This sample Tuberculosis Treatment Research Paper is published for educational and informational purposes only. If you need help writing your assignment, please use our research paper writing service and buy a paper on any topic at affordable price. Also check our tips on how to write a research paper, see the lists of health research paper topics, and browse research paper examples.
Summary Of Principles
Prompt initiation of effective chemotherapy for pulmonary tuberculosis is the most important means by which person-to-person transmission of Mycobacterium tuberculosis is terminated; thus, treatment of tuberculosis is not only a matter of individual health but also is an important public health intervention (Hopewell et al., 2005). Consequently, all providers who undertake treatment of patients with tuberculosis must recognize that not only are they treating an individual, they are assuming an important public health function that also entails a high level of responsibility to the community, as well as to the individual patient. For each individual patient, special consideration should be given to the specific clinical and social context in which tuberculosis treatment is being administered so as to maximize the likelihood of successful treatment completion. Overall, the goals of antituberculosis therapy are multifaceted and include achieving cure without relapse of disease, stopping transmission of M. tuberculosis and preventing the emergence of drugresistant strains.
Directly observed therapy (DOT), that is, providing medications and watching the patient swallow them, is the first step to achieving these goals and is recommended for all patients as it has been shown to increase compliance and completion of therapy (see the section titled ‘Promoting adherence’). Consequently, tuberculosis is generally best treated through public health agencies because of the significant infrastructure and cost of administering DOT. Tuberculosis treatment is generally divided into two phases: the initiation (bactericidal) phase, which lasts 2 months, and the continuation phase, which lasts 4 to 7 months for patients with drug-susceptible disease (Blumberg et al., 2003; WHO, 2003). Antituberculosis treatment should be administered following bacteriologic confirmation of tuberculosis or empirically when there is a high clinical suspicion for disease prior to culture confirmation and, in certain cases, prior to the availability of acid fast bacilli (AFB) smear microscopy results. Tuberculosis should never be treated with a single drug and a single drug should never be added to a failing regimen due to the risk of acquiring drug resistance. Thus, multidrug therapy is always required.
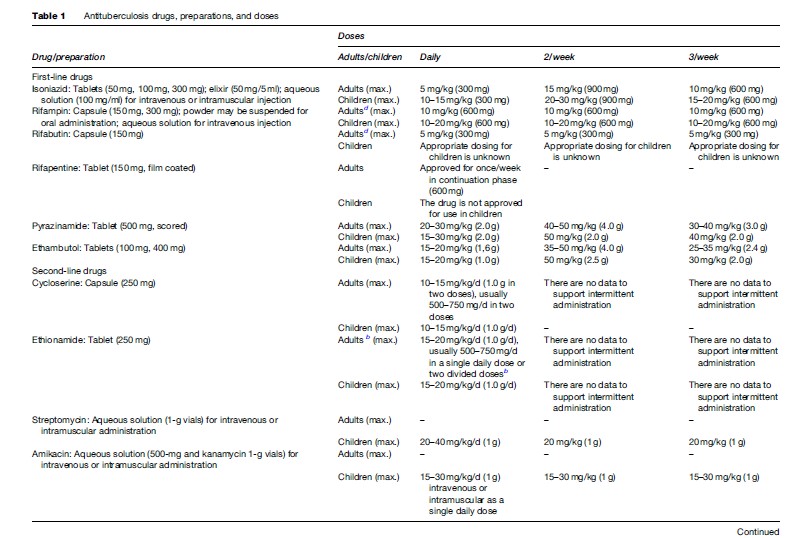
Treatment is generally initiated with an empiric four-drug regimen consisting of isoniazid, rifampin, ethambutol, and pyrazinamide. Recommended dosages and dosing schedules for first and second-line antituberculosis medications are shown in Table 1.
The efficacy of treatment should be monitored by obtaining sputum for AFB smear and culture at least monthly until two consecutive cultures are negative. Cultures should always be obtained after 2 months of treatment as positive cultures at this stage predict the risk of relapse and mandate prolongation of therapy. Drug susceptibility testing should be performed on the initial culture and again if cultures remain positive after 3 months of treatment.
Tuberculosis treatment with combination chemotherapy can be complicated by both mild and serious adverse reactions. Mild adverse reactions can generally be managed with conservative therapy aimed at controlling symptoms, whereas with more severe reactions the offending drug or drugs must be discontinued. Managing serious adverse reactions frequently necessitates expert consultation. Although it is important to recognize the potential for adverse effects, first-line drugs should not be discontinued without adequate justification.
Antituberculosis Drugs
There are 10 drugs currently approved by the U.S. Food and Drug Administration for treating tuberculosis plus six other drugs that are effective but not approved for this indication (Table 1) (Blumberg et al., 2003). An additional agent, thioacetazone, is available and used in some parts of the world, but is not approved for use in the United States. The table lists the drugs, available preparations, and the doses that are recommended (see Table 1).
