This sample Streptococcal Diseases Research Paper is published for educational and informational purposes only. If you need help writing your assignment, please use our research paper writing service and buy a paper on any topic at affordable price. Also check our tips on how to write a research paper, see the lists of health research paper topics, and browse research paper examples.
Streptococci: A Persistent Health Hazard
Early in 1994, in Gloucestershire, England, several people were struck down in a short period of time by what was labeled by the media as ‘flesh-eating disease.’ The causative bacteria were called ‘killer bacteria’ or ‘flesh-eating bacteria,’ and the whole world was shocked to read about this ‘new’ threat. In fact, these bacteria were neither a new phenomenon nor an emerging pathogen. The infections, known medically as necrotizing fasciitis, were caused by group A Streptococcus pyogenes, a bacteria that had been identified many decades before. Streptococci can cause a wide spectrum of diseases in humans and animals and have continued to be a puzzle for clinicians, scientists, and public health personnel. Although bacteria from the streptococcal genus can infect a variety of animals, humans are the primary reservoir for the majority of species that cause serious disease in humans. The pathogenesis of streptococcal disease is so perplexing that the infections caused by these organisms have never been completely understood.
The strategies used by streptococci to outwit their host are quite ingenious. They evade host immune defense by appearing in hundreds of different serotypes; they bind and exploit host proteins for their own advantage and to establish themselves in the host; they trigger their own internalization by host cells so that they can persist and evade the action of antibiotics; they express surface proteins with similarity to host protein, again to evade immune defense, which leads to autoimmune reactions; some of them are real artists in developing antibiotic resistance; they are capable of transferring their genetic material horizontally to other serotypes and species, making epidemiological analysis very difficult; and this list of perplexing properties is far from complete. Streptococci therefore remain a major health hazard and a challenge for scientists and clinicians.
Classification Of Streptococci
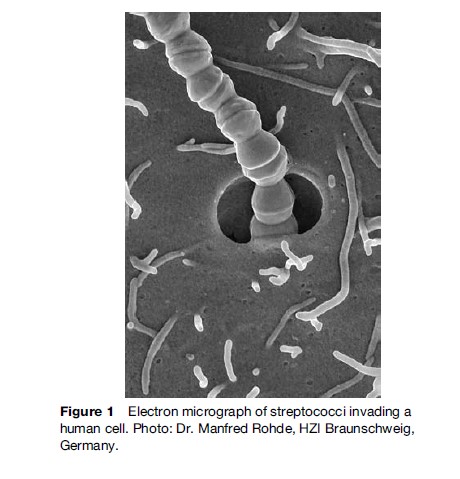
Streptococci are spherical organisms that grow in chains because of incomplete separation after division of the cells (Figure 1). They were first described in 1874 by Billroth, who used the term ‘streptococcus’ (from two Greek words: streptos ¼ chain, kokhos ¼ berry). In the beginning, streptococci were classified according to the disease they caused; however, thanks to advances in diagnostics, and with the availability of modern molecular techniques, many changes have been made in the taxonomy of the Streptococcus genus in the last decade. Historically, a useful identifying characteristic of streptococci has been the reaction they show on blood agar, caused by the lysis of erythrocytes by enzymes released by the streptococcus – a phenomenon known as hemolysis. Based on this characteristic, streptococci were classified into b-hemolytic and non-b-hemolytic groups. In 1934, streptococci were further classified based on the presence of group-specific polysaccharides on the bacterial surface. In this serogrouping, mostly b-hemolytic streptococci were considered. Thirteen different serological groups have so far been identified, out of which groups A, B, C, and G, S. pneumoniae, and viridans group streptococci are most important with regards to human health. The main focus of this research paper is on the diseases caused by different streptococci. The classification of streptococci capable of causing disease in humans and animals is depicted in Table 1.
Diseases Caused By Streptococci
The diseases caused by streptococci have remained a serious health problem for centuries. Because of the large number of pathogenic species (Table 1) in the Streptococcus genus, the spectrum of diseases is also highly diverse, ranging from self-limiting manifestations to life-threatening diseases. Although most of the disease manifestations have been described for b-hemolytic streptococci, the non-b-hemolytic group has been gaining importance as causative organism for a large number of human diseases. Streptococcus pyogenes (group A streptococcus, GAS), which is an exclusively human pathogenic organism, is no doubt the most important in terms of human health. This research paper deals with the diseases caused by important species of streptococci with a major emphasis on group A streptococcal diseases.
Streptococcus Pyogenes (Group A Streptococci)
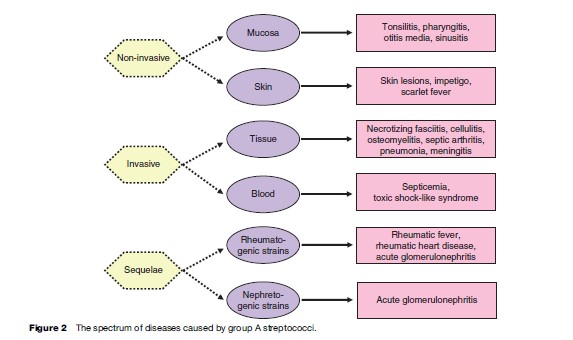
Group A streptococci are the major cause of streptococcal infections in human beings. The organisms colonize oral mucosa and skin and cause a wide spectrum of pyogenic infections, such as tonsillitis, pharyngitis, scarlet fever, and skin inflammation. More serious are the invasive streptococcal infections that, although they have been observed for centuries, have resurged all over the world since 1980. In addition to the high mortality associated with streptococcal invasive diseases, the sequelae of streptococcal infections are also considered a major public health problem and a big research challenge. Rheumatic fever and subsequent rheumatic heart disease are post streptococcal infection complications that involve inflammation of and damage to heart valves, requiring their replacement. About 15 million children between the ages of 5 and 15 suffer from rheumatic heart disease, and approximately 1 million new cases are registered every year. Another complication of streptococcal infection is glomerulonephritis, an inflammation of the kidneys, which very often leads to kidney failure. Figure 2 illustrates the spectrum of the diseases caused by group A streptococci.
