This sample Organohalogen Pollutants and Human Health Research Paper is published for educational and informational purposes only. If you need help writing your assignment, please use our research paper writing service and buy a paper on any topic at affordable price. Also check our tips on how to write a research paper, see the lists of health research paper topics, and browse research paper examples.
What Are Organohalogens?
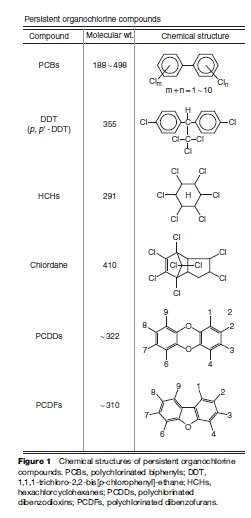
It is axiomatic that human activities change the quality of the environment on a global scale, which can adversely affect life on earth. A prototypical example of such environmental damage and harmful biological effects is that caused by persistent human-made chemicals, particularly organohalogen compounds. Organohalogens are organic compounds that contain chlorine, bromine, fluorine atoms, and the molecules are named chlorinated, brominated, and fluorinated compounds, respectively (Figures 1, 2 and 3). Due to their extreme persistence in the environment, bioaccumulative nature, and long-term health effects in humans, some of these organohalogen compounds such as polychlorinated biphenyls (PCBs, an industrially versatile compound), insecticides such as DDTs, HCHs (BHC), chlordane (CHLs), and industrial byproducts such as chlorinated/brominated dioxins/ dibenzofurans are well-known global environmental contaminants. Although the use of these synthetic chemicals was banned or severely restricted in most developed countries more than three decades ago, they are still found in every component of the global ecosystem and pose a threat to life on earth. Following the ban on the production and usage, residue levels of organohalogens declined, in some cases at a slower rate. Nevertheless, new organohalogens continue to be discovered in the environment. Perfluorinated compounds (PFCs) and polybrominated diphenyl ethers (PBDEs) are widely used in a variety of industrial and consumer products that are considered emerging persistent global environmental pollutants. The historical background of the organohalogens, their physicochemical properties, environmental contamination, human exposure, and effects on human health are described in this research paper with particular focus on chlorinated, brominated, and fluorinated compounds.
Classes Of Organohalogens And Background Information
Chlorinated hydrocarbons were synthesized as early as 1830. Polychlorinated biphenyls (PCBs) were first synthesized in the early 1880s by Schmidt and Schultz (1881) and their commercial production began in 1929.
Commercial PCB formulations were sold under a variety of trade names; for example, in the United States and Great Britain Aroclor was the most common trade name for PCBs. PCB mixtures were named according to their chlorine content. For instance, Aroclor 1254 contains 54% chlorine by weight, and Aroclor 1260 contains 60%. The PCB mixture formulations were different depending on the country of origin and were produced in Germany (Clofen), France (Phenoclor and Pyralene), Japan (Kanechlor), Italy (Fenclor), Russia (Sovol), and Czechoslovakia (Delor). PCB mixtures were produced for a variety of uses such as fluids in electrical transformers, capacitors, heat transfer fluids, hydraulic fluids, lubricating and cutting oils, and as additives in plastics, paints, copying paper, printing inks, adhesives, and sealants. Agricultural insecticide, DDT, was first synthesized before the turn of the 20th century by Zeilder in 1874. Its insecticidal value was discovered, and it was put to field use in the 1940s. Subsequently, this chlorine-containing insecticide replaced compounds with arsenic, lead, and copper, which were in use as insecticides. Application of DDT contributed to rapid reduction of malaria and other insect-borne diseases such as typhoid fever and cholera, and an astonishing associated increase in agricultural productivity in many regions of the world. The great success in the application of DDT during, and in the years following, the Second World War earned Paul Mu¨ ller a Nobel Prize in Medicine. The other organochlorines, HCHs and CHLs, were introduced in 1945 and these insecticides also contributed to human welfare as agricultural and domestic pest control agents. Owing to persistent, bioaccumulative, toxic properties and long-term health effects, organochlorine compounds were banned or severely restricted in the early 1970s in several developed countries (Loganathan and Kannan, 1994). Following this initial restrictive action, persistent, bioaccumulative, and toxic chemicals (PBTs) were the subject of a concerted regional, national, and international effort to limit the production, use, and control the disposal of materials no longer in use.
