This sample Pesticides and Health Research Paper is published for educational and informational purposes only. If you need help writing your assignment, please use our research paper writing service and buy a paper on any topic at affordable price. Also check our tips on how to write a research paper, see the lists of health research paper topics, and browse research paper examples.
Introduction
Pesticides differ from any other chemical substance because they are deliberately spread in the environment with the aim of controlling undesired living species. To this aim, they are necessarily biologically active and therefore characterized by various degrees of toxicity. Since their toxicity is not always specific to the target organisms, the use of these compounds may pose risks to human health, the survival of nontarget species, and the environment.
On the other hand, pesticides are necessary for agriculture, animal breeding, and public health. It has been estimated that without the use of pesticides, up to 50% of the agricultural products, mainly in tropical countries, would be lost. Pesticides are also indispensable tools in public health, mainly for the control of vector-borne diseases and noxious insects. Finally, pesticides have several other uses, including protection of plants and products from biological degradation and amateur uses during leisure time, gardening, for pet care, indoor use, etc. It is therefore clear that the use of pesticides is unavoidable in several domains, but it is necessarily associated with some risk to human health and the environment, requiring assessment to decide on the acceptability of their use on the basis of a risk–benefit analysis.
It is worth underlining that pesticides have been used since the beginning of human history: Various methods for pest control were described by the classical writers. They were used for religious purposes, folk customs, and magic, as well as for controlling plant disease and animal pests. Although the efficacy of these methods may be questioned, the principles of seed treatment, fumigation, tree banding, and the use of preparations to control pests appears to have been widely used (Smith et al., 1975). At the beginning, the first molecules used as pesticides were of natural origin, for example extracted from flowers or other plants, or mined (arsenic, mercury, oil derivatives, etc.).
The first pesticide to be synthesized was 1,1,1-trichloro-2,2-bis[4-chlorophenil]ethane (DDT) in 1874, although it was not used until 1939, when Muller and co-workers discovered its insecticidal properties (IPCS, 1978). In the following years, DDT was used both in public health, mainly against the malaria vector anopheles mosquito, and, since 1946, in agriculture for protection of cotton, deciduous fruits, cereals, and potatoes. The use of DDT was important in the struggle against vector-borne diseases, contributing to the complete eradication of malaria in several regions of the world, and in crop protection, and opened the era of chemical pesticides. However, since the beginning of the 1960s, the bioaccumulation potential of DDT, together with the risk of effects to human and animal health and the environment have been demonstrated, which led to the restriction of its use in many countries. Nowadays DDT use is authorized only in limited and selected situations, only for public health purposes, such as indoor residual application or bed net treatment in tropical countries, within antimalaria activities (WHO, 2003). At the end of the 1960s and the beginning of the 1970s, organophosphorus compounds (OP) largely replaced DDT. These compounds immediately showed very good insecticidal activity, accompanied by low or absent potential for accumulation in biological organisms and in the environment; however, their introduction into the market caused a rapid growth of cases of acute poisoning and fatalities, attributable to the high toxicity of these compounds. For these reasons, research pointed to the development of molecules that were more specific for their targets, with lower toxic potential for humans and nontarget species; consequently other acetylcholinesterase inhibitors, such as the carbamate insecticides, were introduced on the market. Meanwhile, OP compounds that were more easily degradable by biological enzymes and, in particular, carboxylesterase, were developed. Since carboxylesterase is found in mammalian tissues, but poorly represented in insects, these OP compounds show good insecticidal activity and low toxicity to mammals. However, their toxicity for untargeted nonmammalian species such as bees remained unchanged. In 1978, a compound similar to natural pyrethrum, fenvalerate, was synthesized and introduced on the market. Later, other pyrethroids were developed; there are currently roughly 40 available on the market. These compounds are now largely used around the world, both in agriculture and in public health programs such as elimination of insects in aircraft flying from countries in which vector-borne diseases are endemic and indoor residual application in tropical countries for malaria prevention (WHO, 2003). The history of the development of insecticides, similar to the history of the development of other pesticides, such as fungicides and herbicides, clearly shows that the research has always been oriented at two major, complementary objectives: Efficacy in pest control and reduction of human and environmental risk. This task is not so easily achieved. Therefore, risk assessment of pesticide use is a fundamental component of pest management strategies, and regulations of pesticide production and use have been implemented over time, with the ban or restriction of use of the most dangerous and toxic compounds. At present, together with pharmacological substances, pesticides are the group of chemicals characterized by the largest and most comprehensive amount of toxicological (and, for pesticides, ecotoxicological) information. Based on this information, risk assessment and management can be undertaken either before a compound is introduced on the market (premarketing phase) or after its introduction (postmarketing phase), or both.