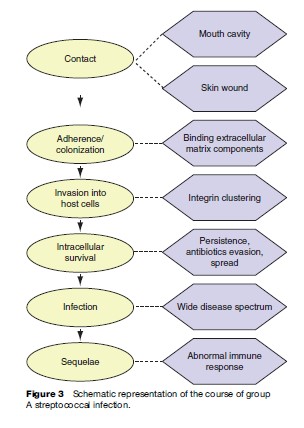
Course Of Group A Streptococcal Infections
Infection with GAS begins with contact, either in the oral cavity or to a skin wound. This is followed by adherence and colonization of bacteria in the oral mucosa or at the fibrin clots of the wound. The further progression of the disease from these sites depends on the ability of the isolates to avoid phagocytosis by immune cells. The form of the disease that develops is determined by the invasive capabilities of the isolates and the genetic susceptibility of the host. Three to six months after the primary infection, postinfection complications such as rheumatic fever or glomerulonephritis are induced in predisposed individuals. The course of group A streptococcal infections is illustrated in Figure 3.
To adhere to and invade host cells as well as to evade host immune defenses, group A streptococci express a number of virulence factors. Besides adhesins and invasins, M proteins are the major virulence factors. M protein is a major surface protein of streptococci, which enables the organism to resist phagocytosis. A group of proteins called complement is usually involved in the recognition of and response to bacterial invaders. Inhibition of complement activation is a common mechanism by which M protein protects streptococci from phagocytosis, with different types of M protein binding differentially to fibrinogen and the complement-regulating factors C4BP and factor H. Binding of these molecules leads to inhibition of both the classic and alternate complement pathways. Because of variation in the N-terminal region, M protein exists as approximately 100 different serotypes. With a few exceptions, one strain expresses only one type of M protein. Anti-M protein antibodies provide type specific protection against infection in animal models. Because of these properties, M protein has been regarded as a choice protective antigen. M protein therefore has been a focus of interest for the last two decades. These studies have led to elucidation of the structure and function of M protein. The complete sequence of various M serotypes is known, and B and T cell epitopes (protein sequences that can be defensively recognized by the human immune system) have been identified. The structural features of M protein include repetitive sequences and a conserved anchor. The four repeat blocks of M protein, A, B, C, and D, differ from each other in their size and sequence. A comparative sequence analysis between M protein and different human proteins showed a significant homology with myosin and tropomyosin, which are proteins found in the human heart. These similarities can have immunological consequences, although the exact role of M protein in the induction of rheumatic heart disease has not yet been elucidated.
The adherence of streptococci to host cells is an essential step for the initiation of an infection. This interaction occurs between adhesins on the bacterial surface and the specific receptor in the host cells. In the case of group A streptococci, the adherence mechanism is interesting in the sense that the adherence does not take place directly between adhesins and host cell receptors, but is mediated through the host protein fibronectin. Fibronectin is a high-molecular-weight glycoprotein, which is present in soluble form in plasma and other body fluids, and in insoluble form in the extracellular matrix. It is a multifunctional protein and plays an important role in the interactions of cells with the extracellular matrix. Because of its ability to bind to both host cells and streptococci, it is considered as an essential bridging molecule in streptococcal adherence.
Binding of fibronectin by streptococci was found to be via a surface protein that was designated SfbI protein. The sequence analysis shows that the fibronectin-binding domain of SfbI consists of 37 amino acids, which occurs as repetitive domain. Other functional epitopes, such as signal sequence, membrane anchor, and so forth, are similar to many other Gram-positive surface proteins. SfbI protein plays an important role in pathogenesis and is capable of competitively inhibiting the binding of streptococci to fibronectin and their adherence to human epithelial cells. Anti-SfbI antibodies can also block fibronectin binding to streptococci and can react with many different group A streptococcal strains. The presence of the SfbI gene correlates very well with the fibronectinbinding capacity and the epithelial cell adherence of streptococci. SfbI protein also has an additional binding domain for fibronectin, which is structurally different from the fibronectin binding repeats. This binding domain, designated ‘spacer domain,’ consists of 30 amino acids. Fibronectin contains three domains that interact differently with the binding domains on SfbI. The repeat region has a high affinity for the 30-kDa N-terminal fibronectin fragment, and a low affinity for the 120-kDa C-terminal fragment. The spacer region binds with high affinity to the 45-kDa fibronectin fragment, which is located between the 30-kDa and 120-kDa fragments.
