versus olanzapine. International Clinical Psychopharmacology, 20, 105-112.
- Davidson, J. R. (2006). Pharmacotherapy of social anxiety disorder: What does the evidence tell us? The Journal of Clinical Psychiatry, 67, 20-26.
- Dell’osso, B., Mundo, E., & Altamura, A. C. (2006). Quetiapine augmentation of selective serotonin reuptake inhibitors in treatment-resistant obsessive-compulsive disorder: A six-month follow-up case series. CNS Spectrums, 11, 879-883.
- Edgerton, J. E., & Campbell, R. J., III. (1994). American psychiatric glossary (7th ed.). Washington, DC: American Psychiatric Press.
- Eison, A. S., & Temple, D. L., Jr. (1986). Buspirone: Review of its pharmacology and current perspectives on its mechanism of action. The American Journal of Medicine, 80(3B), 1-9.
- Fein, J. D., & Hamann, K. L. (2005). Images in clinical medicine: Stevens-Johnson syndrome. The New England Journal of Medicine, 352, 1696.
- Fochtmann, L. J., & Gelenberg, A. J. (2005). Guideline watch: Practice guideline for the treatment of patients with major depressive disorder (2nd ed.). Arlington, VA: American Psychiatric Association. Retrieved December 11, 2006, from http://www.psych.org/psych_pract/treatg/pg/MDD. watch.pdf
- Glazer, W. M., Morgenstern, H., Schooler, N., Berkman, C. S., & Moore, D. C. (1990). Predictors of improvement in tardive dyskinesia following discontinuation of neuroleptic medication. The British Journal of Psychiatry: The Journal of
- Mental Science, 157, 585-592. Goldsmith, D. R., Wagstaff, A. J., Ibbotson, T., & Perry, C. M. (2004). Spotlight on lamotrigine in bipolar disorder. CNS Drugs, 18, 63-67.
- Hansen, L., & Kingdom, D. (2006). Akathisia as a risk factor for suicide. British Journal of Psychiatry, 188, 192.
- Hirsch, M. (2002, August). Nefazodone and liver damage. Harvard Mental Health Letter, 19(2), 8.
- Howland, R. H. (2005). Pharmacotherapy for insomnia. Journal of Psychosocial Nursing, 43(12), 13-16.
- Julien, R. M. (2005). A primer of drug action: A comprehensive guide to the actions, uses, and side effects of psychoactive drugs (10th ed.). New York: Worth.
- Keck, P. E., Jr., & McElroy, S. L. (2003). Redefining mood stabilization. Journal of Affective Disorders, 73, 163-169.
- Keller, M. B., McCullough, J. P., Klein, D. N., Arnow, B., Dunner, D. L., Gelenberg, A. J., et al. (2000). A comparison of nefazodone, the cognitive behavioral-analysis system of psychotherapy, and their combination for the treatment of chronic depression. The New England Journal of Medicine, 342, 1462-1470.
- Kennedy, S. H., Fulton, K. A., Bagby, M. R., Greene, A. L., Cohen, N. L., & Rafi-Tari, S. (2006). Sexual function dur-ing bupropion or paroxetine treatment of major depressive disorder. Canadian Journal of Psychiatry, 51, 234-242.
- Krystal, A. D. (2004). The changing perspective on chronic insomnia management. Journal of Clinical Psychiatry, 65(Suppl. 8), 20-25.
- Lieberman, J. A. (1994). Clinical biological studies of atypical antipsychotics: Focus on the serotonin/dopamine systems. Journal of Clinical Psychiatry Monograph Series, 12(2), 24-31.
- Lingam, V. R., Lazarus, L. W., Groves, L., & Oh, S. H. (1988). Methylphenidate in treating poststroke depression. Journal of Clinical Psychiatry, 49, 151-153.
- Lingford-Hughes, A. R., Welch, S., & Nutt, D. J. (2004). Evidence-based guidelines for the pharmacological management of substance misuse, addiction, and comorbidity: Recommendations from the British Association for Psychopharmacology. Journal of Psychopharmacology, 18, 293-335.
- Livingstone, C., & Rampes, H. (2006). Lithium: A review of its metabolic adverse effects. Journal of Psychopharmacology, 20, 347-355.
- Marangell, L. B., & Martinez, J. M. (2006). Concise guide to psychopharmacology (2nd ed.). Arlington, VA: American Psychiatric Publishing.
- Masi, G., Milone, A., Canepa, G., Millepiedi, S., Mucci, M., & Muratori, F. (2006). Olanzapine treatment in adolescents with severe conduct disorder. European Psychiatry, 21, 51-57.
- Merriman, S. H. (1999). Monoamine oxidase drugs and diet. Journal of Human Nutrition and Dietetics, 12, 1-8.
- Meyer, J. S., & Quenzer, L. F. (2005). Psychopharmacology: Drugs, the brain, and behavior. Sunderland, MA: Sinauer Associates.
- Miller, M. C. (2004, August). What is Symbyax, the new drug being marketed for the treatment of bipolar depression? Harvard Mental Health Letter, 21(2), 8.
- Morrell, M. J., Isojarvi, J., Taylor, A. E., Dam, M., Ayala, R., Gomez, G., et al. (2003). Higher androgens and weight gain with valproate compared with lamotrigine for epilepsy. Epilepsy Research, 54, 189-199.