Classification Of Pesticides
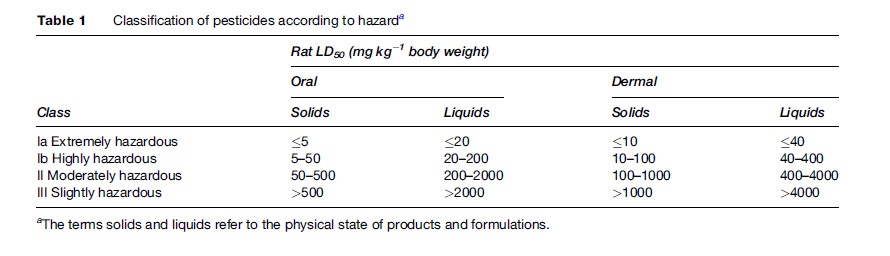
In most countries, pesticides are classified according to their acute mammalian toxicity, as suggested by WHO (WHO, 2006) (Table 1). The classification refers to the technical compounds and for each active ingredient it may vary according to the formulation. Different criteria are used when neither the oral nor the dermal is the most relevant route of absorption (e.g., fumigants). This classification is useful for identifying pesticides posing a hazard of acute poisoning, still a major problem in developing countries. However, if the active ingredient produces irreversible damage (such as terata or tumors), is volatile, causes markedly cumulative effects, or is found after direct observations to be particularly hazardous or significantly allergenic to man, then adjustments are made by placing the compound in a class indicating a higher hazard.
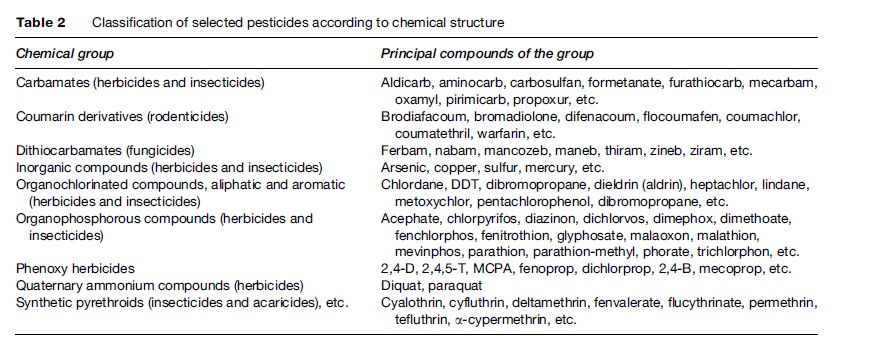
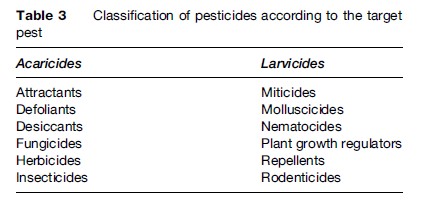
Pesticides can also be classified according to their chemical structure (Table 2) or according to the target pest, as reported in Table 3. Application of pesticides against individual pests implies the use of equipment and work practices and therefore different kinds of exposure.