Group A streptococci are traditionally considered to be extracellular pathogens because they can colonize different tissues to cause extracellular infections. However, group A streptococci can also cause invasive infections, so it is thought that they might also act as intracellular pathogens. These properties have now been described for a large number of group A streptococcal isolates. The invasion of streptococci is correlated to the source of the isolate. Isolates from throat and skin infections are not only very invasive but are also capable of surviving intracellularly. It is thought that this is a way in which group A streptococci avoid the action of antibiotics and are able to persist in the host and cause recurrent infections. A number of invasive streptococcal isolates show a strong SfbI-mediated fibronectin binding. SfbI therefore plays a role not only in the adherence but also in the invasion of streptococci. Latex beads coated with purified SfbI proteins are rapidly internalized by epithelial cells, indicating that SfbI per se is sufficient to trigger invasion. In this process, the fibronectin-binding spacer domain plays a decisive role. This happens through a cooperative binding of both binding domains of SfbI protein to fibronectin. The binding of the 30-kDa fibronectin domain to the repeat region of SfbI protein activates binding of the 45-kDa domain to the spacer region. This activation is a prerequisite for the invasion of streptococci into eukaryotic cells.
Life-Threatening S. Pyogenes Infections
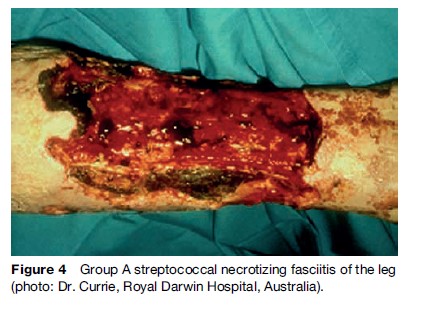
In addition to milder infections such as pharyngitis, group A streptococci are capable of causing life-threatening infections such as necrotizing fasciitis, septicemia, and streptococcal toxic shock syndrome. The progression of these diseases is extremely fast. Necrotizing fasciitis is a deep infection of the subcutaneous tissue that leads to the destruction of fascia, which are sheets of tissue lying under the skin (Figure 4). After the initial indication of infection, the disease develops at a very high speed. At this point of time in general a systemic infection also occurs, which leads to multiple organ failure and death. Even with aggressive therapy, the mortality rate is about 50%. Cellulitis and myositis can also be caused by invasive streptococci. These infections can lead to a serious complication, streptococcal toxic shock syndrome, often found in young adults. Typical symptoms of this syndrome are extreme pain, high fever, and finally septic shock. The secreted components of streptococci, such as erythrogenic toxins, and superantigens, which influence the immune cells to secrete large amounts of the proinflammatory molecules IL-1a/b, IL-8, and TNFa, are mainly involved in the development of toxic shock. In invasive infection, as well as in postinfection streptococcal sequelae, it is likely that host genetic susceptibility plays an important role.
Sequelae Of S. Pyogenes Infections
Rheumatic fever and glomerulonephritis are important sequelae of group A streptococcal infections. Although the exact mechanisms of rheumatic fever are not yet elucidated, it is assumed that this disease results from an abnormal immune response to an untreated or not fully treated streptococcal pharyngitis, which leads to the generation of autoimmune antibodies that cross-react with host tissue. The most common manifestation of rheumatic fever is arthritis, which occurs in around 75% of patients and mostly affects adolescents and adults. Other manifestations include chorea, a condition mainly affecting children and characterized by emotional disturbances, uncoordinated movements, and muscle weakness, and carditis, with rheumatic heart disease developing in around 30% of rheumatic fever patients. Rheumatic fever and rheumatic heart disease are observed in all parts of the world, with the global incidence estimated to be 1.3 cases per 1000 people, although the incidence rate varies greatly between countries and different population groups. For example, in Sudan and China, the rates are estimated to be 100 and 150 per 100 000 respectively, while in developed countries the rate has dropped dramatically since the 1950s to <1.0 per 100 000, although spontaneous outbreaks of rheumatic fever still do occur. Approximately half of all children with heart disease suffer from rheumatic fever and rheumatic heart disease, making them the most common cause of acquired heart disease in children. A unique epidemiological observation is the high incidence of rheumatic heart disease in the aboriginal population in Australia and the Maoris in New Zealand, which at an estimated 9.6 per 1000 is much higher than the incidence in the nonindigenous populations. The reasons for this are not yet known, but it seems that environmental factors and genetic susceptibility might be responsible.
The relationship between group A streptococci and rheumatic fever has been established for many decades, but the exact pathogenesis, despite intensive studies, remains unknown. Host factors, bacterial factors, and an abnormal immune response may all contribute toward induction of rheumatic fever. Family predisposition has been a topic of discussion for a long time. Although controversial, there is some evidence that genetic predisposition plays an important role. Individuals with rheumatic fever and their family members express certain antigens on the surface of their immune cells, known as HLA. A correlation between rheumatic fever and HLA has often been reported where the HLA-DR locus is involved. Some B-cell antigens have also been implicated in rheumatic fever. Monoclonal antibodies that were raised in mice against B-cells from rheumatic fever patients react with B-cells of 100% of the patients, but only with 10% of the control group. The proportion of the population that is predisposed to rheumatic fever is not yet known. Putative genetic markers such as the B-cell antigen D8/17 are generally found less frequently in controls compared to rheumatic fever patients and relatives of those patients, further implicating the role of genetics in susceptibility; however, there is variation depending on the study size and the geographical region.
In a heterogeneous population, outbreaks of pharyngitis result in rheumatic fever in approximately 3% of cases. The incidence rate of rheumatic fever following sporadic group A streptococcal pharyngitis is generally much lower than in the outbreaks.