- Nasrallah, H. A., Ketter, T. A., & Kalali, A. H. (2006). Carbamazepine and valproate for the treatment of bipolar disorder: A review of the literature. Journal of Affective Disorders, 95, 69-78.
- National Institutes of Health. (2005). NIH state-of-the-science conference statement on manifestations and management of chronic insomnia in adults. Retrieved December 11, 2006, from http://consensus.nih.gov/2005/2005InsomniaSOS026html.htm
- Nishino, S., Mishima, K., Mignot, E., & Dement, W. C. (2004). Sedative-hypnotics. In A. F. Schatzberg & C. B. Nemeroff (Eds.), Textbook of psychopharmacology (3rd ed., pp. 651-670). New York: American Psychiatric Publishing.
- Oztas, P., Aksakal, A. B., Oztas, M. O., & Onder, M. (2001).Severe acne with lithium. The Annals of Pharmacotherapy, 35, 961-962.
- Pandina, G. J., Aman, M. G., & Findling, R. L. (2006). Risperidone in the management of disruptive behavior disorders. Journal of Child and Adolescent Psychopharmacology, 16, 379-392.
- Papakostas, G. I., Petersen, T. J., Burns, A. M., & Fava, M. (2006). Adjunctive atomoxetine for residual fatigue in major depressive disorder. Journal of Psychiatric Research, 40, 370-373.
- Papakostas, G. I., Petersen, T. J., Nierenberg, A. A., Murakami, J. L., Alpert, J. E., Rosenbaum, J. F., et al. (2004). Ziprasidone augmentation of selective serotonin reuptake inhibitors (SSRIs) for SSRI-resistant major depressive disorder. Journal of Clinical Psychiatry, 65, 217-221.
- Passavanti, G., Buongiorno, E., De Fino, G., Rutigliano, G., Giannattasio, M., Coratelli, P. (1989). Lithium induced polyuria and polydipsia. Advances in Experimental Medicine and Biology, 252, 215-231.
- Preston, J. D., O’Neal, J. H., & Talaga, M. C. (2005). Handbook of clinical psychopharmacology for therapists (4th ed.). Oakland, CA: New Harbinger Publications.
- Preston, J. D., O’Neal, J. H., & Talaga, M. C. (2006). Child and adolescent clinical psychopharmacology made simple. Oakland, CA: New Harbinger Publications.
- Roth, T., Stubbs, C., & Walsh, J. K. (2005). Ramelteon (TAK-375), a selective MT1/MT2-receptor agonist, reduces latency to persistent sleep in a model of transient insomnia related to a novel sleep environment. Sleep: Journal of Sleep and Sleep Disorders, 28, 303-307.
- Schaffer, A., Zuker, P., & Levitt, A. (2006). Randomized, doubleblind pilot trial comparing lamotrigine versus citalopram for the treatment of bipolar depression. Journal of Affective Disorders, 96, 95-99.
- Sharma, T. (2001). Quetiapine—Efficacy in different domains. European Neuropsychopharmacology: The Journal of the European College of Neuropsychopharmacology, 11, S385-S390.
- Simpson, D., & Plosker, G. L. (2004). Spotlight on atomoxetine in adults with attention-deficit hyperactivity disorder. CNS Drugs, 18, 397-401.
- Stahl, S. M. (2006). Positive findings for negative symptoms of schizophrenia: No longer untreatable? Acta Psychiatrica Scandinavica, 114, 301-302.
- Stephens, R. J., Bassel, C., & Sandor, P. (2004). Olanzapine in the treatment of aggression and tics in children with Tourette’s syndrome—A pilot study. Journal of Child and Adolescent Psychopharmacology, 14, 255-266.
- Tandon, R., & Jibson, M. D. (2005). Comparing efficacy of first-line atypical antipsychotics: No evidence of differential efficacy between risperidone, olanzapine, quetiapine, ziprasidone, and aripiprazole. International Journal of Psychiatry in Clinical Practice, 9, 204-212.
- Tiihonen, J. (2006). Fatal agranulocytosis four years after discontinuation of clozapine. The American Journal of Psychiatry, 163, 161-162.
- Trivedi, M. H., Fava, M., Wisniewski, S. R., Thase, M. E., Quitkin, F., Warden, D., et al. (2006). Medication augmentation after the failure of SSRIs for depression. The New England Journal of Medicine, 354, 1243-1252.
- Vestergaard, P., & Schou, M. (1989). Weight gain with lithium. Journal of Clinical Psychopharmacology, 9, 227.
- Wheatley, D. P., van Moffaert, M., Timmerman, L., & Kremer, C. M. (1998). Mirtazapine: Efficacy and tolerability in comparison with fluoxetine in patients with moderate to severe major depressive disorder. The Journal of Clinical
- Psychiatry, 59, 306-312. Ziegenbein, M., Sieberer, M., Calliess, I. T., & Kropp, S. (2006). Aripiprazoleinduced extrapyramidal side effects in a patient with schizoaffective disorder. Australian and New Zealand Journal of Psychiatry, 40, 194-195.
See also:
Free research papers are not written to satisfy your specific instructions. You can use our professional writing services to order a custom research paper on any topic and get your high quality paper at affordable price.





