This sample Lupus Erythematosus Research Paper is published for educational and informational purposes only. If you need help writing your assignment, please use our research paper writing service and buy a paper on any topic at affordable price. Also check our tips on how to write a research paper, see the lists of health research paper topics, and browse research paper examples.
Lupus erythematosus (LE), an inflammatory autoimmune disease, is the result of multiple events involving both susceptible genes and environmental impacts. The clinical manifestations of the disease may vary in severity from limited cutaneous lesions (cutaneous lupus erythematosus, CLE) to severe systemic disease, especially involving progressive renal damage (systemic lupus erythematosus, SLE). A common feature of the disease is the breakdown of tolerance to self-antigens, a consequence of which is the production of antibodies reactive with multiple self-proteins. Patients with a systemic organ manifestation of the disease have high levels of circulating autoantibodies against a number of nuclear antigens (ANA) including double-stranded DNA (dsDNA) and ribonucleoproteins. However, the pathogenetic mechanisms of LE are still not well understood, although there is accumulating evidence that aberrant apoptosis plays a pivotal role in the pathogenesis of various subtypes of the disease. Elucidation of further relevant humoral and cellular factors may lead to future development of effective strategies to prevent abnormal reactivity in patients with LE.
Epidemiology Of Lupus Erythematosus
In Europe, the annual incidence of SLE ranges from 3.3 to 4.8 cases per 100 000 persons per year, and in the United States, the annual incidence of SLE has been reported to range from 2.0 to 7.6 cases per 100 000 persons per year. However, the incidence of SLE in the general population varies according to the characteristics of the population studied, such as predominant age, sex, race, ethnicity, and national origin. Furthermore, most of the studies focus on SLE and a systematic epidemiological analysis of the various subtypes of CLE has not been conducted. The incidence of CLE has only been estimated to be two to threefold higher than the incidence of SLE. In addition, cutaneous manifestations appear in 73–85% of patients with SLE and can occur at any stage of the disease; conversely, only a small percentage of patients with CLE subsequently develop a systemic manifestation of the disease. In most patients with SLE, the clinical symptoms of the disease appear between the age of 15 and 40 years, with a mean age of 29–32 years. The disease affects both females and males, with a female:male ratio of approximately 10:1 for SLE.
Classification Of Cutaneous Lupus Erythematosus
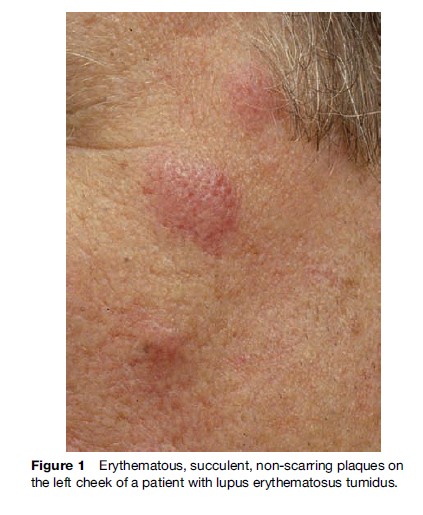
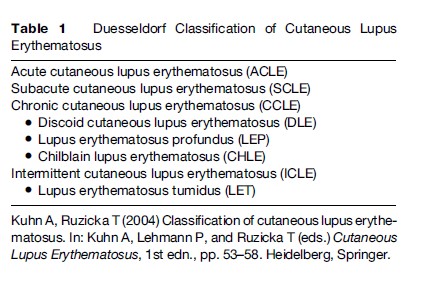
The clinical expression of the skin lesions in LE shows a great variety; consequently, this has led to the practice of identifying different subsets of the disease. Although the clinical expression of skin lesions in LE shows a great variety, lesions frequently appear at the beginning of the disease. Historically, the disease was first recognized and evaluated by its visible cutaneous symptoms before the analysis and study of its systemic organ manifestations established the complex nature of LE. However, the development of a unifying concept for skin manifestations of the disease has proven difficult. In 1977, Gilliam developed a classification system that divided the cutaneous lesions into LE-specific and LE-nonspecific manifestations by histological analysis of skin biopsy specimens. The LE-specific cutaneous findings encompass the various subtypes of CLE, which were subdivided into three different categories as defined by constellations of clinical features, histological changes, laboratory abnormalities, and average duration of skin lesions: Acute CLE (ACLE), subacute CLE (SCLE), and chronic CLE (CCLE). In contrast, skin lesions, such as urticarial-like vasculitis and livedo reticularis, are some of the most common LE-nonspecific cutaneous lesions and are mostly associated with SLE, reflecting potentially serious complications. Since the initial formulation of the Gilliam nomenclature and classification system more than two decades ago, several attempts have been made to improve on this system and to provide new approaches to the problem of classification of the cutaneous manifestations of LE. In recent years, a further subtype with characteristic clinical, histological, and photobiological features, named LE tumidus (LET), has been analyzed and defined as a separate entity of CLE (Figure 1). The course and prognosis in these patients is generally more favorable than in those with other subtypes of CLE, and therefore a modified classification system, including LET as the intermittent subtype of CLE (ICLE), was suggested in 2004 (Table 1).