In contrast to host factors, the involvement of bacterial factors in the induction of rheumatic fever is less controversial. The concept of rheumatogenicity, that only some definite strains are capable of causing rheumatic fever, has existed for many decades. Many studies have shown the association of certain M types, such as 1, 3, 5, 6, 14, 18, 19, 24, 27, and 29, with rheumatic fever. The involvement of M protein was underlined by the fact that the strains associated with rheumatic fever express definite epitopes, induce a strong M protein-specific immune response, and show homologies between M epitopes and host proteins. The strains associated with rheumatic fever have a thick capsule and generally do not express opacity factor. In spite of these studies, the role of M serotypes remains a matter of some controversy. In many endemic areas with high incidence of rheumatic fever, the strains are either nontypable or express M types that are traditionally associated with skin infections. In the last few years, there has been a growing belief that rheumatogenicity is strain-specific rather than M type-specific. Group A streptococci are very efficient in transferring their genetic material horizontally, so that theoretically every strain can be associated with rheumatic fever. Besides M protein, other streptococcal components have been associated with rheumatic fever including capsule, cell wall-associated carbohydrates, and cell membrane. These components show cross-reactivity with host proteins such as cardiac myosin, heart valve glycoproteins, and sarcolemmal membrane. It has also been demonstrated that certain serotypes are able to bind and aggregate collagen, which is an important component of the extracellular matrix. This may lead to the production of anticollagen antibodies, leading to an autoimmune response and subsequent rheumatic fever. The role of collagen in rheumatic fever is supported by the fact that the serum samples from patients with acute rheumatic fever or chronic rheumatic heart disease show substantially high titers of anticollagen antibodies.
In addition to other host and bacterial factors, an abnormal immune response plays an important role in the induction of rheumatic fever. Immunological cross-reactivity between certain streptococcal antigens and the host tissue leads to damage of the heart, joints, and brain. Such cross-reactive antibodies are often found in the sera of rheumatic fever patients. Although humoral immunity is presumed to be an important component of the immune response in rheumatic fever, there is increasing evidence that the primary damage is caused by cellular immunity. Cellular infiltrates from the heart tissue of rheumatic fever patients show a predominance of T lymphocytes, and very often macrophages are found in the myocardial lesions. Both of these cell types are components of cellular immunity. Moreover, in rheumatic fever, many markers are observed that activate inflammation via the cellular immune system. Increased numbers of circulating CD4þ lymphocytes and increases in the levels of cytokines such as IL-1 and IL-2, IFNg, TNF-a, and their receptors are observed in rheumatic fever. Most probably, the normal mechanism for suppressing inappropriate cellular immunity is not functioning in rheumatic fever patients. This leads to an uncontrolled activation and tissue damage. The induction of rheumatic fever and rheumatic heart disease is illustrated as a simple schematic diagram in Figure 5.
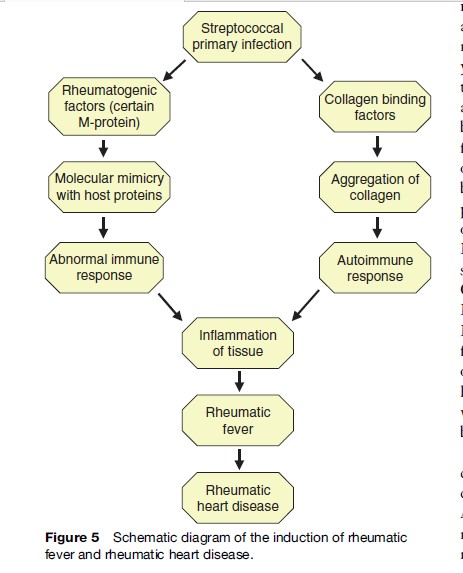
Other immune mechanisms have also been implicated in the pathogenesis of rheumatic fever. Some streptococcal antigens, such as exotoxins, act as superantigens, molecules that are able to directly bind to the MHC class II receptors on antigen-presenting cells and to the receptors on T cells. Cross-linking of these receptors by the superantigen leads to T-cell activation and subsequent release of inflammatory factors that can influence the disease pathogenesis. It is also possible that some copathogens, such as coxsackie virus, may act together with the streptococci to cause rheumatic fever.
Glomerulonephritis is another sequelae of streptococcal infection, which has a much lower incidence than rheumatic fever. Although for glomerulonephritis the exact mechanisms are not known, as is the case with rheumatic fever it is clear that some definite streptococcal strains are nephritogenic and, as in rheumatic fever, it is likely that genetic predisposition plays a role in developing the disease. One hypothesis is that the nephritogenic streptococci express pathogenicity factors that have high affinity for kidney cells and accumulate in the glomeruli, leading to the formation of immune complexes. Alternate hypotheses include the involvement of inappropriate cellular immune responses and complement responses, or the proliferation and apoptosis of glomerular cells. It is possible that the pathogenesis occurs as a result of a combination of these factors.
Therapy, Prophylaxis, And Other Control Strategies
Penicillin has been the usual treatment for different streptococcal infections since the 1940s. It is not only used to treat streptococcal pharyngitis but also to prevent recurrent episodes of acute rheumatic fever. This secondary prophylaxis generally involves intramuscular administration of penicillin every 3–4 weeks. Alternatively a daily oral dose of penicillin, sulfa drugs, or erythromycin can be taken, but this is thought to be a less effective method of prevention. In spite of the fact that group A streptococci have not yet developed resistance to penicillin, this therapy has not been successful in controlling streptococcal infection and the sequelae. Development of a vaccine against group A streptococci is urgently needed and is a major challenge in streptococcal research. In the last few years, many potential vaccine candidates have been identified. Because of the protective action of anti-M protein antibodies, many groups have tried to develop M protein-based vaccines. These efforts have not been very successful because of two basic problems. First, M protein itself, or at least a part of it, can induce the negative sequelae because it possesses cross-reactive epitope for certain host proteins, and second, M protein occurs in a large number of serotypes, and the protection generated is generally M serotype-specific. Some progress has been made to solve these problems. Vaccine prototypes containing C-repeat peptides, which are highly conserved between M types, have shown protection in mouse models. Multivalent M protein vaccines consisting of peptides from the variable regions of multiple M serotypes, without the human cross-reactive epitopes linked to sequelae, have also yielded promising results. M protein-based vaccine prototypes are currently undergoing clinical trials but are still in the optimization phase.