Characteristics And Trends Of Human Exposure To Pesticides
Since they are deliberately spread in the environment, pesticides and their residues are nowadays ubiquitous environmental contaminants, and most of the human population can be exposed, even outside the workplace. Worker exposure to pesticides can take place either in the production and packaging of these compounds or during their use in agriculture and public health in indoor or outdoor applications. Sources and patterns of exposure vary significantly among different population subgroups. In particular, the skin is the main route of exposure for agricultural workers, while in industrial workers the main route of exposure is generally the respiratory tract. The general population is mainly exposed through the ingestion of low amounts of complex pesticide mixtures present as residues in food commodities, even though, in some cases, a significant intake of specific compounds can take place, for example in case of ingestion of heavily contaminated food. Environmental exposure may take place due to proximity to pesticide treatments, indoor application, and the presence of pesticides in materials such as leather objects and wooden furniture. In these cases, the main route of absorption is the respiratory tract, but also significant skin absorption, mainly in children, may take place. Of course, the levels of exposure measured in workers are usually some orders of magnitude higher than those observed in the general population, even though the characteristics of the exposure significantly vary among different worker subgroups. In particular, exposure of industrial pesticide workers is similar to any other occupational exposure in the chemical industry: it takes place in confined spaces, its levels are usually constant over time, it can be better controlled, and a relatively small number of well-identified compounds is usually involved. On the other hand, agricultural workers are usually exposed to a large number of compounds, in some cases not fully known and identified, exposure is intermittent, related to application modalities and schedules, and exposure levels are extremely variable, depending on weather conditions, type of application, and work practice (Maroni et al., 2000). Greenhouse activities are associated with levels of exposure much more similar to other confined environments. During formulation of commercial products, i.e., mixing one or more active ingredients at the appropriate concentration with other formulants, exposure has characteristics that are intermediate between industrial production and pesticide use in agriculture. In common with industrial production, exposure takes place in confined environments and it is continuous over time but, similarly to agriculture, it involves a large number of compounds, it typically occurs in small productive units, it is organized in campaigns, and therefore workers experience short-term exposure to several pesticides. In public health and agriculture, the use of a large variety of compounds is usually the rule, although in some specific applications, such as cotton defoliation or malaria control programmes, a single product may be present. Among agricultural workers, professional applicators can be considered as a group that is particularly at risk, because they are exposed to a high number of different pesticides, but their exposure is more prolonged rather than intermittent over short periods of time (hours or days), as in the case of other agricultural workers.
Pesticide Exposure Monitoring
Determination of the extent of exposure either as the amount of compound potentially able to come into contact with the human body and then be absorbed (environmental monitoring) or as the dose that actually has been absorbed in the organism (biological monitoring) is a fundamental component in risk assessment activities.
Unfortunately, both environmental and biological monitoring are substantially limited in their use in pesticide exposure in agriculture for several reasons.
Environmental monitoring stemming from agricultural activities taking place outdoors and exposure levels varying greatly over time, make it very difficult to collect representative measures. It is worth mentioning that, since the main route of pesticide absorption in agricultural workers is the skin, the most reliable environmental monitoring techniques designed to measure the amount of airborne chemical substances are not useful: the more complicated, less reliable, and expensive approach of measuring dermal exposure is necessary. For pesticide workers submitted to industrial exposure, the environmental monitoring approach is similar to the approach used for any other chemical substance.
It is evident that biological monitoring is potentially the most adequate tool for monitoring the pesticide exposure of agricultural workers. Unfortunately, biological monitoring also has several limitations, such as exposure levels varying over time, which complicates choosing the appropriate sampling time, and the representativeness of the results and consequently whether they can be extrapolated, the lack of biological exposure limits and even reference values for most of the compounds used, and the poor knowledge of the toxic kinetics of most pesticides in humans.
Since measuring the levels of exposure of agricultural workers is a very complicated task that is unfeasible in many situations, the use of mathematical models and other estimation methods is being proposed to obtain surrogates for exposure data aimed at risk assessment activities.