The next class of organohalogens of concern is brominated compounds, including polybrominated diphenyl ethers (PBDEs) and polybrominated dibenzo-p-dixions/ furans (PBDDs/PBDFs). PBDEs constitute an important group of flame retardants. PBDEs are added to consumer products so the products will not catch fire or will burn more slowly if exposed to flame or heat. PBDEs are added to plastics, upholstery, fabrics, and foams and in common products such as computers, television sets, mobile phones, furniture, and carpet pads. Nearly 90% of electrical and electronic appliances contain PBDEs, which are added as flame retardants that afford upto 15 times greater escape time in case of a fire. PBDDs/PBDFs are relatively less toxic than chlorinated dioxins and are formed during heating or incineration of polybrominated biphenyls (PBBs) and PBDEs. Low levels of PBDDs/PBDFs detected in environmental samples suggest relatively lower exposure to biota (fish) and humans to these compounds.
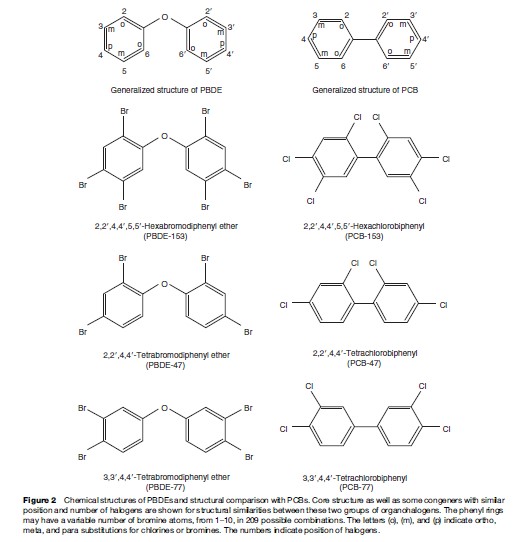
In contrast to PCBs, PBDEs are currently being produced and used in household materials. PBDEs are primarily indoor pollutants. PBDEs leach into the environment when household wastes decompose in landfills or are incompletely incinerated. Human health concerns stem from the fact that PBDEs are persistent, bioaccumulative, and structurally similar to PCBs (Figure 2). PBDE concentrations are rapidly increasing in the global environment and in human blood, breast milk, liver, etc. Although these chemicals are ubiquitous in the environment and bioaccumulate in wildlife and humans, little is known about their potential toxic properties (Kodavanti, 2005).
Perfluorinated compounds are another group of organic compounds in the persistent organohalogen family. PFCs are used in a variety of specialized consumer and industrial products (Senthil Kumar, 2005). PFCs are used in metal-plating baths, surfactants, cleaning products, rust inhibitors, fire-fighting applications, starting materials for polymers, herbicide and insecticide formulations, cosmetics, shampoos, pharmaceuticals, water and oil repellent coatings for fabrics and paper, greases and lubricants, paints, polishes, upholstery, textiles, carpets, soil/stainresistance coatings, mining and oil well surfactants, acid mist suppressants, electronic etching baths, alkaline cleaners, floor polishes, photographic film, and denture cleaners and adhesives. PFCs are also used in paper protection, including food contact applications (plates, food containers, bags, and wraps) and non-food-contact applications (folding cartons, masking papers; Kannan et al., 2004).
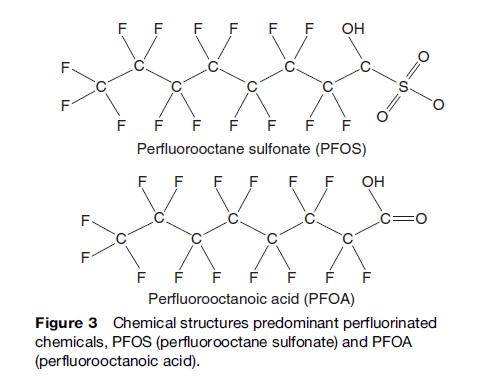
The chemical stability and nondegradable nature of PFCs, coupled with their widespread use, has led to global environmental contamination and accumulation of PFCs in aquatic and terrestrial organisms, including humans. Perfluorooctane sulfonate (PFOS, Figure 3) and perfluorooctanoic acid (PFOA, Figure 3) have been routinely detected in environmental matrices, wildlife, and human tissues (Kannan et al., 2004). Detectable amounts of PFOS were also found in human blood samples obtained from individuals residing in a number of countries. These findings have raised concerns about environmental contamination by perfluorinated compounds and their possible impacts on ecosystems and on human health. Although perfluorinated chemicals are ubiquitous in the global environment and bioaccumulate in wildlife and humans, their toxic properties are still under investigation.