An alternative strategy for the development of streptococcal vaccine is the use of streptococcal adhesins as candidates. SfbI protein, the major adhesin of group A streptococci, is capable of inducing a protective immune response against different streptococcal strains in a mouse lethality model. The fibronectin-binding domains of SfbI proteins represent important protective antigens. There are many other advantages of SfbI protein-based vaccines: (1) SfbI protein is expressed by more than 70% of the clinical isolates belonging to different serotypes from different geographical areas, so the narrow specificity of an M protein-based vaccine is avoided, (2) the fibronectin-binding domains of SfbI protein are highly conserved, (3) antibodies against SfbI protein do not show any cross-reactivity with human tissue, and (4) SfbI protein acts as a strong mucosal adjuvant. Therefore, SfbI protein seems to be a promising vaccine candidate that should soon go to phase I trials. Beside M protein and SfbI protein, other vaccine prototypes based on FBP54 protein and C5a peptidase are also in a development phase.
Streptococcus Agalactiae (Group B Streptococci)
Group B streptococci (GBS), with the sole species Streptococcus agalactiae, were for the first time described in 1887, when they were isolated from a case of bovine mastitis. Until 1937, they were not regarded as human pathogens, but were then identified as a cause of sepsis in the newborn. In the last couple of decades, they have become a serious health problem and a major cause of bacterial meningitis in newborns, leading to invasive disease in approximately 1 out of every 1000 live births. The infection of newborns with GBS not only is characterized by high mortality rates, but a large percent of survivors also suffer from subsequent neuronal damage. GBS occur as symptomless flora of the genital tract in up to 25% of adult women, and infection of the newborn generally occurs during delivery, when the newborn become exposed to the bacteria in the birth canal. GBS infection of the mother is also involved in preterm rupture of the amniotic membrane leading to premature labor. It is also possible for the organism to spread from the vagina into the amniotic fluid during pregnancy, where aspiration of contaminated fluid by the fetus can lead to invasive disease, or even intrauterine death. Because of the reduced number of alveolar macrophages and incomplete immunity in the newborn, group B streptococci can easily colonize the lungs. From here, they invade the blood and form a systemic infection. The precise mechanisms of how GBS reach the circulation from the lung are not yet known. Infection of the newborn is divided into two different types depending on the length of time between birth and development of disease. In early onset disease, the symptoms appear between 24–72 h after birth. The mortality in these cases is 89% for sepsis, and 62% for meningitis. Early onset disease is also characterized by a high rate of neurological damage in the survivors. In late-onset disease, the symptoms can appear many weeks after birth, and meningitis is the major manifestation.
At 10–20%, mortality is much lower than with early onset disease; however, morbidity is high, with survivors suffering from neurological sequelae such as blindness and deafness. GBS has also emerged as an important pathogen among nonpregnant adults, in particular the elderly and patients with chronic underlying disease, with clinical manifestations including bacteremia, pneumonia, and skin or soft-tissue infection. However, the incidence of these cases is not as high as in newborns.
Prevention Of Group B Streptococcal (GBS) Disease
Based on the structure of its polysaccharide capsule, GBS are divided into nine different serotypes, of which serotypes Ia, Ib, II, and III are most commonly involved in disease. Approximately 40% of isolates from invasive diseases are Ia type, and 27% are type III. The major problem with GBS infections is the fast and dramatic progression of the disease, which cannot be sufficiently treated with antibiotics. As many women of childbearing age have been colonized with GBS in the vaginal area, every pregnant woman should be tested for this organism before delivery. In the case of positive results, the woman is normally treated with antibiotics to reduce the risk of transferring the bacteria to the newborn during delivery. Because of increasing resistance of bacteria to these antibiotics and the risk of allergic reaction to the mother and the newborn, it is desirable to develop an alternative to antibiotic therapy. Clinical studies have shown that infants whose mothers have a high titer of anti-GBS antibodies are rarely infected. Therefore, vaccinating women of childbearing age to passively protect the newborn seems to be a promising strategy.
A GBS vaccine able to induce specific antibodies both in the serum and at mucosal surfaces would provide two levels of protection to the neonate. First, transfer of maternal antibodies toward GBS across the placenta would provide passive immunity to GBS, and second, prevention of colonization in the reproductive tract would protect the neonate from infection in utero or during childbirth. Because the capsule of the GBS is an important virulence factor, efforts have been made to use it as a vaccine candidate. These efforts were unsuccessful because of the antigenic variation and the low immunogenicity of capsular polysaccharides. The interest has therefore shifted toward the use of a surface protein as a vaccine candidate. Major surface proteins of GBS are a and b antigen of the C protein complex. Of the two proteins, a is potentially more useful as a vaccine component, being present in approximately 50% of all GBS isolates, compared to only 10% for b. More recently, another surface protein, Rib, has been identified. Rib is expressed on almost all serotype III strains of GBS; therefore, most strains of GBS not expressing a express Rib. In fact 90% of all GBS strains responsible for invasive disease express either Rib or a, making a combination of these proteins potentially useful for a broad-specificity GBS vaccine. Sip, a surface protein with an unknown function, is also of interest because it is expressed by GBS of all serotypes, and therefore might also provide broad protection. In animal models these proteins have been shown to induce a protective immune response, making them promising vaccine candidates. However, in spite of the urgent need for a GBS vaccine, development is still in an early phase.