Toxic Effects Of Pesticides
Acute Poisonings
Acute pesticide poisoning can derive from intentional, occupational, or accidental exposure to pesticides, but worldwide figures of pesticide poisonings are not available. Pesticides are estimated to be responsible for less than 4% of deaths from all types of accidental poisoning, as based on reports from poison control centers. In a study conducted in Central American countries from 1992 to 2000, an apparent increase in pesticide poisoning cases from 6.3 to 19.5 per 100 000 population was observed, together with an increased mortality rate from 0.3 to 2.1 per 100 000. This apparent increase in the number of cases in recent years may reflect increased use but also availability of better statistics (PAHO, 2002). Attempts to quantify the extent of pesticide poisoning frequently do not separate occupational, accidental, and intentional poisoning and also fail to define, at least qualitatively, the severity of poisoning. However, available data indicate that suicide attempt is by far the most frequent cause of pesticide poisoning, at least in developing countries where it represents 44–91% and 26–60% of acute pesticide poisonings in South-East Asia and in Central America, respectively (Besbelli, 2001; PAHO, 2002). Suicide attempts usually, but not exclusively, involve organophosphorus compounds (Besbelli, 2001). Severity of poisoning varies from country to country, and mortality rates due to poisoning of from less than 1% to 24% or more have been observed. This variability is in part linked to the different percentage of suicide attempts. The majority of unintentional pesticide poisonings are occupational, although cases occur in the general population due to improper use or storage of pesticides intended for amateur uses or in-house pest control ( Jeyaratnam, 1990). Occupational poisonings account for 5–32% of the cases in South-East Asia and roughly 36% in Central American countries (Besbelli, 2001; PAHO, 2002).
Chronic And Long-Term Pesticide Effects
Long-term exposures occur in farmers, professional pesticide users, and, to a much lesser extent, in the general population via residues in food and water and by environmental exposure due to indoor and outdoor use for pest control. The identification of subjects who might have been occupationally or nonoccupationally chronically exposed to pesticides is relatively easy; however, the identification of the source of exposure is difficult, particularly in agriculture, and the toxicological evidence and quantitative information of exposure is seldom available. Moreover, extrapolation from current data to assess past exposures as well as the risk associated with a given pesticide is difficult since active ingredients and application practices differ and change with time. This is particularly true in the general population where data on exposure and biological monitoring are s
Carcinogenicity
Carcinogenicity potential for pesticides is evaluated in experimental animals before the registration of the compound and its marketing. However, this applies to molecules that have been introduced or evaluated in the last 30–35 years. The results of these studies are generally not published in the open literature because they are proprietary data of the manufacturers and are only available to the regulatory bodies. Cancer risk assessment for pesticides for regulatory purposes is based on evidence from cancer studies generally conducted in the rat and the mouse, although a recent revision showed that carcinogenicity studies in mice do not necessarily provide information adequate to raise conclusions on carcinogenic risk to humans (Doe et al., 2006). Evidence from in vitro and in vivo genotoxicity studies is also taken into account. However, since the carcinogenic effect is expected to occur decades after the exposure, evaluations of the carcinogenic potential have also been conducted on the basis of epidemiological data. In this respect, a number of pesticides have been evaluated, especially by the International Agency for Research on Cancer (IARC). The criteria used by the IARC for choosing the compounds to be evaluated include evidence of human exposure, some experimental evidence of carcinogenicity, and/or some evidence or suspicion of a risk to humans. The results obtained so far by IARC include approximately 60 pesticide active ingredients, most of which are no longer in common use. Among those still in some use, ethylene dibromide was classified as 2A (the agent is probably carcinogenic to humans), and its use is highly restricted; amitrole and dichlorvos were classified as 2B (the agent is possibly carcinogenic to humans). The classification of dichlorvos has been criticized, and recently the Scientific Panel on Plant Health, Plant Protection Products and their Residues of the European Food Safety Authority considered the carcinogenic potential of dichlorvos in animals not relevant to humans, on the basis of mechanistic considerations (EFSA, 2006).
The epidemiology of cancer in agricultural workers is a very complex issue. Although a Medline search done with the terms pesticides, cancer, and epidemiology yielded a total of 665 publications, the actual results of epidemiological studies have been inconsistent, and a clear picture of the epidemiology of cancer in relation to agricultural exposure has yet to emerge. Potential carcinogenic threats in agriculture can be attributable to different types of substances and risk factors. In fact, farmers may come in contact with a variety of substances, including pesticides, solvents, oils and fuels, dusts, paints, welding fumes, zoonotic viruses, microbes, and fungi (Alavanja et al., 2005; Alexander et al., 2005). However, the results of epidemiological studies suggest that farmers had consistent deficits for cancers of the colon, rectum, liver, nose, and bladder, while excessive rates of malignancy include Hodgkin’s disease, leukemia, non-Hodgkin’s lymphoma, multiple myeloma, and cancers of the lip, stomach, prostate, skin (nonmelanotic), brain, and connective tissues. The etiologic factors that may contribute to these inconsistent excesses in the agricultural environment have not been identified.