Current Treatment Regimens
There are a number of published guidelines for the treatment of tuberculosis (Blumberg et al., 2003). All are based on the same principles, all recommend the same drugs, and all agree that 6 months is the minimum duration of treatment for bacteriologically confirmed tuberculosis. The principal difference between the guidelines lies in the frequency of administration recommended (daily vs. intermittent), which is primarily determined by the availability of resources for supervision.
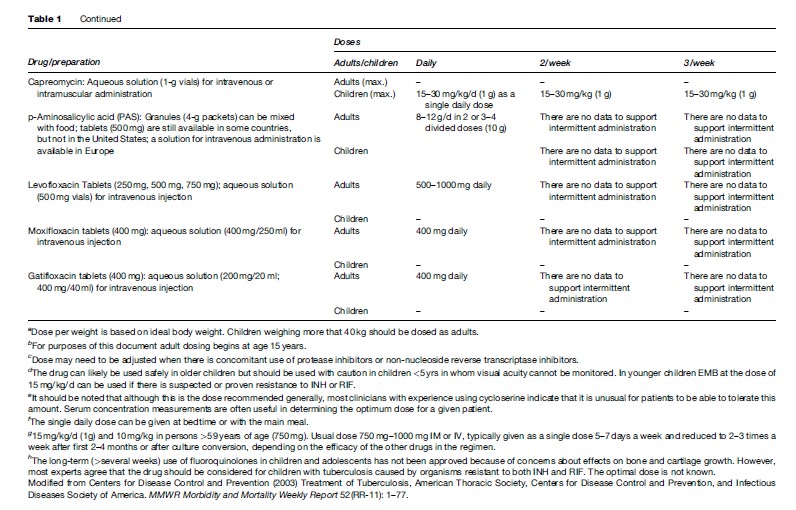
The treatment regimens that are recommended currently by the American Thoracic Society (ATS), Centers for Disease Control and Prevention (CDC), and the Infectious Diseases Society of America (IDSA) are shown in Table 2 (Blumberg et al., 2003). These recommendations are intended to guide treatment in areas where mycobacterial culture, drug susceptibility testing, chest radiography, and second-line drugs are available routinely. The treatment recommendations of the World Health Organization (WHO, 2003) are directed toward resource-limited countries in which the above facilities are not available routinely (Table 3).
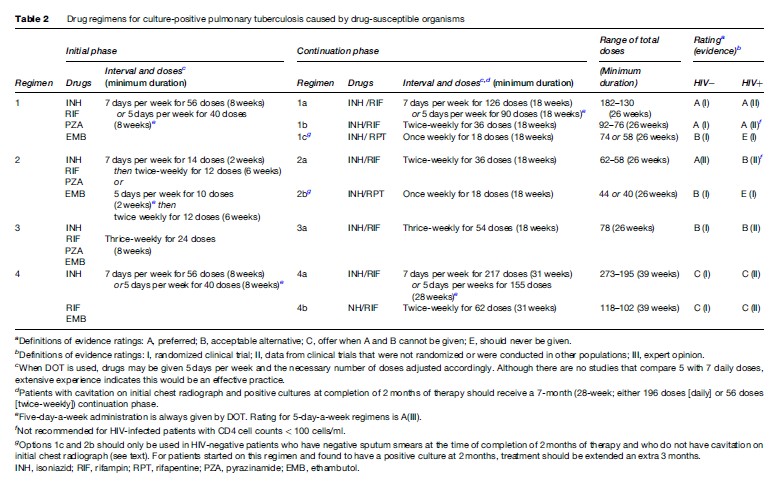
The basic treatment regimen recommended by the ATS, CDC, IDSA, and WHO for previously untreated patients with either pulmonary or extrapulmonary tuberculosis consists of an initial phase of isoniazid, rifampin, pyrazinamide, and ethambutol for 2 months, followed by 4 months of isoniazid and rifampin (Blumberg et al., 2003; WHO, 2003). The recommendation of a four-drug initial phase is based, in part, on findings of the British Medical Research Council (Mitchison and Nunn, 1986), and strongly suggests that where the prevalence of initial resistance to isoniazid is likely to be high, treatment regimens should include an initial 2-month, 4-drug initial phase, and rifampin plus isoniazid should be given for a 4-month continuation phase. The results of this regimen are nearly as good in the presence of resistance to isoniazid as for fully susceptible organisms.