Chlorinated Compounds
Physicochemical Properties
Human-made organochlorine compounds possess unique properties that render them highly persistent in the global environment, causing chronic toxicity to wildlife and humans. PCBs are colorless to light yellow, have no smell, and are tasteless oily liquids or solids. Some PCBs are volatile and may exist as a vapor in air. The physicochemical properties of PCBs vary widely and depend on the number and positions of chlorine atoms in the biphenyl rings. PCBs resist both acids and alkalis and have thermal stability. This has made them useful in a wide variety of industrial applications including dielectric fluids in transformers and capacitors, heat transfer fluids, and lubricants. Generally, PCBs are relatively insoluble in water and the solubility decreases with increased chlorination. PCBs are readily soluble in nonpolar organic solvents and biological lipids. When PCBs are burned at high temperatures, the products of combustion include polychlorinated dibenzofuran (PCDFs) and polychlorinated dibenzo-p-dioxins (PCDDs), which are more hazardous than PCBs itself. DDT compounds, HCH isomers, and chlordane compounds have properties similar to certain higher-chlorinated and lower-chlorinated PCBs, respectively. The physical and chemical stability of the organochlorines contributes to their persistence in the environment and is responsible for the environmental and health problems caused by these compounds.
Environmental Contamination And Human Exposure
PCBs entered the air, water, and soil during the manufacture and use in a variety of applications. PCB wastes are placed in landfills. PCBs also entered the environment from accidental spills and leaks during the transport of PCB-containing materials. Once in the environment, PCBs do not readily break down and therefore remain there for a very long period of time. PCBs can easily cycle between air, water, and soil. For example, PCBs can enter the air by evaporation from both water and soil. In air, PCBs can be carried long distances and have been found in snow and seawater in areas far away from where PCBs were released into the environment, such as the Arctic and Antarctic environments. This has resulted in extended contamination and distribution over the global environment, as evidenced by their detection in remote environmental media and biota such as the Arctic and the Antarctic atmosphere, hydrosphere, and biosphere. Because of their persistent and lipophilic properties, PCBs bioaccumulated in various lower trophic organisms (plankton), bivalve mollusks, fish, reptiles, marine mammals, birds, and terrestrial mammals. In concurrence with these findings, these compounds have been detected in fish and other food products. The primary route of human exposure of PCBs and chlorinated pesticides was through consumption of contaminated foods such as dairy products, meat, and freshwater fish. Unexpectedly high levels of these compounds were found in human adipose tissues, blood, and milk (Loganathan et al., 1993). Chlorinated pesticides such as DDT compounds, HCB, HCHs, and chlordane compounds also possess similar properties to certain PCBs; these compounds also contaminated the global environment and biota. Organochlorine pesticides also showed a similar pattern of bioaccumulation and biomagnification in the food chain and levels in humans to that observed for PCBs.
Effects On Human Health
The available biomedical data and laboratory animal studies provide strong evidence of the toxic health effects of PCBs and chlorinated pesticides. Information on health effects of PCBs is available from studies of people exposed accidentally, by consumption of contaminated rice oil in Japan in the year 1968 (outbreak of Yusho) and Taiwan in the year 1979 (the Yu-Cheng incident), by consumption of contaminated fish and meat products and via general environmental exposures. Major symptoms of Yusho disease consisted of acneform eruptions, pigmentation of the skin, nails, and conjunctiva, increased discharge from the eyes, and numbness of the limbs (Yusho Support Center, 2007). Health effects that have been associated with exposure to PCBs in humans and/or animals include liver, thyroid, dermal, and ocular changes, immunological alterations, neurodevelopmental changes, reduced birth weight, reproductive toxicity, and cancer. PCB exposures have been associated with low birth weight and learning and behavioral deficits in children of women who consumed PCB-contaminated fish from Lake Michigan. Further, coplanar PCBs (chlorine substitutions in para and meta positions in the biphenyl molecule) cause dioxin-like toxicity via AhR-mediated toxicity, leading to the development of cancer. However, non-dioxin-like PCBs seem to exert neurotoxicity through their effects on thyroid hormones and intracellular signaling processes (Kodavanti and Tilson, 1997). Serious environmental and health problems, especially in birds, were attributed to DDT and its metabolites (DDE, DDD). In addition, 4,40 -DDE, which is a major metabolite of 4,40 -DDT, has been linked to eggshell thinning and diminished reproductive success in a variety of bird species, including peregrines, hawks, gulls, eagles, terns, cormorants, and many other species. In addition, abnormalities in male sexual development in humans have been associated with the exposure of estrogenic chemicals such as DDT. It is known that 4,40 -DDE does not bind to the estrogen receptor but inhibits androgen binding to the androgen receptor, resulting in inhibition of androgeninduced transcriptional activity and an androgenic effect in mammals.