A recent, large, prospective cohort study of private applicators, commercial applicators, and spouses of farmer applicators conducted in Iowa and North Carolina, the Agricultural Health Study, showed a low overall cancer incidence rate, probably as a result of low overall smoking prevalence and other lifestyle factors. These data are in agreement with the body of the published literature, which suggests that agricultural workers, as a general rule, are healthier than the general population, with an overall death rate and a total cancer mortality less than expected, but with a higher incidence of certain specific neoplasms: leukemia, myeloma, non-Hodgkin’s lymphoma, and lip, stomach, skin, brain, and prostate cancers (Alavanja et al., 2005; Alexander et al., 2005). Based on these data, one might conclude that there is not, at present, convincing evidence that repeated exposure to pesticides causes cancer in humans, but some areas of concern deserve further investigation.
Effects On Children
Children (especially in the first 6–12 months after birth) are considered by some to be at higher risk of toxic effects from pesticide exposure as their metabolic processes are immature, and they are less able to detoxify chemicals. In some instances, however, metabolic immaturity may be beneficial, because the metabolic pathways that activate their toxic metabolites are not yet developed. Available data suggest that the possible increased susceptibility is evident at high doses, whereas young animals do not appear to be more susceptible to low doses causing no toxic effects in adults.
Long-Term Neurological Effects
Pesticide exposure has been implicated as the cause of several neurological disorders including diseases of the central nervous system (parkinsonism), neurobehavioral changes, diseases of the peripheral nervous system, and suicide.
Among the studies where parkinsonism and pesticide exposures were considered, there were 31 case–control studies (Li et al., 2005). These included studies with different criteria for definition of parkinsonism such as nonspecific signs, or cardinal signs of Parkinson’s, or cardinal signs and positive reaction to L-DOPA, or, in addition to the latter, also progressivity of disease. In addition, the assessments of exposure to pesticides varied greatly in these studies. In fact, 20% of the studies only considered pesticides as a single variable, 22% identified the pesticidal class, 25% considered duration and frequency of pesticide exposure, and 33% took into account both the pesticidal class and the duration/frequency of exposure. The general conclusion from these studies is that there is a very weak, if any, relationship between pesticide exposure and Parkinson’s disease.
In 38 studies of chronic OP pesticide exposures, behavioral and neurological endpoints were measured and assessed (Kamel and Hoppin, 2004). Also in these studies, assessment of exposure was very crude and included the identification of past poisoning (22%), work as a pesticide applicator (55%), cumulative exposure (15%), and AChE measurements (8%). Toxicological endpoints in 18 studies included behavioral symptoms, affect, cognitive and psychomotor functions; in six studies neurological endpoints included vibration sensitivity, balance, tremor, and nerve function; and in 14 studies both groups of endpoints were used. Most of the studies identified one or more endpoints to be significantly affected by exposure. However, the results were inconsistent, reported changes were usually mild, and a clear syndrome could not be identified. Sometimes changes were reported to be reversible, sometimes irreversible, because the features were observed a long time after cessation of exposure. The significance of these changes, if real, remains unclear. In addition, electrophysiological results were usually examined together on a group basis and correlation with clinical data was almost always missing (Colosio et al., 2003; Lotti and Moretto, 2005).
Dermatitis
The most common clinical form of pesticide-related skin diseases is contact dermatitis, either allergic or irritant. Many compounds have been reported to cause either the allergic form, the irritant form, or both (Moretto, 2002). Sometimes, the irritant effect might be due to co-formulants rather than to the active ingredient. Less commonly occurring diseases are erythema multiforme, ashy dermatosis, acne, porphyria cutanea, and hair and nail disorders (Spiewak, 2001).
Strategies For Prevention Of Pesticide Risks
Premarketing Phase
The premarketing phase concerns all the activities that can be carried out before a product is introduced on the market and authorized for use. In developed countries, strict requirements must be satisfied before authorization, and the cost of satisfying these requirements is very high and borne by the manufacturers. In developing countries, considerable amounts of pesticides are imported from industrialized countries, but effective premarketing legislation is not always in place. This lack of proper legislation and adequate control infrastructures to enforce legislation in developing countries often leads to the use of the most hazardous and inexpensive compounds and to poor control of adverse effects to humans and the environment.