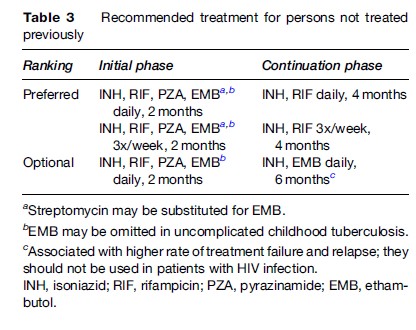
Variations in the pattern of administration of the basic regimen do exist and are designed primarily to enable observation of medication ingestion (Table 2). In circumstances in which DOT beyond the initial phase is not feasible, the WHO guidelines recommend a continuation phase of daily isoniazid and ethambutol. This is intended to minimize the likelihood of rifampin resistance developing. However, in a comparative clinical trial the isoniazid/ethambutol continuation phase regimen was significantly less effective than the isoniazid/rifampin continuation phase regimen ( Jindani, 2004). Moreover, it has been shown that in patients with HIV infection the outcome is significantly better when the regimen contains rifampin throughout (Korenromp et al., 2003).
Initial Susceptibility Testing
Where quality-assured laboratory facilities are available, drug susceptibility testing against first-line medication should be performed. This is of particular importance in areas of high prevalence of drug resistance or among persons in whom epidemiological circumstances indicate a risk of drug resistance. If resistance is identified, regimens can be tailored to suit the specific pattern. In certain situations, expert consultation may be needed to guide empiric selection of an expanded treatment regimen for individuals suspected of having drug-resistant tuberculosis, even before susceptibility results are available.
Promoting Adherence
Although it is well known that patients with tuberculosis can be difficult to manage, this difficulty does not absolve treating clinicians of their responsibility to render the patient noninfectious and permanently cured. In effect, successful treatment of tuberculosis requires not only the selection of an appropriate regimen (prescribed in appropriate doses and for appropriate durations) but also assiduous efforts in assuring patient adherence to the prescribed regimen.
The reasons for poor adherence to treatment for tuberculosis are complex and numerous, and prediction of who will be adherent and who will not is unreliable (Burkhart and Sabate, 2003). Numerous factors including cultural and linguistic barriers as well as lifestyle circumstances, such as homelessness and substance abuse, among a variety of other issues, can interfere with adherence (Moss et al., 2000; Blumberg et al., 2003). It is clear that there is no single approach to management that is effective for all patients, conditions, and settings. Consequently, interventions that target adherence must be tailored or customized to the particular situation of a given patient (Blumberg et al., 2003) and may include setting clinic hours to suit the patient’s schedule; providing treatment support in the clinic, the patient’s home, or other locations; and offering incentives and enablers, such as transportation reimbursements. This patient-centered, individualized approach to treatment support is a core element of all tuberculosis care and control efforts.
A central element in ensuring completion of therapy is giving the drugs under DOT (Blumberg et al., 2003). Pill counting or testing for drug metabolites in urine may also be useful for assessment of adherence. Use of DOT has been shown to be associated with improved outcomes of therapy in several cohort analyses (Weis et al., 1994; Zhao et al., 1996; Chaulk and Kazandjian, 1998). Patient-centered approaches using the full range of accepted measures to ensure medication ingestion are central to the WHO’s global tuberculosis control strategy, known as DOTS (DOTS is the acronym for Directly Observed Treatment, short-course and is the brand name for the WHO recommended strategy for TB control), and comprised of four elements in addition to DOT (WHO, 2002). Although concerns have been raised that the direct observation of medication ingestion does not lead to better outcomes compared with self-supervised treatment (Volmink and Garner, 2006), the full DOTS strategy has been very successful in high-incidence countries such as Peru and China (Suarez et al., 2001; Xianyi et al., 2002).
Predictors Of Outcome
The optimal surrogate marker for predicting poor outcome has yet to be identified, however, several factors have been found to be associated with poor outcomes, namely treatment failure (culture-positive tuberculosis after 4 months of treatment) or relapse (a second episode of tuberculosis after attaining culture-negative cure at completion of treatment) (Mitchison, 1993; Benator et al., 2000; Blumberg et al., 2003). Culture status at the end of the initiation phase is currently the most validated marker for risk of poor treatment outcome (Perrin et al., 2007). The U.S. Public Health Service Study 22 found that sputum culture positivity at the time of completion of the initial phase of treatment in combination with the presence of cavitation on the initial chest film were highly predictive of either treatment failure or relapse (Benator et al., 2000). For this reason, patients with cavitation on the initial chest film who have positive sputum cultures at the end of the initial phase of treatment should have the continuation phase of treatment extended from 4 to 7 months, making a total of 9 months as recommended by the ATS/CDC/ IDSA treatment guidelines (Blumberg et al., 2003). However, it should be noted that these recommendations differ from those of WHO, as in many parts of the world radiographic examination and cultures are not available. Additional factors that have been associated with poor outcomes include inadequate weight gain while on treatment (Khan et al., 2006), the use of alcohol, younger age (but above 18 years), and poor clinic attendance (Blumberg et al., 2003).