Significance Of Photosensitivity In Cutaneous Lupus Erythematosus
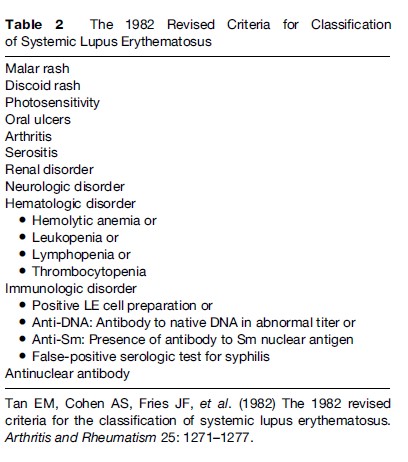
It has long been known that aberrant reaction to sunlight is present in almost all patients with a cutaneous or systemic manifestation of LE. Therefore, photosensitivity is listed as one of the American College of Rheumatology (ACR) criteria for the classification of SLE, although it is poorly defined as ‘‘a result of an unusual reaction to sunlight by patient history or physician observation’’ (Tan et al., 1982: 1274).Therefore, a detailed clinical history is important for the diagnosis and assessment of photosensitivity in patients with LE, including several key components, such as the morphology of the rash, duration, distribution, and the relationship to sun exposure and specific symptoms (e.g., pain, pruritus, burning, blistering, and swelling). Each of these symptoms may provide clues to the nature of the photosensitive eruption and, thus, the diagnosis. In addition, photoprovocation tests with long-wave UV light (UVA) and sunburn UV light (UVB) irradiation have been found to be an objective way to evaluate photosensitivity in patients with CLE. A standardized protocol for phototesting has been developed and optimized by taking into account multiple factors, such as light source, test area of irradiated skin, dose of UV exposure, and frequency of irradiation. In 2001, photoprovocation test reactions were evaluated in more than 400 patients with different subtypes of CLE and most of the patients developed characteristic skin lesions using combined UVA and UVB. Altogether, skin lesions were observed in 54% of patients with LE; 42% of these patients reacted to UVB irradiation only and 34% to UVA irradiation only. Interestingly, there were substantial differences in the clinical subtypes of LE with regard to response to the different UV wavelength. Patients with ICLE have been found to be the most photosensitive subtype, as phototesting revealed characteristic skin lesions in 72% of these patients. In contrast, pathologic skin reactions were induced by UV irradiation in 63% of patients with SCLE, in 60% of patients with ACLE, and in 45% of patients with CCLE. Moreover, photoprovocation tests are also helpful for the education of patients on protective measures. It has been demonstrated that broadband sunscreens can suppress the induction of skin lesions by UV irradiation in patients with CLE. Therefore, consequent protection against UV light as well as other physical and mechanical injuries may be of significant value for the course and prognosis of both CLE and SLE (Table 2).