A key element in this phase is the toxicology and ecotoxicology testing of the product, addressed at preparing a toxicological and ecotoxicological profile of the substance to be marketed. A predefined set of toxicity tests is required and carried out, generally with minor differences, by all industrialized countries. It includes information on acute toxicity (oral, inhalation, dermal), skin and eye irritation, skin sensitization, short-term toxicity, mutagenicity, long-term toxicity, carcinogenicity, and effects on reproduction. Specific effects, i.e., neurotoxicity or immunotoxicity, are studied only if suggested by the body of data collected on the substance or based on its chemical and physical properties. Based on the toxicological data collected, and on the intended use of the compound, a risk assessment is carried out that leads to the establishment of exposure limits such as the Acceptable Daily Intake (ADI; estimated maximum amount of an agent, expressed on a body mass basis, to which an individual in a (sub)population may be exposed daily over its lifetime without appreciable health risk), the Acute Reference Dose (ARfD) (the following definition of the ARfD was adopted by the 2002 JMPR (FAO/WHO, 2003): The ARfD of a chemical is an estimate of the amount of a substance in food and/or drinking-water, normally expressed on a body-weight basis, that can be ingested in a period of 24 h or less, without appreciable health risk to the consumer, on the basis of all the known facts at the time of the evaluation), and the Acceptable Operator Exposure Level (AOEL) (according to the European Union Directive 97/57/EC (establishing Annex VI to Directive 91/414/EEC), the AOEL is defined as ‘.. . the maximum amount of active substance to which the operator may be exposed without any adverse health effects. The AOEL is expressed as milligrams of the chemical per kilogram body weight of the operator’). The uses of the compounds should be compatible with such exposure limits in order to allow the registration and authorization for use of the pesticide. As a consequence, it is very important to develop suitable methods for exposure estimation. The evaluation of pesticide operator exposure, for example, is an integral part of risk assessment, for both regulatory assessment purposes and postregistration surveillance of pesticide use. Risk is assessed through estimation of operator exposure using generic exposure databases and taking into account the intended uses and comparing this estimate with the AOEL. If the AOEL is exceeded, authorization might not be granted for the compound.
The manufacturer may participate in the preventive process by producing safer formulations (for example, granules or soluble packages), by withdrawal of the most toxic compounds from the market, and by the preparation of easily understandable guidance to the adequate use of the product.
Postmarketing Phase
Once a pesticide is placed on the market, workers, the general population, and the environment are monitored as part of surveillance activities. For the general population, monitoring pesticides and their residues in food and drinks is the most relevant activity carried out in the postmarketing phase. This can estimate the potential for pesticide exposure in the general population and, based on the results, specific commodities may be banned. A general overview of the results produced by the residue-monitoring systems indicates that, at least in the countries where monitoring activities are carried out on a routine basis, the proportion of samples (food items) found to be irregular is generally rather low and decreases over time. For example in the EU, from the 5–6% frequency of detection of irregular samples typically observed in the mid-1990s, the current frequency of detection has decreased to 2–3% and in some countries to 1%. Two main reasons can cause a sample to be defined as irregular: A sample contains a permitted pesticide residue but in a concentration exceeding the respective maximum residue limit (MRL), or a food item contains the residue of a pesticide that is not authorized for use on that crop. Data collected in industrialized and developed countries show, with a few exceptions, that pesticide contamination in food commodities is acceptably controlled by food safety activities, and therefore it might be concluded that the risk for consumers’ health is adequately considered in these countries. On the other hand, concern remains for the chemical contamination of food in developing countries and in some countries in transition, where local systems for pesticide prevention in food are in some cases weak or absent.