Monitoring Response
Sputum cultures should have converted to negative within 2 to 3 months of chemotherapy in 75–90% of patients taking a regimen that includes isoniazid and rifampin (Blumberg et al., 2003). Failure of the sputum cultures to become negative by this time should prompt further investigation, and may indicate that either the patient is not taking the drugs or, much less commonly, the organisms are resistant to the drugs being used. Patients who continue to have M. tuberculosis in their sputum after 2–3 months of treatment should be started on DOT, if not already being supervised in this manner, and should have drug susceptibility tests performed, if facilities are available. If resistance is found, the regimen should be modified based on the results. If sputum samples are still positive after 4 months of therapy, the regimen should be considered to have failed and a new regimen begun, ideally, based on recent drug susceptibility test results (Blumberg et al., 2003).
Management Of Relapse
Most relapses occur within 6 to 12 months after completion of therapy (Blumberg et al., 2003). In general, the management of patients who experience relapse despite the completion of a DOT regimen that contained isoniazid and rifampin involves reinstituting the same regimen previously used until susceptibility results are available. This approach is possible because organisms known at the outset of treatment to be sensitive to the first-line antituberculosis drugs commonly retain full drug susceptibility when isoniazid and rifampin were part of the original regimen. However, if the patient is severely ill at least three new agents should be added to the regimen for the possibility of drug resistance. Drug susceptibility testing should be performed and the regimen modified if resistance is detected. In addition, retreatment should be given under DOT.
Patients who experience relapse after completing a regimen that did not contain rifampin should be considered to harbor organisms that are resistant to the drugs that were used, at least until susceptibility is proven. The likelihood of resistance is directly related to the duration of previous treatment (Costello et al., 1980; Suwanogool et al., 1984). The likelihood of resistance to isoniazid increases approximately 4% per month of prior treatment and resistance to streptomycin increases at approximately 2.5% per month of prior treatment.
Drug-Resistant Tuberculosis
Drug resistance can be present de novo in the initial isolate or acquired during treatment. Features known to be associated with the acquisition of drug resistance while on treatment include the initial size of the bacillary population (i.e., presence of pulmonary cavities), the drug regimen prescribed (i.e., inappropriate drugs, insufficient dosage) and noncompliance of an otherwise adequate regimen (Blumberg et al., 2003). If drug-resistant organisms are suspected or confirmed by susceptibility testing, assistance should be sought from a clinician who has appropriate expertise in such situations. The basic principle of treatment for patients whose organisms demonstrate resistance to one or more of the first-line drugs, but not both to isoniazid and rifampin (see section titled ‘Multidrug resistant and extensively drug-resistant tuberculosis’) is to administer a regimen that consists of any remaining effective first-line drugs plus at least two (but generally three to four) agents to which there is demonstrated susceptibility (Blumberg et al., 2003). Unfortunately, there are no evidence-based guidelines as to the relative effectiveness of various regimens nor the optimal duration of treatment, however, the likelihood of treatment success does appear to be correlated to whether the organism is susceptible to rifampin (Espinal et al., 2000). When the organism is susceptible to isoniazid and rifampin, and is treated with a regimen that contains these drugs, likelihood of success is considered to be very high. In the presence of resistance to isoniazid, treatment with rifampin and ethambutol, supplemented by pyrazinamide, is likely to be successful (Blumberg et al., 2003).
Multidrug-Resistant And Extensively Drug-Resistant Tuberculosis
Multidrug-resistant (MDR) tuberculosis (MDRTB) is defined by the presence of resistance to both isoniazid and rifampin. Extensively drug-resistant (XDR) tuberculosis (XDRTB) is defined as MDRTB that is also resistant to a fluoroquinolone and at least one of three injectable second-line drugs: amikacin, capreomycin, or kanamycin. It is estimated that worldwide there are 400 000 incident cases of MDRTB and XDRTB identified each year (Raviglione and Smith, 2007). Since 1994, the WHO and the International Union Against Tuberculosis and Lung Disease have been conducting drug surveillance monitoring in 35 countries through a network of Supranational Reference Laboratories that aid National Reference Laboratories in conducting quality-assured drug susceptibility testing. These surveys have shown that drug resistance in general is ubiquitous (Aziz and Wright, 2005). The surveys are being repeated every 3 to 5 years to determine trend and in certain areas of the world rates of multidrug resistance continue to be alarmingly high (Pablos-Mendez et al., 1998; Espinal et al., 2001; Aziz and Wright, 2005). These so-called hotspots include Latvia, the Delhi region in India, Estonia, the Dominican Republic, and Argentina.
A recent analysis of 17 690 isolates from 49 countries by the CDC and WHO found that 2% of the isolates met the definition of XDRTB. Moreover, XDRTB was identified in all regions. For Latvia and the United States, where drug susceptibility results were available for all the tuberculosis cases, 4% and 19% of their MDR cases also met the XDR definition, respectively. As part of these projects an outbreak of HIV-associated XDRTB was reported in 2006 from the KwaZulu-Natal Province of South Africa. Of 221 MDRTB cases identified, 53 (23%) were also resistant to a fluoroquinalone (ciprofloxacin) and an injectable (kanamycin) (Gandhi et al., 2006). Only half reported a prior history of treatment for tuberculosis. HIV test results were available in 44 patients and all were found to be seropositive, and mortality was alarmingly high with 52 of the 53 patients dying within a median of 16 days from the initial sputum collection.