Brominated Compounds
Physicochemical Properties
Similar to PCBs and other chlorinated pesticides, polybrominated diphenyl ethers (PBDEs) are synthetic chemicals that do not occur in nature. PBDEs comprise two phenyl rings linked by oxygen (thus the designation as ether; Figure 2). PBDEs are structurally similar to PCBs (Figure 2) and are quite resistant to physical, chemical, and biological degradation. Also, PBDEs cause adverse effects similar to PCBs on nervous, immune, and endocrine systems and influence metabolism of chemicals endogenous to the body as well as the metabolism of foreign chemicals. Compared to chlorine atoms, bromine atoms are in general lost more easily from the molecule (more reactive), rendering PBDEs susceptible to various types of degradation and metabolism more readily than PCBs.
Environmental Contamination And Human Exposure
PBDE residues have been detected in indoor air, house dust, and foods. PBDE exposure to humans may be possible via multiple sources (air, water, food, and dust). PBDEs are found in higher levels in house dusts in the United States than Europe. The contents of vacuum cleaner bags were used to assess household dust exposure to PBDEs in a total of 20 U.S. and German homes. This study found that PBDE-47, PBDE-99, and PBDE-209 were present in the highest concentrations and that the U.S. samples were approximately 50 times higher than samples from Germany. High concentrations of PBDEs are also found in sewage sludge, with levels in the United States running 10–100 times higher than those in Europe. Over half of the sewage sludge produced annually in the United States is applied to land as fertilizer (US Environmental Protection Agency, 1999). Thus, application of sewage sludge may represent a source of exposure to humans and wildlife, through direct contact or uptake by plants. For example, a recent survey of U.S. foods showed PBDEs in infant soy formula and beef, suggesting that plants as well as animals can be contaminated with PBDEs.
Significant levels of PBDEs may be found in outdoor air, even at rural locations. PBDE levels were about half those of PCBs in early spring. It is currently well established that there may be significant levels of PBDEs in indoor air and dust. PBDE concentrations in indoor films were 15–20 times higher than in outdoor films. Levels in the U.S. dust were 50 times higher than those in Germany.
Levels of PBDEs in human tissues, specifically blood, milk, and fat, have increased exponentially since the 1970s in several countries, including the United States, Canada, and Sweden (Schecter et al., 2005). Currently, the doubling time for PBDE levels in the human body is estimated to be 3–5 years. In recent years in Sweden, breast milk levels are decreasing, presumably as a result of a decrease in the use of PBDE-containing products. The European Union has decreased PBDE use by two-thirds in recent years. Levels of PBDEs among individuals in North America, as measured in blood, breast milk, or adipose tissue, are 10–70 times higher than in Europe or Japan (Shechter et al., 2005). High levels of PBDEs in North America were attributed to the maximum use of penta BDE mixture (>90%) when compared to the rest of the world. Like other lipophilic compounds, PBDEs readily cross the placenta into the fetus. This provides the opportunity for PBDEs to interfere with developmental processes, producing developmental effects.
Effects On Human Health
There have been no epidemiological studies on the health effects of environmental exposure to PBDEs. Although there are studies in animals documenting adverse effects, there are not adequate data on administered doses that do not produce overt toxicity. In addition, extrapolation of a dose in a rodent (rat or mouse) to a comparable dose (or exposure) in humans requires either extensive pharmacokinetic data in both rodents and humans, or a series of assumptions. Currently, there is not sufficient information to extrapolate effect data from rodents to humans. Reliance on a series of assumptions obviously introduces substantial uncertainty and probably inaccuracy. One approach that may be useful to circumvent the difficulties associated with rodent-to-human extrapolation is to compare current observed levels of PBDEs in humans to the levels of PCBs that are known to produce adverse effects in humans. One of the most sensitive (perhaps the most sensitive) endpoints for adverse PCB effects is developmental neurotoxicity. It appears that this may be the case for PBDEs as well, based on the current available data on structural similarities and adverse effects seen in in vitro as well as in in vivo in animal models. In addition, the mechanisms of action for PBDE and PCB neurotoxicity may be the same, because the limited data available suggest that PCBs and PBDEs are approximately equipotent on neuronal intracellular signaling.