Another source of exposure for the general population is surfaces such as floors and other touchable surfaces; this is particularly true for toddlers when they play on the floor and have frequent hand-to-mouth and object-to-mouth activity. A number of studies have examined the residential exposure to pesticides. Most of these studies have been conducted in the United States and, to a lesser extent, in Europe. In many instances, external exposure was accompanied by measurements of urinary metabolites of pesticides. Sources of intake are considered to be both from the environment and from food and water containing pesticide residues, and it was rarely possible to clearly distinguish the different sources. Certain conditions have been shown to cause increased exposure of residents to pesticides. These include indoor use of pesticides, contamination of the boots and clothing of agricultural workers, and hand-to-mouth behavior in children. However, in general, the urinary levels of pesticides or their metabolites in residents was lower, sometimes significantly lower, than those found in workers (Bouvier et al., 2005). Most risk assessments are based on surrogate data and conservative assumptions. In the absence of measured exposure data or representative data on analogous substances, exposure is estimated using modeling approaches (EPA, 1997, 2000, 2001a, 2001b).
The epidemiological surveillance of acute pesticide poisonings is another fundamental tool in postmarketing prevention and provides the basis for setting priorities and defining the need for further actions, including withdrawal from the market or restriction of uses of the compounds showing unacceptable risks for humans or for the environment.
Postmarketing prevention in the workplace is made by exposure assessment and health surveillance, which includes medical surveillance and biological monitoring. Health surveillance includes the pre-employment (or preplacement) medical examination, before the subject is placed in a job involving pesticide handling; the periodical medical examination, related to the specific exposure and work conditions; and the medical consultation or examination when a worker returns to his or her workplace after a significant absence for disease. Basically, the pre-employment medical examination is carried out in order to determine the physical ability of a worker to do the job for which he or she is recruited, to identify any medical condition that can be worsened by pesticide exposure, and to set up a baseline for comparison in any further evaluation. At the periodical medical examination, the main objectives are:
- to detect, as early as possible, any specific adverse health effect that might be attributable to the occupational exposures taken into account;
- to detect any significant change in the health status that may compromise the ability to continue the assigned job, further deteriorate the worker’s health if continued, and reveal any increased susceptibility for work-related exposure conditions.
It must be remembered that particularly in developing countries, where pesticides pose the highest levels of risk to human beings, animals, and the living environment, the foundation of risk prevention is information, training, and education. These activities should be preferably carried out in the agricultural settings but, unfortunately, at present health-care structures in agriculture are lacking both in the developing and the developed world.
Bibliography:
- Alavanja MC, Sandler DP, Lynch CF, et al. (2005) Cancer incidence in the agricultural health study. Scandinavian Journal of Work Environment & Health 31(supplement 1): 39–45.
- Alexander BH, Bloemen L, and Allen RH (2005) Sessions on the epidemiology of agricultural exposure and cancer. Scandinavian Journal of Work Environment & Health 31(supplement 1): 5–7.
- Besbelli N (2001) Harmonized collection of data on human pesticide exposures. Seventh GINC Tokyo Meeting for Information Exchange and Collaboration in Asia on Chemical Management and Pesticide Poisoning. http://www.nihs.go.jp/GINC/meeting/7th/meet-rep.html (accessed October 2007).
- Bouvier G, Seta N, Vigoroux-Villard A, Blanchard O, and Momas I (2005) Insectide urinary metabolites in nonoccupationally exposed populations. Journal of Toxicology and Environmental Health Part B 8: 485–512.
- Colosio C, Tiramani M, and Maroni M (2003) Neurobehavioral effects of pesticides: State of the art. Neurotoxicology 24: 577–591.
- Doe JE, Boobis AR, Blacker A, et al. (2006) A tiered approach to systemic toxicity testing for agricultural chemical safety assessment. Critical Reviews in Toxicology 36: 37.
- EPA (1997) Standard Operating Procedures (SOPs) for Residential Exposure Assessments. Washington DC: United States Environmental Protection Agency. http://www.epa.gov/oscpmont/ sap/meetings/1997/september/sopindex.htm (accessed October 2007).
- EPA (2000) Intro and background: Session III: models – residential exposures – REx model. In: Scientific Advisory Panel Meeting, 26–29
- September 2000, Arlington, Virginia, Washington DC: United States Environmental Protection Agency. http://www.epa.gov/scipoly/sap/ meetings/2000/september/rex_background_memo2.pdf (accessed October 2007).
- EPA (2001a) National Home and Garden Pesticide Use Survey. Washington DC: United States Environmental Protection Agency (No. RTI/5100/17–01f).