Treatment Of MDR And XDR Tuberculosis
In general, the outcome of treatment depends largely on the number of agents to which the organisms are susceptible, the promptness and appropriateness of therapy, the number of previous courses of therapy, and the HIV status of the patient. Consequently, the outcome of treatment in persons with tuberculosis caused by MDR organisms is generally less good than that of treatment of disease caused by susceptible organisms. Treatment of XDR organisms is even more complex and is estimated to cost upwards of $500 000 per case. Consultation with an expert is strongly advised in treating patients with MDRTB and XDRTB. The regimen selected represents the last best chance for cure and must be implemented correctly. The regimen should be based on the results of drug susceptibility tests, however, the results are often not known until several weeks after therapy is started. In such instances, treatment should be empirically determined on the basis of the patient’s history of prior therapy, avoiding reliance on agents taken previously and on the prevailing resistance patterns in the community or subpopulation of which the patient is a member.
In a group of patients with MDRTB who had undergone multiple previous courses of chemotherapy, the rate of successful outcome was only slightly better than 50% (Goble et al., 1993). However, both in New York and in San Francisco non-HIV-infected patients with MDRTB who had not been extensively treated previously had a much better rate of success (92% and 97%, respectively), albeit with a much shorter follow-up time (Telzak et al., 1995; Burgos et al., 2005). In the report from San Francisco, the prognosis for patients with HIV infection and MDRTB was very poor, with all 11 patients dying during the period of the study, although this group of patients was treated prior to the availability of highly active antiretroviral therapy (HAART) (Burgos et al., 2005). Whether HAART would improve the outcome is not known. In the cohort of 53 patients with XDRTB from the KwaZulu-Natal Province, 15 were receiving antiretroviral therapy and yet 98% of the cohort died (Gandhi et al., 2006).
Treatment Of Extrapulmonary Tuberculosis
Tuberculosis can involve virtually any organ or tissue in the body. Nonpulmonary sites tend to be more common among children and persons with impaired immunity. To establish the diagnosis of extrapulmonary tuberculosis, appropriate specimens including pleural fluid; pericardial or peritoneal fluid; pleural, pericardial, and peritoneal biopsy specimens; lymph node tissue; and bone marrow, bone, blood, urine, brain, or cerebrospinal fluid should be obtained for AFB staining, mycobacterial culture, and drug susceptibility testing (Blumberg et al., 2003).
Central Nervous System Tuberculosis
Tuberculous meningitis is the most severe form of extrapulmonary tuberculosis and the most common form of central nervous system tuberculosis (Hosoglu et al., 2002). The clinical presentation is nonspecific making early diagnosis difficult. Moreover, conventional approaches such as AFB smear and culture from cerebrospinal fluid for speciation and susceptibility testing are often nondiagnostic. In view of the catastrophic consequences and high incidence of mortality in poorly treated tuberculous meningitis, the potential for resistant organisms should be taken into account when treatment is initiated. If there are no epidemiological indicators of possible resistance, a regimen of isoniazid, rifampin, pyrazinamide, and ethambutol should be effective. The recommended length of the continuation phase is 7 months for a total treatment duration of 9–12 months, although there are no clinical trials that serve to define the optimum treatment duration (Blumberg et al., 2003). Isoniazid penetrates the blood–brain barrier readily, and although data are limited, in the presence of meningeal inflammation, rifampin, pyrazinamide, and streptomycin enter the cerebrospinal fluid in concentrations sufficient to inhibit growth of the organism. Ethambutol penetrates poorly, and doses of 25 mg/kg body weight produce subinhibitory concentrations.
Corticosteroid treatment has a beneficial effect in patients with tuberculous meningitis and cerebral edema (O’Toole et al., 1969; Girgis et al., 1991; Thwaites et al., 2004). Given the severity of the process, reasonably good data supporting corticosteroid use in more severe forms of the disease, and a paucity of information in patients with less severe tuberculosis meningitis, corticosteroid treatment – specifically with dexamethasone – is recommended for all patients, especially those with alterations in their level of consciousness (Thwaites et al., 2004). The recommended dose of dexamethasone is 12 mg/day for 3 weeks then decreased gradually during the next 3 weeks.
The other major central nervous system form of tuberculosis, the tuberculoma, presents a more subtle clinical picture than does tuberculous meningitis. The response to antituberculosis chemotherapy is good, and corticosteroids are indicated only if there is an increase in intracranial pressure.