Diseases Caused By Group C And Group G Streptococci
Group C and group G streptococci belong to heterogeneous streptococcal species that include nonpathogenic commensals as well as serious disease-causing bacteria. Group C streptococci can be divided into two morphological groups, the large and the small colony variants. The small colony variants, which include Streptococcus milleri group, form the normal flora in the mouth cavity and gastrointestinal and urinary tracts of human beings, but are also capable of causing serious infections. The large colony variants, which include species like Streptococcus equi and Streptococcus dysgalactiae, are traditionally considered to be animal pathogens, although they can cause bacteremia, cellulitis, peritonitis, septic arthritis, pneumonia, and endocarditis in human beings. Although the incidence rate of human infection by group C streptococci is much lower than those by group A and group B streptococci, the mortality of approximately 25% is very high. Group G streptococci were also initially not considered as human pathogens and were regarded as normal flora of human skin, throat, and gastrointestinal tract. However, in the last few years the incidence of life-threatening human infections by group G streptococci has substantially increased.
As with group A streptococci, adherence and invasion play an important role in the pathogenesis of group C and group G streptococci. They, too, are capable of binding to many host proteins. Fibronectin-binding proteins and M-like proteins, as well as different enzymes and toxins, are the major pathogenicity factors. The precise pathogenic mechanisms of these organisms have not yet been fully understood.
Group C and group G streptococci have recently been associated with acute rheumatic fever. The aboriginal population of Australia, where streptococcal pharyngitis is extremely rare, shows the highest incidence worldwide of rheumatic heart disease. The antibodies isolated from this population against group C and group G streptococci show a strong cross-reactivity to cardiac myosin. This association between group C and G streptococci and rheumatic fever might explain the large number of
rheumatic fever cases without a known history of group A streptococcal infections. If the studies in other endemic areas also confirm the involvement of group C and group G streptococci in rheumatic fever, it would then be essential to test for their presence in the throat cultures and to treat accordingly.
Streptococcus Pneumoniae
Another important species of genus Streptococcus is S. pneumoniae (the ‘pneumococcus’). These organisms are exclusively human pathogens and form part of the normal nasopharyngeal flora in 10% of adults and about 40% of healthy children. Carriage does not always lead to invasive disease, but it can lead to infection of others, which is proportional to the frequency and intimacy of contact between people. In most cases carriage is asymptomatic, and after a period of weeks the pneumococcus is cleared by the host immune system. However, under certain conditions, the bacteria move from the nasopharynx to other sites in the body, leading to disease. The pneumococcus is able to cause several diseases ranging from serious, life-threatening conditions such as bacteremia, pneumonia, and meningitis to less severe diseases such as sinusitis and otitis media that, although not usually associated with high mortality, are nevertheless a major drain on the public health system of developed countries. In the United States alone, 7 million cases of otitis media and more than half a million cases of pneumonia are registered every year, with about 5–10% mortality. Worldwide, more than 20 million cases of pneumonia with about 1 million deaths are registered every year.
Pneumococcal Virulence Factors
Pneumococci are divided into 90 different serotypes depending on the composition of the polysaccharide capsule that coats the bacteria, and serotype distribution is different in different geographical areas. The pneumococcal capsule is an antiphagocytic factor and plays an important role in pathogenesis, with unencapsulated strains having severely reduced virulence in animal models. Other pathogenicity factors include neuraminidases, pneumolysin, autolysins, pneumococcal surface antigen A, and the family of choline-binding proteins that bind to choline residues found on the pneumococcal surface. Pneumolysin, a cytotoxic protein, is present in the cytoplasm and is secreted after the action of autolysins. Its main mode of action is as a cytotoxin, binding to host cell membranes, where it forms transmembrane pores and leads to cell lysis, damaging the bronchial epithelium and directly inhibiting phagocytosis by immune cells. However, it also has other roles in virulence; it can directly activate complement, and at sublytic concentrations it can stimulate cells of the immune system to produce cytokines, leading to inflammation.
The proteins that are anchored on the pneumococcal surface through a choline-mediated mechanism also play a role in pathogenesis. Nine such proteins have so far been identified, including pneumococcal surface protein A (PspA) and secretory IgA binding protein (SpsA). PspA is a strongly immunogenic protein that specifically binds human lactoferrin, and has the ability to inhibit complement. PspA has been shown to be a protective antigen in animal models, and is broadly cross-reactive, with antibodies against one PspA type able to protect against pneumococci with different PspA types. SpsA is a multifunctional protein that is reported to act as an adhesin in the nasopharynx via its ability to bind human secretory IgA. It is also able to bind the complement regulatory factor H, and this may help the pneumococcus to avoid being killed by complement. SpsA has a conserved functional domain for binding to secretory IgA and secretory component, and is expressed by all clinically relevant strains, making it a promising vaccine candidate.
Besides proteins with a membrane anchor mechanism, pneumococci express and secrete proteins without such an anchor. One of these proteins is Eno, an a-enolase of pneumococci, which is an important enzyme of glycolytic pathway. After secretion, Eno is capable of reassociating to the bacterial surface. An important property of Eno is its binding to plasmin and plasminogen, which might allow the bacteria to invade into the tissues by the degradation of the extracellular matrix. This is the first example of a metabolic enzyme that also acts as a possible pathogenicity factor.