A study in the Netherlands collected breast milk shortly after birth from 209 mothers who intended to breastfeed their newborns. The study also included 209 mothers who did not intend to breastfeed. The neuropsychological function of the children was tested beginning in infancy, with periodic assessment up to 9 years of age. Deficits associated with PCB exposure were observed in both breastfed and nonbreastfed infants on a number of cognitive and other behavioral functions (Vreugdenhil et al., 2004). PCB levels in breast milk were based on the four most prevalent congeners, representing about 60% of the total PCBs. The median concentration was 414 ng/g lipid, with a range of 158–969 ng/g. Dividing by 0.6 results in a median of 690 ng/g and a range of 263–1615 ng/g. A similar study in Germany, with equivalent breast milk PCB levels, also documented adverse effects on cognition associated with increased PCB exposure (Winneke et al., 1998).
Milk concentrations of PBDEs in the United States are currently well below the levels reported in the Dutch and German PCB studies. The median PBDE concentration in the Texas study was 34 ng/g lipid, with a range of 6–419 ng/g (Schecter et al., 2003). In other studies, the median for breast milk levels was 58 ng/g, with a range of 9.5–1078 ng/g. The median value in breast milk is approximately ten times higher for PCBs than for current concentrations of PBDEs; however, the concentration ranges overlap. The current doubling time for levels of PBDEs in milk in North America is as short as 2.6 years. Assuming that the current exponential increase continues, doubling times will continue to decrease. In this case, levels that are known to produce developmental neurotoxicity would be reached in less than 10 years. It is also important to remember that a no-effect level for PCBs has not been determined. Therefore, it is not known whether current concentrations of PBDEs in the environment are producing adverse effects, assuming that PBDEs have effects at approximately the same concentrations as PCBs. In addition, it is likely that the effects of PCBs and PBDEs are additive, at least for neurotoxicity. Therefore, the levels of PBDEs currently in the environment may well be producing adverse effects.
Although the U.S. Government has not banned penta or octaBDE, they have been voluntarily withdrawn by manufacturers. This withdrawal should result in at least a retardation of the doubling time. Since the vast majority of the PBDEs currently in use are the decaBDE mixture, it is expected that its metabolic products will make up an increasingly important fraction of the PBDE concentrations in the human body, if production does not cease. Based on current information, it is not possible to estimate how quickly decaBDE and its products would increase.
Fluorinated Compounds
Physicochemical Properties
Perfluorinated compounds are emerging new environmental pollutants. Most fluorinated organohalogens possess amphiphilic (ionic and neutral) properties. Owing to their thermodynamically strong covalent C–F bonds, these compounds were initially considered nonmetabolizable and nontoxic. In many cases, a given perfluorinated compound may readily convert to one or more additional perfluorinated compounds. For example, because of the reactivity of its sulfonyl group, perfluorooctane sulfonate – which is used in fire-fighting foams, herbicides, insecticides, paints, lubricants, and carpeting – readily converts to perfluorooctanoic acid, potassium perfluorooctane sulfonate, and perfluorooctane sulfonamide. To further complicate the situation, many perfluorinated compounds easily isomerize. Because the physicochemical and biochemical properties such as vapor pressure, water solubility, lipophilicity, metabolic degradability, particle affinity, etc., of organohalogen compounds differ from isomer to isomer, the environmental distribution, persistence, and toxicity of various perfluorinated isomers will vary significantly. Isomer-specific analysis is therefore needed to address the environmental risk posed by these compounds. Perfluorinated compounds are water-soluble in the several parts per million range. PFCs with unique surface modification properties readily bind to surfaces including blood globulins.