Lymphatic Tuberculosis
Tuberculous lymphadenitis is a common form of extrapulmonary tuberculosis. Cervical adenopathy is most common, but inguinal, axillary, mesenteric, mediastinal, and intramammary involvement have also been described (Golden and Vikram, 2005). A 6-month regimen is recommended for treatment of tuberculous lymphadenitis (Campbell and Dyson, 1977; Campbell, 1990; Jawahar et al., 1990). However, even with effective regimens, the rate of response is much slower than with pulmonary tuberculosis. Lymph nodes may enlarge, new nodes may appear, and fistulas may develop during treatment that ultimately proves effective. This transient worsening may be a manifestation of the immune reconstitution syndrome. Overall, true bacteriologic relapse after completion of therapy is unusual.
Corticosteroid treatment has been used to shrink intrathoracic nodes and relieve bronchial obstruction, primarily in children. Corticosteroids also increase the rate of resolution of radiographic changes thought to be due to bronchial narrowing by lymph nodes or endobronchial lesions in children with primary tuberculosis (Nemir, 1967). Apart from this indication, there is no clear role for corticosteroids in lymphatic tuberculosis.
Pleural Tuberculosis
Two forms of tuberculous involvement of the pleura are seen; a hypersensitivity to bacilli present in the pleural space resulting in pleurisy, and a distinct form that represents true empyema (Baumann et al., 2007). Treatment of the hypersensitivity variety of tuberculous pleural effusion consists of standard antituberculosis drug regimens (Blumberg et al., 2003). Repeat thoracenteses may be required to relieve symptoms, but drainage via tube thoracostomy is rarely necessary. In general, the amount of residual pleural scarring from this form is small. The use of corticosteroids may increase the rate of resolution and decrease the residual fluid, but such treatment is rarely indicated.
The second variety of tuberculous involvement of the pleura is a true empyema. This is much less common than tuberculous pleurisy with effusion and results from a large number of organisms spilling into the pleural space, usually from rupture of a cavity or an adjacent parenchymal focus by way of a broncho-pleural fistula. Although standard chemotherapy should be instituted for tuberculous empyema, it is unlikely to clear the pleural space infection, probably because penetration of the antituberculosis agents into the pleural cavity is limited. For this reason, surgical drainage is often necessary and may be required for a prolonged period of time both as treatment for the infection and because of the frequent association with a broncho-pleural fistula. Drainage may be accomplished with a standard thoracostomy tube. In selected patients, creation of an Eloesser flap, in which a small portion of rib overlying the empyema space is resected and the skin is sutured to the pleura, is the procedure of choice. Corticosteroids have no role in treating this form of pleural tuberculosis.
Genitourinary Tuberculosis
The principal means of diagnosing genitourinary tuberculosis is the isolation of M. tuberculosis from urine (Christensen, 1974). In general, renal tuberculosis is treated with a standard 6-month regimen (Gow and Barbosa, 1984). Nephrectomy is seldom indicated except in patients who have tuberculosis caused by MDR organisms and who can tolerate removal of a kidney. Nephrectomy may also be indicated for patients who have recurrent pyogenic bacterial infections in a kidney destroyed by tuberculosis, for persistent pain, and for massive hematuria. Surgical or endoscopic procedures may also be necessary to correct ureteral strictures and to augment the capacity of a contracted bladder.
Bone And Joint Tuberculosis
In musculoskeletal tuberculosis, the spine, hip, and knee are the most common sites of M. tuberculosis infection (Davidson and Horowitz, 1970; Ludwig and Lazarus, 2007). Spinal tuberculosis (Pott’s disease) involves the thoracic spine in 50% of cases (Watts and Lifeso, 1996). Standard chemotherapy of 6 to 9 months’ duration is highly successful in skeletal tuberculosis, but surgery is occasionally a necessary adjunct. The role of emergency spinal cord decompression in such patients is unclear, and if paraplegia is already present, the benefit of surgical intervention is even less clear. Longer duration of treatment has been suggested by some experts because of the difficulties in assessing response. Several controlled studies have documented that chemotherapy conducted largely on an ambulatory basis is effective in curing spinal tuberculosis without the need for immobilization. Moreover, there is no well-defined surgical procedure of choice. Surgery may be indicated in other forms of articular tuberculosis when there is extensive destruction of the joint or surrounding soft tissues, in which case synovectomy and joint fusions may be necessary.
Abdominal Tuberculosis
A 6-month regimen is effective for patients with peritoneal or intestinal tuberculosis (Singh et al., 1969; Demir et al., 2001). Surgery may be necessary to establish a diagnosis and, in addition, is necessary to relieve intestinal obstruction if it should occur. Corticosteroids have been advocated in tuberculous peritonitis to reduce the risk of adhesions causing intestinal obstructions, but this recommendation is controversial because the frequency of obstruction is generally low, and in small studies the use of corticosteroids did not reach statistical significance in preventing fibrotic complications (Singh et al., 1969).