Treatment And Prevention Of Pneumococcal Disease
The high mortality rate of the S. pneumoniae infection has remained constant in the last 30 years, despite the availability of antibiotics. One of the reasons is the dramatic spread of penicillin-resistant pneumococci in the last few years. The increase in antibiotic resistance in countries like Spain and South Africa, where 60% of the isolates are resistant, is a matter of serious concern. The rise in penicillin resistance has led to increased use of other antimicrobial drugs, but many pneumococci are now developing resistance to these as well, and are therefore multiply resistant. In some parts of the world, up to 89% of penicillin-resistant isolates are also resistant to other antimicrobials. Molecular fingerprinting techniques have shown diversity among resistant strains from around the world, but has also shown that many multiply resistant strains belong to recognizable clonal groups such as one that was initially isolated in Spain, known as the Spanish serotype 23F clone. Isolates of the Spanish clone have been recovered in many countries throughout Europe, Southeast Asia, the United States, and South Africa. To stop the further spread of resistant pneumococci, it is essential to understand the molecular events leading to the development of resistance and to identify the factors that allow the fast spread of these strains.
Another problem in the control of S. pneumoniae infection is the suboptimal efficacy of the current vaccines, which are mainly based on capsular polysaccharides. A polysaccharide vaccine has been in use for many years, based on 23 polysaccharide types that are present in 90% of the isolates from Europe and the United States. However, polysaccharide is not an effective immunogen, and it stimulates a poor immune response in children, the elderly, and immunocompromised patients such as those with HIV. Thus the protection provided by the polysaccharide vaccine is lowest in those groups that are at highest risk of pneumococcal infection. Certain population groups such as Native Americans also demonstrate a reduced rate of protection following vaccination with pneumococcal polysaccharides. The immunogenicity of the capsule antigens can be increased by their conjugation with immunogenic proteins, and recently a conjugate vaccine has been released that is based on the seven most prevalent serotypes. This should provide better protection in children; however, because the number of capsular serotypes included in the vaccine is limited, there is a chance that serotypes that are not currently associated with high levels of disease might become more involved in pneumococcal disease. The ideal pneumococcal vaccine would be based on an antigen that is common among serotypes, so proteins such as PspA and SpsA, whose functional groups are highly conserved, are promising candidates for use as conjugate vaccine prototypes.
Viridans Group Streptococci (Oral Streptococci)
The viridans group of streptococci are also known as the oral streptococci because they are frequently found in the oral cavity. This group can itself be subdivided into smaller groups such as the mutans group and the mitis group (Table 1). They are generally nonhemolytic and form a part of the normal oral flora of humans, although they can be found in other sites of the body. Oral streptococci generally live in microbial communities called biofilms that are attached to the mucosa and tooth surface, forming plaque. Living in biofilms protects them from the antibacterial properties of saliva, and helps them to anchor to surfaces of the mouth and avoid being washed away during swallowing. Viridans group streptococci are usually not considered to be pathogenic, but during dental procedures and under certain other conditions it is possible for them to invade host tissues and cause systemic disease such as bacteremia. Thus the term ‘opportunistic pathogen’ is perhaps more accurate to describe them. They are able to cause serious disease in immunocompromised people and newborns, and they are a leading cause of dental disease.
Diseases Caused By Oral Streptococci
Dental Diseases
Oral streptococci cause dental disease (caries) by dissolving the tooth enamel, exposing the underlying layers and pulp of the tooth. Although it has been known for over a century that plaque bacteria use dietary carbohydrates to generate the acid that weakens tooth enamel, the identification of the responsible organisms is still controversial. Of all the species of bacteria found in dental plaque, only the mutans streptococci have been shown to be associated with dental caries. Increased levels of mutans streptococci are considered as strong predictors of current or future decay. Although mutans streptococci are generally accepted as being of prime importance, there have been indications that other oral streptococci might also contribute to dental caries. Besides dental caries, periodontal diseases, which affect the gums and bone supporting the tooth, are also caused by microbes, especially oral streptococci. Some members of the anginosus group, such as Streptococcus constellatus, have been associated with periodontitis.
Abscesses
Many oral streptococci gain access to the pulp of the tooth and the region around the root and multiply within the confined space to produce an abscess. A wide range of species can be isolated from dental abscesses; among the most common are streptococci of the anginosus group. There has been increasing evidence that these streptococci are also involved in other abscesses. The peritonsillar as well as deep-neck abscesses are serious infections capable of causing life-threatening complications. Further, empyema thoracis as well as lung and brain abscesses are associated with high mortality. In a prospective study, the streptococci of anginosus group were identified as the cause of liver abscesses. In this abscess, the infection was monobacterial, whereas in other abscesses mixed infections were involved. The striking capability of the anginosus group to cause abscesses has been the target of investigation. It seems that polymorphonuclear leukocytes (PMNs) are unable to kill the bacteria completely, adding to the ability of these bacteria to persist in the host and cause purulent infection. The exact mechanisms behind this resistance to phagocytosis are unclear, but some species of the anginosus group have been found to produce a toxin that may lyse PMNs. In addition, the anginosus group of bacteria produce a spectrum of degrading enzymes that allow them to multiply in locations where they must obtain nutrients by breakdown of the host tissues. However, the exact mechanisms of abscess formation as well as expression of virulence traits in response to the environment are still not fully understood.