Altered Apoptosis And Inducible Nitric Oxide Synthase In Cutaneous Lupus Erythematosus
Apoptosis is a form of programmed cell death that is characterized by several distinct morphological changes, such as cell shrinkage, plasma membrane blebbing, and nuclear condensation, in response to the activation of several factors and receptors specific for the apoptotic pathway. The biochemical hallmark of apoptotic cell death is the degradation of DNA into oligosome-sized fragments by specific endonucleases and the formation of small vesicles from the cell surface, also known as apoptotic bodies. In 1994, a potentially crucial role in the initiation of the autoimmune reaction cascade was attributed to UVinduced keratinocyte apoptosis by Casciola-Rosen and coworkers. More recently, the potential significance of apoptosis in the pathogenesis of CLE has been emphasized by further investigation. Using different staining techniques to detect nuclei with DNA damage, an increased number of apoptotic keratinocytes was found in skin lesions of various subtypes with this disease. Furthermore, a significant increase of apoptotic nuclei was also seen in UV-induced lesions of patients with various manifestations of CLE after phototesting. This was in striking contrast to healthy controls with a proper clearance of apoptotic cells after UVA and UVB irradiation. The hypothesis that clearance of apoptotic cells in the skin of the majority of patients with CLE is either impaired or delayed is in analogy to the growing evidence that defects in the clearance of apoptotic cells may be important in triggering the immune response in SLE. Impaired clearance for dying cells may explain accumulation of apoptotic and subsequently of secondary necrotic cells in various tissues of these patients. Interestingly, lymph node biopsies from patients with SLE have been investigated to determine whether a defect in engulfment of apoptotic cell material can also be observed in germinal centers. A characteristic feature of lymphatic germinal centers is the presence of specialized phagocytes, usually referred to as tingible body macrophages (TBM). Under healthy conditions TBM remove apoptotic cells very efficiently in the early phase of apoptosis. However, in a subgroup of patients with SLE apoptotic cells accumulate in the germinal centers. This may be due to impaired phagocytic activity or caused by the absence of TBM. Instead of normal clearance, apoptosis might progress allowing cells to enter the late stages of apoptotic cell death, including secondary necrosis.
Moreover, systemic autoimmunity has been noted in mice deficient for molecules potentially involved in the clearance of apoptotic cells including serum amyloid P (SAP), c-Mer, C4, IgM, or C1q. SAP is a member of a family of proteins termed pentraxins that bind to apoptotic cells and then interact directly with phagocyte receptors or with C1q. C1q and a related protein, mannose binding lectin (MBL), function as collectins, which are proteins with globular lectin-like heads and collagenlike tails that bind to and flag late-apoptotic cells for disposal by phagocytosis. Interestingly, the surface blebs of apoptotic keratinocytes bind C1q, an early component of the complement cascade. The C1q-binding protein that was initially identified to be present in apoptotic plasma membrane blebs is calreticulin, and autoantibodies to calreticulin can interfere with this binding. The binding of C1q to apoptotic cells has been postulated to facilitate the clearance of these cells by macrophages that express a C1q cell surface receptor. A potential role for C1q in the clearance of apoptotic debris and in the genesis of CLE is suggested by two observations. First, patients with complete congenital C1q deficiency frequently develop LElike photosensitive eruptions at an early stage. Second, mice with C1q deficiency show an SLE-like disease associated with an accumulation of apoptotic cells in the kidneys. However, the clearance of UV-induced apoptotic keratinocytes was not observed to be altered in C1qdeficient mice.
Nitric oxide (NO), a pleiotropic molecule synthesized by a family of nitric oxide synthases, is an important regulator of apoptosis and has an implication in the course of various autoimmune diseases. Interestingly, this molecule appears to have differential effects upon the various cell types within the skin and has been shown to play a role in the pathogenesis of CLE. Aberrant regulation of inducible nitric oxide synthase (iNOS) expression has been noted in UV-induced lesions of patients with CLE. In contrast to healthy controls, patients with various subtypes of this disease were noted to have significantly delayed and prolonged expression of iNOS, suggesting that the kinetics of iNOS induction and the time span of local iNOS expression might play an important role in the pathogenesis of CLE. Moreover, the clinically normalappearing skin of patients with active SLE also demonstrated elevated levels of iNOS in both the epidermis and adjacent vascular endothelium. It has further been reported that elevated levels of iNOS and serum nitrite correlated with established indices of disease activity and titers of anti-dsDNA antibodies in patients with SLE.
Regulatory T Cells And Chemokines For Lymphocyte Recruitment In Cutaneous Lupus Erythematosus
Naturally arising CD4+CD25+ regulatory T cells (Treg) represent a major lymphocyte population actively maintaining immunological tolerance to self and non-self-antigens. In the experimental mouse model of LE (NZB/ NZW F1), the frequency of Treg is lower than in control animals. Recently, a decreased number of peripheral Treg was also found in SLE patients and a significant correlation was detected between the number of Treg and disease activity. Whether the suppressive function of Treg cells is impaired in patients with SLE is currently under investigation. Furthermore, a decrease of Treg only at the site of inflammation in skin lesions of patients with CLE has recently been observed. This reduction of Treg in the dermal infiltrate was independent of the subtype of the disease and not reduced in other inflammatory skin diseases such as psoriasis vulgaris. Therefore, a reduction in the number of Treg in skin lesions of patients with CLE might be caused by specific factors of this disease. Nevertheless, studies on CLE did not encompass a general Treg defect as supported by a normal frequency of circulating Treg subpopulation and by a normal capacity to suppress Tcon proliferation. The reduction of Treg in CLE probably reflects a limitation of the disease to the skin, whereas a systemic decrease of Treg might explain the widespread autoimmunity in patients with SLE resulting in multiple organ involvement.