Pericardial Tuberculosis
A 6-month regimen is recommended for treatment of pericardial tuberculosis. Because of its potentially lifethreatening nature, antituberculosis agents should be instituted promptly once the diagnosis is made or strongly suggested. It appears that the likelihood of pericardial constriction is greater in patients who have had symptoms longer; thus, early therapy may reduce the incidence of this complication. Several studies have suggested that corticosteroids have a beneficial effect in treating both tuberculous pericarditis with effusion and constrictive pericarditis (Strang et al., 1987; Mayosi et al., 2002). However, a meta-analysis of studies examining the effects of corticosteroids in tuberculous pericarditis concluded that although steroids could have an important effect, the studies were too small to be conclusive (Mayosi et al., 2002). Nevertheless, in patients with proven tuberculous pericarditis who are receiving adequate antituberculosis therapy and who have no major contraindications to the use of corticosteroids should receive them. The optimum regimen is not known, but prednisone – 60 mg/day for 4 weeks – followed by 30 mg/day for 4 weeks, 15 mg/day for 2 weeks, and 5 mg/day for 1 week is the recommended regimen (Blumberg et al., 2003). Corticosteroid therapy should not be used if there is a strong suspicion that the infection is caused by a drug-resistant organism unless adequate antituberculosis chemotherapy can be ensured.
In general, if hemodynamic compromise occurs, pericardiectomy is necessary. Although pericardiocentesis generally improves the circulatory status, the improvement is usually temporary. Pericardial windows with drainage into the left pleural space also generally provide only temporary relief. The criteria for selecting patients for pericardiectomy are not clear, apart from those patients who have severe hemodynamic compromise.
Disseminated Tuberculosis
Standard antituberculosis chemotherapeutic regimens should be employed for disseminated tuberculosis unless meningitis is present in which case the recommended duration is 9–12 months (Blumberg et al., 2003). Corticosteroids may be useful for severe pulmonary disease with respiratory failure.
New Drugs For Tuberculosis
The primary goal of new antituberculosis drug development will be to shorten the minimum duration of treatment for bacteriologically proven tuberculosis from the current 6 months to 1–2 months, and to develop more effective treatments for latent tuberculosis infection (O’Brien and Nunn, 2001). Forty years have passed since the last novel antituberculosis drug, rifampicin, was introduced (Glickman et al., 2006). The recent identification of XDRTB and continued escalation of MDRTB further underscores the critical need for the development of new drugs. However, it is worth noting that the response to MDRTB and XDRTB should be primarily the strengthening of current tuberculosis control programs to prevent additional cases, as well as the development of new diagnostics for the rapid identification drug resistance. Otherwise, in the absence of effective tuberculosis control practices, resistance is likely to develop rapidly against these new drugs. The recent explosion of knowledge concerning the biochemistry, genomics, and proteomics of M. tuberculosis will no doubt contribute to identification of new drug targets as well as rapid diagnostics (Zhang, 2005). As of this writing there are 11 compounds undergoing preclinical and clinical testing (Glickman et al., 2006; Raviglione, 2006). Of the drugs in human trials, the quinolones are closest to completing evaluation. There is preliminary evidence that inclusion of a quinolone, probably moxifloxacin because of its greater potency, may be able to shorten the duration of treatment (Burman et al., 2006; Rosenthal et al., 2006). As of this writing, a multicenter Phase III treatment duration shortening trial that includes moxifloxacin is under way.
Bibliography:
- Aziz MA and Wright A (2005) The World Health Organization/ International Union Against Tuberculosis and Lung Disease Global Project on Surveillance for Anti-Tuberculosis Drug Resistan A model for other infectious diseases. Clinical Infectious Diseases 41(supplement 4): S258–S262.
- Baumann MH, Nolan R, Petrini M, Lee YC, Light RW, and Schneider E (2007) Pleural tuberculosis in the United States: Incidence and drug resistance. Chest 131: 1125–1132.
- Benator D, Bhattacharya M, Bozeman L, et al. (2002) Rifapentine and isoniazid once a week versus rifampicin and isoniazid twice a week for treatment of drug-susceptible pulmonary tuberculosis in HIVnegative patients: A randomised clinical trial. The Lancet 360: 528–534.
- Blumberg HM, Burman WJ, Chaisson RE, et al. (2003) American Thoracic Society/Centers for Disease Control and Prevention/ Infectious Diseases Society of America: Treatment of tuberculosis. American Journal of Respiratory and Critical Care Medicine 167: 603–662.
- Burgos M, Gonzalez LC, Paz EA, et al. (2005) Treatment of multidrugresistant tuberculosis in San Francisco: An outpatient-based approach. Clinical Infectious Diseases 40: 968–975.
- Burkhart PV and Sabate E (2003) Adherence to long-term therapies: Evidence for action. Journal of Nursing Scholarship 35: 207.
- Burman WJ, Goldberg S, Johnson JL, et al. (2006) Moxifloxacin versus ethambutol in the first 2 months of treatment for pulmonary tuberculosis. American Journal of Respiratory and Critical Care Medicine 174: 331–338.