- EPA (2001b) General Principles for Performing Aggregate Exposure and Risk Assessments. Washington DC: Office of Pesticide Programs United States Environmental Protection Agency. http://www.epa. gov/pesticides/trac/science/aggregate.pdf (accessed October 2007).
- European Food Safety Authority (2006) Opinion of the Scientific Panel on Plant Health Plant Protection Products and their Residues on the request from EFSA related to the evaluation of dichlorvos in the context of Directive 91/414/EEC. The European Food Safety Authority Journal 343: 1.
- Food and Agriculture Organization/WorldHealth Organization (FAO/ WHO) (2003) Pesticide Residues in Food – 2003. Report of the Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Core Assessment Group on Pesticide Residues. Geneva September 2003. . http://www.fao.org/ ag/AGP/AGPP/Pesticid/JMPR/Download/2005_rep/ report2005jmpr.pdf.
- Food Agriculture Organization/WorldHealth Organization (FAO/ WHO) Pesticide Residues in Food – 2005. Report of the Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Core Assessment Group on Pesticide Residues. Geneva, 20–29 September 2005. http://www.fao.org/ag/AGP/AGPP/Pesticid/JMPR/Download/2005_rep/report2005jmpr.pdf (accessed October 2007).
- International Programme on Chemical Safety (IPCS) (1978) Environmental Health Criteria: no. 9. DDT and derivatives. Geneva, Switzerland: IPCS.
- Jeyaratnam J (1990) Acute pesticide poisoning: a major global health problem. World Health Statistics Quarterly 43: 139–144.
- Kamel F and Hoppin JA (2004) Association of pesticide exposure with neurologic dysfunction and disease. Environmental Health Perspectives 112: 950–958.
- Li AA, Mink PJ, McIntosh LJ, Teta MJ, and Finley B (2005) Evaluation of epidemiologic and animal data associating pesticides with Parkinson’s disease. Journal of Occupational and Environmental Medicine 47: 1059–1087.
- Lotti M and Moretto A (2005) PNS effects and delayed polyneuropathy. In: Gupta RC (ed.) Toxicology of Organophosphate and Carbamate Pesticides, pp. 361–370. San Diego, CA: Elsevier.
- Maroni M, Colosio C, Ferioli A, and Fait A (2000) Biological monitoring of pesticide Exposure: A review. Toxicology 143: 1–123.
- Moretto A (2002) Occupational aspects of pesticide toxicity in humans. In: Marrs T and Ballantyne B (eds.) Pesticide Toxicology and International Regulation, pp. 431–472. London: Wiley.
- Pan American Health Organization (PAHO) (2002) Epidemiological situation of acute pesticide poisoning in Central America, 1992–2000. Epidemiological Bulletin 23: 5–9.
- Smith AE and Secoy DM (1975) Forerunners of pesticides in classical Greece and Rome. Journal of Agricultural and Food Chemistry 23: 1050–1057.
- Spiewak R (2001) Pesticides as a cause of occupational skin diseases in farmers. Annals of Agricultural and Environmental Medicine 8: 1–5.
- World Health Organization (WHO) (2006) The WHO Classification of Pesticides by Hazard. http://www.who.int/ipcs/publications/ pesticides_hazard_rev_3.pdf (accessed October 2007).
- World Health Organization (2003) Joint (WHO)/Convention Task Force on the Health Aspects of Air Pollution (2003). Health Risks of Persistent Organic Pollutants from Long Range Transboundary Air Pollution, pp. 19–61. Amsterdam, the Netherlands: Drukkerij Wilco.
- British Crop Protection Council (2006) The Pesticide Manual. Hampshire, UK: British Crop Protection Council.
- Klaassen CD (ed.) (2001) Casarett and Doull’s Toxicology. The Basic Science of Poisons. 6th edn. New York: McGraw-Hill.
- Krieger R (ed.) (2001) Handbook of Pesticide Toxicology. 2nd edn. San Diego, CA: Academic Press.
- Meister Pro (2005) Farm Chemicals Handbook. Willoughby, OH: Meister Publishing Company.
See also:
Free research papers are not written to satisfy your specific instructions. You can use our professional writing services to buy a custom research paper on any topic and get your high quality paper at affordable price.