Environmental Contamination And Human Exposure
Although perfluorinated compounds have been produced and used in a wide variety of industrial and consumer products for more than five decades, these compounds have recently been recognized as a group of environmental pollutants about which little is known. Because of their unique properties, PFCs are environmentally persistent and have been detected in ice, water, sediment, sewage sludge, etc., and in aquatic organisms, terrestrial and marine animals, and humans. Organofluorine compounds were first found in human blood in the 1960s. PFCs have been detected in human blood samples throughout the world (reviewed by Houde et al., 2006). However, the major routes of PFOS exposure in humans are not fully understood. Among other possibilities, consumer products such as nonstick cookware and popcorn bags may be a source of exposure in addition to food products such as fish. A recent study of human blood samples from around the world found the highest levels of PFOS in the United States and Poland, intermediate levels in Korea, Belgium, Malaysia, Brazil, Italy, and Colombia, and the lowest in India (Kannan et al., 2004).
Effects On Human Health
There have been no epidemiological studies on the health effects of environmental exposure to PFCs. There were few reports in animals indicating adverse health effects associated with exposure to PFCs. In PFOS production employees in Decatur, Alabama, and Antwerp, Belgium, serum hepatic enzymes, cholesterol, or lipoproteins were not altered when serum PFOS levels were less than 6 ppm. However, when PFOS levels were noted to be more than 6 ppm in the year 1995 (0.0–12.83) and in 1997 (0.1–9.93) (Olsen et al., 1999), evidence of peroxisome proliferating effect in individuals exceeding 6 ppm serum concentrations was noticed. Another study showed that the PFOS concentrates in liver and serum causing hypolipidemia at cumulative dosages. In humans, PFOA and PFDA can alter expression or secretion of a differentiated gene product (IgM). At high exposure levels, both chemicals increased solubilization of proteins from lymphoblastic cell lines. Another PFOA-related modulation in hepatic responses is obesity. The cross-sectional study in occupationally PFOA-exposed humans showed 10% increase in mean estradiol levels.
Humans exhibit a weak response to the peroxisome proliferating PFCs, which is in part due to a relatively low level of peroxisome proliferator-activated receptor (PPAR) alpha expression in human liver. PFOA induced apoptosis in human HepG2 cells with the involvement of reactive oxygen species (ROS), mitochondria, and caspase-9 in PFPA-induced apoptosis. In addition, PFOA was reported to have modulated hepatic responses to obesity and alcohol consumption among the production workers.
Summary And Conclusions
Increasing levels of chlorinated, brominated, and fluorinated persistent organic compounds in the environmental media (air, water, soil, sediment) and in human tissues including adipose tissue, breast milk, and placenta continue to be a cause of human health and ecological concern. For persistent organic pollutants (POPs), especially PCBs and dioxins, consumption of contaminated fish and other food materials has been the main route of exposure to humans. The exposure pathway for these chlorinated organic compounds in humans is mainly from the outdoor environment and biota. However, for the emerging environmental pollutants such as brominated (PBDEs) and perfluorinated compounds (PFCs), the human exposure pathway is predominantly from indoor contamination. Given the widespread use of PBDEs in household items, indoor contamination may be a significant source of human exposure. Similarly, perfluorinated compounds are used in a variety of consumer products and exposures from the indoor environment and consumption of PFCcontaminated foodstuffs may contribute to exposures. The human exposures and health effects by these organohalogens will continue for a long period of time, even after a ban on their production, as has been evidenced for organochlorines.