Although the pathogenetic role of skin-infiltrating lymphocytes is undoubted, their recruitment and activation pathways in inflammatory skin diseases, such as CLE, are still elusive. A superfamily of small chemotactic proteins has been shown to regulate lymphocyte trafficking under inflammatory conditions, and it has been demonstrated that UV irradiation induces the expression of T cell attracting chemokines. Furthermore, the CXC chemokine receptor R3 ligands CXCL9, CXCL10, and CXCL11 have been identified as the most abundantly expressed genes in the skin of patients with CLE. Additionally, it has been reported that the CCR4 ligand TARC/CCL17 was strongly expressed in skin lesions and elevated in the serum of patients with CLE. The functional relevance of lymphocytic CCR4 expression could be confirmed by TARC/CCL17-specific in vitro migration assays, suggesting that CCR4 and TARC/CCL17 are involved in the pathophysiology of CLE disease.
Treatment And Prevention Of Cutaneous Lupus Erythematosus
In patients with CLE, it is important to provide instructions concerning methods of protection from sunlight and artificial sources of UV irradiation as well as avoidance of potentially photosensitizing drugs. Topical corticosteroids are the mainstay of treatment for patients with all different subtypes of CLE; however, they are of limited value in the therapy of widespread skin lesions due to well-known side effects, such as skin atrophy. Recently, different groups have found administration of calcineurin inhibitors to be useful in CLE. In addition, physical therapy, such as cryotherapy or lasers and dermatosurgical methods, may be useful adjuncts and can be invaluable in enhancing quality of life for patients with this disease. The mainstay of treatment for widespread skin manifestations in patients with CLE, irrespective of the subtype of this disease, is antimalarial agents. Our understanding of the use of combinations of antimalarial agents and proper dosing according to the ideal body weight limits problems with toxicity. Further therapies, such as dapsone, retinoids, and thalidomide, are helpful for patients with resistant disease; however, side effects need to be taken into consideration. Recent advances in biotechnology resulted in the development of several novel systemic agents for the treatment of autoimmune diseases. It is conceivable that the prevention of apoptotic keratinocytes by protective measures and strategies may significantly reduce disease activity in CLE; however, further progress and controlled clinical trials are necessary for the approval of new therapeutic strategies.
In summary, consequent protection against UV light and also other physical and mechanical injuries may be of significant value for the course and prognosis of CLE, especially since such injuries may even initiate systemic manifestations of the disease, which was previously limited to the skin. Further steps in this research will consider the pathogenetic mechanisms in CLE photosensitivity to develop more specific pharmaceuticals beyond UV filters, such as antioxidants or DNA repair enzymes, which will be able to counteract the detrimental effects of UV irradiation on the disease.
Bibliography:
- Kuhn A and Ruzicka T (2004) Classification of cutaneous lupus erythematosus. In: Kuhn A, Lehmann P, and Ruzicka T (eds.) Cutaneous Lupus Erythematosus, 1st edn., pp. 53–58, Heidelberg, Springer.
- Tan EM, Cohen AS, Fries JF, et al. (1982) The 1982 revised criteria for the classification of systemicc lupus erythematosus. Arthritis and Rheumatism 25: 1271–1277.
- Albrecht J, Berlin JA, Bravermann IM, et al. (2004) Dermatology position paper on the revision of the 1982 ACR criteria for systemic lupus erythematosus. Lupus 13: 839–849.
- Callen JP (2004) Update on the management of cutaneous lupus erythematosus. British Journal of Dermatology 151: 731–736.
- Casciola-Rosen LA, Anhalt G, and Rosen A (1994) Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface blebs on cultured keratinocytes. Journal of Experimental Medicine 197: 1317–1330.