Systemic Infections
Bacteremia And Sepsis
Oral streptococci gain access to the circulation frequently. More than half of the blood culture isolates obtained after dental procedures are oral streptococci. Any procedure associated with breach of soft tissues results in high frequency of detectable bacteremia by oral streptococci. There is strong evidence for an oral source for disseminated infections. Fingerprinting techniques have demonstrated a correlation between oral isolates and isolates from blood-infected prostatic devices and heart valve lesions. The ability of oral streptococci to contribute to systemic disease is observed most dramatically in patients with neutropenia. In these patients, oral streptococcal bacteremia is frequently associated with the development of septic shock and death. Although S. oralis and S.mitis are predominant in these patients, the involvement of other oral streptococci, especially from the anginosis group, cannot be ignored. Therefore oral streptococci must be considered to be clinically significant systemic pathogens capable of producing shock and death after gaining access to the blood. Furthermore, these microorganisms are occasionally associated with communityacquired pneumonia and childhood meningitis.
Infective Endocarditis
The most widely recognized systemic consequence of oral streptococcal bacteremia is infective endocarditis, an infection of the heart valves and surrounding structures. Approximately half of all cases are attributed to oral streptococci. Infective endocarditis is characterized by septic platelet-rich vegetations on injured heart valves. Unsuccessful intervention may result in obstruction of infected valves, dissemination of septic thrombi to other organs, and death. Among the oral streptococci, the mitis group is predominant in this disease. The streptococci move through the blood to selectively attach to and colonize injured heart valves. On the valves, the virulence of these streptococci may again change, reflecting a unique pattern of gene expression in vivo. It has been shown that numerous streptococcal genes are expressed on the heart valves in animal experimental endocarditis, which are not expressed in vitro. Approaches in molecular biology may reveal a variety of previously unknown characteristics. Although the exact mechanism used by oral streptococci to cause infective endocarditis is not clear, there are indications that virulence in infective endocarditis may require expression of adhesins for injured valve tissue, mediators of growth, promotion of thrombus, a niche protected by immune clearance, and resistance to platelet microbial protein.
Summary
Streptococcal infections remain a serious health problem worldwide. Since antibiotics alone have not been able to control these infections, the development of an effective vaccine and other control strategies will be the focus of future research. A prerequisite is the precise understanding of the pathogenesis of streptococcal infections. An important starting point is the understanding of the pathogenesis of rheumatic fever, the mechanisms of the association of group C and group G streptococci in rheumatic heart disease, and the process by which pneumococci convert themselves from harmless commensals to highly virulent pathogens. These results can contribute toward development of a suitable streptococcal vaccine and an improved pneumococcal vaccine. In the last few years, alternative therapeutic strategies that target the critical infection mechanisms have been gaining interest. To control streptococcal infections, it also will be essential to study genetic susceptibility and identify the genetic markers of patients. The sequencing of the human and streptococcal genomes will make it easier to identify the genes involved in pathogenesis and susceptibility and to study their regulation. A bottleneck will be the lack of suitable animal models. The use of susceptible mouse strains may solve the problem of invasive infections models, but the development of a model for rheumatic heart disease remains a challenge. For this, transgenic and knockout mice may be helpful. In spite of great expectations, many more research efforts will be required to develop effective vaccines and alternative control strategies and to bring them into the clinical phases.
Bibliography:
- Bogaert D, De Groot R, and Hermans PW (2004) Streptococcus pneumoniae colonisation: The key to pneumococcal disease. Lancet Infectious Diseases 4: 144–154.
- Carapetic JR, Steer AC, Mulholland EK, and Weber M (2005) The global burden of group A streptococcal diseases. Lancet Infectious Diseases 5: 685–694.
- Chhatwal GS and McMillan DJ (2005) Uncovering the mysteries of invasive streptococcal diseases. Trends in Molecular Medicine 11: 152–155.
- Chhatwal GS, McMillan D, and Talay SR (2006) Pathogenicity factors in group C and G streptococci. In: Fischetti VA, et al. (eds.) Gram Positive Pathogens, pp. 213–221. Washington, DC: ASM Press
- Chhatwal GS and Preissner KT (2006) Extracellular matrix interactions with gram-positive pathogens. In: Fischetti VA, et al. (eds.) Gram Positive Pathogens, pp. 88–99. Washington, DC: ASM Press
- Cunningham MW (2000) Pathogenesis of group A streptococcal infections. Clinical Microbiology Reviews 13: 470–511.
- Gillespie SH and Balakrishnan I (2000) Pathogenesis of pneumococcal infection. Journal of Medical Microbiology 49: 1057–1067.
- Hakenbeck R and Chhatwal GS (eds.) (2007) Molecular Biology of Streptococci. Norfolk, UK: Horizon Bioscience.
- McMillan DJ and Chhatwal GS (2005) Prospects for a group A streptococcal vaccine. Current Opinion in Molecular Therapeutics 7: 11–16.
- Mitchell TJ (2000) Virulence factors and the pathogenesis of disease caused by Streptococcus pneumoniae. Research in Microbiology 151: 413–419.
- Paton JC (2004) New pneumococcal vaccines: Basic science and developments. In: Tuomanen E (ed.) The Pneumococcus, pp. 383–402. Washington, DC: ASM Press
- Stevens DL and Kaplan EL (eds.) (2000) Streptococcal Infections: Clinical Aspects, Microbiology, and Molecular Pathogenesis. Oxford, UK: Oxford University Press.
See also:
Free research papers are not written to satisfy your specific instructions. You can use our professional writing services to buy a custom research paper on any topic and get your high quality paper at affordable price.