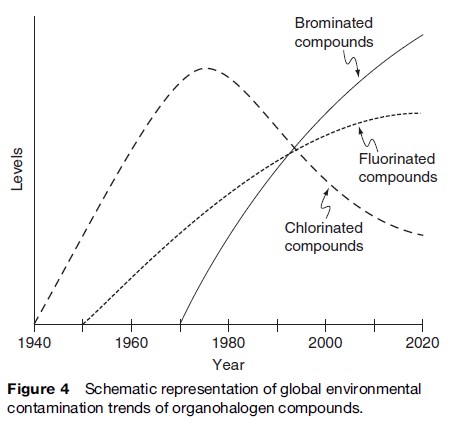
A schematic representation of time perspectives of environmental contamination and exposure to humans is shown in Figure 4. Chlorinated compounds such as PCBs and pesticides very rapidly contaminated the environment and biota during the periods of their use for agricultural and public health purposes. The contamination levels declined after the ban or severe restrictions placed on the production and use of these compounds in most developed countries. However, developing countries still continue to use these inexpensive chemicals for agricultural pest control and to control insects that spread malaria, typhoid, dengue fever, etc. Thus, developing countries form the point source for continued global contamination with the organochlorine compounds. Therefore, future chronic toxic effects in humans and wildlife by organochlorine compounds cannot be ruled out. In contrast, brominated and fluorinated compounds are being produced in large quantities and used globally by both developed and developing countries. These compounds are heavily used in indoor appliances and materials. Human exposure pathways for PBDEs and PFCs are direct and intimate. Considerable data have been amassed on the presence of PBDEs and PFCs in indoor environmental media (air, water, dust, lint, clothing, food packaging materials, etc.) and human tissues (blood, breast milk, liver, fetus, etc.). Based on PBDE and PFC use, their recalcitrant property, bioaccumulation, and biomagnification potential, it can be predicted that the environmental contamination, and human exposure and health effects by these compounds will continue to increase for several decades in both developed and developing countries (Figure 4). These factors need consideration in the effort to minimize exposure to humans from indoor pollution, and dietary exposure to protect human health from possible long-term health effects caused by the organohalogen compounds.
Bibliography:
- Houde M, Martin JW, Letcher RJ, Solomon KR, and Muir DCG (2006) Biological monitoring of perfluoroalkyl substances: A review. Environmental Sciences and Technology 40: 3463–3473.
- Kannan K, Corsolini S, Falandysz J, et al. (2004) Perfluorooctanesulfonate and related fluorochemicals in human blood from several countries. Environmental Sciences and Technology 38: 4489–4495.
- Kodavanti P (2005) Neurotoxicity of persistent organic pollutants: Possible mode(s) of action and further considerations. Dose Response 3: 273–305.
- Kodavanti PR and Tilson HA (1997) Structure-activity relationships of potentially neurotoxic PCB congeners in the rat. Neurotoxicology 18: 425–442.
- Loganathan BG and Kannan K (1994) Global organochlorine contamination: An overview. AMBIO 23: 187–191.
- Loganathan BG, Tanabe S, Hidaka Y, Kawano M, Hidaka H, and Tatsukawa R (1993) Temporal trends of persistent organochlorine residues in human adipose tissue from Japan, 1928–1985. Environmental Pollution 81: 31–39.
- Olsen GW, Burris JM, Mandel JH, and Zobel LR (1999) Serum perfluorooctane sulfonate and hepatic and lipid clinical chemistry tests in fluorochemical production employees. Journal of Occupational and Environmental Medicine 41: 799–806.
- Schecter A, Pavuk M, Papke O, Ryan JJ, Birnbaum L, and Rosen R (2003) Polybrominated diphenyl ethers (PBDEs) in US mothers’ milk. Environmental Health Perspectives 111: 1723–1729.
- Schecter A, Papke O, Tung K, Joseph J, Harris T, and Dahlgren J (2005) Polybrominated diphenyl ether flame retardants in the US population: Current levels, temporal trends, and comparison with dioxins, dibenzofurans, and polychlorinated biphenyls. Journal of Occupational and Environmental Medicine 47: 199–211.
- Schmidt H and Schultz G (1881) Uber benzidin (a-di-amidophenyl). Annalen der Chemie Liebigs 207: 320.
- Senthil Kumar K (2005) Fluorinated organic chemicals: A review. Research Journal of Chemistry and Environment 9: 50–79.
- US Environmental Protection Agency (1999) Biosolids Generation, Use and Disposal in the United States. EPA530-R-99–009. Washington, DC: Environmental Protection Agency.
- Vreugdenhil HJI, Mulder PGH, Emmen HH, and Weisglas-Kuperus N (2004) Effects of perinatal exposure to PCBs on neuropsychological functions in the Rotterdam cohort at 9 years of age. Neuropsychology 18: 185–193.
- Winneke G, Buchloski A, Heinzow B, et al. (1998) Developmental neurotoxicity of polychlorinated biphenyls (PCBs): Cognitive and psychomotor functions in 7-month old children. Toxicology Letters 102–103: 423–438.
- Yusho Support Center (2007) Left Behind the Yusho. A Report by Yusho Support Center. Tokyo, Japan: Yusho Support Center.
See also:
Free research papers are not written to satisfy your specific instructions. You can use our professional writing services to buy a custom research paper on any topic and get your high quality paper at affordable price.