- Costner MI, Sontheimer RD, and Provost TT (2003) Lupus erythematosus. In: Sontheimer RD and Provost TT (eds.) Cutaneous Manifestations of Rheumatic Diseases, 1st edn., pp. 15–64. Philadelphia, PA: Williams and Wilkins.
- Dutz JP, Sontheimer RD, and Werth VP (2007) Pathomechanisms of cutaneous lupus erythematosus. In: Wallace DJ and Hahn BH (eds.) Dubois’ Lupus Erythematosus, 7th edn., pp. 550–573. Philadelphia, PA: Williams and Wilkins.
- Fas SC, Fritzsching B, Suri-Payer E, and Krammer PH (2006) Death receptor signaling and its function in the immune system. Current Directions in Autoimmunity 9: 1–17.
- Franz B, Fritzsching B, Riehl A, et al. (2007) Low number of regulatory T cells in skin lesions of patients with cutaneous lupus erythematosus. Arthritis and Rheumatism 56: 1910–1920.
- Gaipl US, Kuhn A, Sheriff A, et al., (2006) Clearance of apoptotic cells in human SLE. Current Directions in Autoimmunity 9: 173–187.
- Hochberg MC (1997) Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis and Rheumatism 40: 1725.
- Kuhn A, Fehsel K, Lehmann P, et al. (1998) Aberrant timing in epidermal expression of inducible nitric oxide synthase after UV irradiation in cutaneous lupus erythematosus. Journal of Investigtive Dermatology 111: 149–153.
- Kuhn A, Sonntag M, Richter-Hintz D, et al. (2001) Phototesting in lupus erythematosus: A 15-year experience. Journal of the American Academy of Dermatology 45: 86–95.
- Kuhn A, Lehmann P, and Ruzicka T (eds.) (2004) Cutaneous Lupus Erythematosus, 1st edn. Heidelberg, Germany: Springer.
- Kuhn A and Beissert S (2005) Photosensitivity in lupus erythematosus. Autoimmunity 38: 519–529.
- Kuhn A, Herrmann M, Kleber S, et al. (2006) Accumulation of apoptotic cells in the epidermis of patients with cutaneous lupus erythematosus after ultraviolet irradiation. Arthritis and Rheumatism 54: 939–950.
- Kuhn A, Krammer PH, and Kolb-Bachofen V (2006) Pathophysiology of cutaneous lupus erythematosus: Novel aspects. Rheumatology 45(supplement 3): 14–16.
- Kuhn A, Ruzicka T (2004) Classification of cutaneous lupus erythematosus. In: Kuhn A, Lehmann P, and Ruzicka T (eds.) Cutaneous Lupus Erythomatosus, 1st edn. pp. 53–58. Heidelberg, Springer.
- LeFeber WP, Norris DA, Ryan SR, et al. (1984) Ultraviolet light induces binding of antibodies to selected nuclear antigens on cultured keratinocytes. Journal of Clinical Investigation 74: 1545–1551.
- Meller S, Winterberg F, Gilliet M, et al. (2005) Ultraviolet radiation-induced injury, chemokines, and leukocyte recruitment: An amplification cycle triggering cutaneous lupus erythematosus. Arthritis and Rheumatism 52: 1504–1516.
- Miyara M, Amoura Z, Parizot C, et al. (2005) Global natural regulatory T cell depletion in active systemic lupus erythematosus. Journal of Immunology 175: 8392–8400.
- Roos A, Xu W, Castellano G, et al. (2004) Mini-review: A pivotal role for innate immunity in the clearance of apoptotic cells. European Journal of Immunology 34: 921–929.
- Sontheimer RD, Racila E, and Racila DM (2005) C1q: Its functions within the innate and adaptive immune responses and its role in lupus autoimmunity. Journal of Investigative Dermatology 125: 14–23.
- Stege H, Budde MA, Grether-Beck S, et al. (2002) Evaluation of the capacity of sunscreens to photoprotect lupus erythematosus patients by employing the photoprovocation test. European Journal of Dermatology 12: VII–IX.
- Wenzel J, Henze S, Worenkamper E, et al. (2005) Role of the chemokine receptor CCR4 and its ligand thymusand activation-regulated chemokine/CCL17 for lymphocyte recruitment in cutaneous lupus erythematosus. Journal of Investigative Dermatology 124: 1241–1248.
See also:
Free research papers are not written to satisfy your specific instructions. You can use our professional writing services to buy a custom research paper on any topic and get your high quality paper at affordable price.