- Campbell IA (1990) The treatment of superficial tuberculous lymphadenitis. Tubercle 71: 1–3.
- Campbell IA and Dyson AJ (1977) Lymph node tuberculosis: A comparison of various methods of treatment. Tubercle 58: 171–179.
- Centers for Disease Control and Prevention (2006) Emergence of mycobacterium tuberculosis with extensive resistance to second-line drugs – worldwide, 2000–2004. MMWR Morbidity and Mortality Weekly Report 55: 301–305.
- Centers for Disease Control and Prevention (2007) Extensively drugresistant tuberculosis – United States, 1993–2006. MMWR Morbidity and Mortality Weekly Report 56: 250–253.
- Chaulk CP and Kazandjian VA (1998) Directly observed therapy for treatment completion of pulmonary tuberculosis: Consensus statement of the public health tuberculosis guidelines panel. Journal of the American Medical Association 279: 943–948.
- Christensen WI (1974) Genitourinary tuberculosis: Review of 102 cases. Medicine (Baltimore) 53: 377–390.
- Costello HD, Caras GJ, and Snider DE Jr (1980) Drug resistance among previously treated tuberculosis patients, a brief report. American Review on Respiratory Diseases 121: 313–316.
- Davidson PT and Horowitz I (1970) Skeletal tuberculosis. A review with patient presentations and discussion. American Journal of Medicine 48: 77–84.
- Demir K, Okten A, Kaymakoglu S, et al. (2001) Tuberculous peritonitis–reports of 26 cases, detailing diagnostic and therapeutic problems. European Journal of Gastroenterology and Hepatology 13: 581–585.
- Espinal MA, Kim SJ, Suarez PG, et al. (2000) Standard short-course chemotherapy for drug-resistant tuberculosis: Treatment outcomes in 6 countries. Journal of the American Medical Association 283: 2537–2545.
- Espinal MA, Laszlo A, Simonsen L, et al. (2001) Global trends in resistance to antituberculosis drugs. World Health Organization-International Union Against Tuberculosis and Lung Disease Working Group on Anti-Tuberculosis Drug Resistance Surveillance. New England Journal of Medicine 344: 1294–1303.
- Gandhi NR, Moll A, Sturm AW, et al. (2006) Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet 368: 1575–1580.
- Girgis NI, Farid Z, Kilpatrick ME, Sultan Y, and Mikhail IA (1991) Dexamethasone adjunctive treatment for tuberculous meningitis. Pediatric Infectious Disease Journal 10: 179–183.
- Glickman SW, Rasiel EB, Hamilton CD, Kubataev A, and Schulman KA (2006) Medicine. A portfolio model of drug development for tuberculosis. Science 311: 1246–1247.
- Goble M, Iseman MD, Madsen LA, Waite D, Ackerson L, and Horsburgh CR Jr (1993) Treatment of 171 patients with pulmonary tuberculosis resistant to isoniazid and rifampin. New England Journal of Medicine 328: 527–532.
- Golden MP and Vikram HR (2005) Extrapulmonary tuberculosis: An overview. American Family Physician 72: 1761–1768.
- Gow JG and Barbosa S (1984) Genitourinary tuberculosis. A study of 1117 cases over a period of 34 years. British Journal of Urology 56: 449–455.
- Hopewell PC and Pai M (2005) Tuberculosis, vulnerability, and access to quality care. Journal of the American Medical Association 293: 2790–2793.
- Hopewell PC, Pai M, Maher D, Uplekar M, and Raviglione MC (2006) International standards for tuberculosis care. Lancet Infectious Diseases 6: 710–725.
- Hosoglu S, Geyik MF, Balik I, et al. (2002) Predictors of outcome in patients with tuberculous meningitis. International Journal of Tuberculosis and Lung Disease 6: 64–70.
- Jawahar MS, Sivasubramanian S, and Vijayan VK (1990) Short course chemotherapy for tuberculous lymphadenitis in children. British Medical Journal 301: 359–362.
- Jindani A, Nunn AJ, and Enarson DA (2004) Two 8-month regimens of chemotherapy for treatment of newly diagnosed pulmonary tuberculosis: International Multicentre Randomized Trial. Lancet 364: 1244–1251.
- Khan A, Sterling TR, Reves R, Vernon A, and Horsburgh CR (2006) Lack of weight gain and relapse risk in a large tuberculosis treatment trial. Am J Respir Crit Care Med 174: 344–348.
- Korenromp EL, Scano F, Williams BG, Dye C, and Nunn P (2003) Effects of human immunodeficiency virus infection on recurrence of tuberculosis after rifampin-based treatment: An analytical review. Clinical Infectious Diseases 37: 101–112.
- Ludwig B and Lazarus AA (2007) Musculoskeletal tuberculosis. Dis Mon 53: 39–45.
- Mayosi BM, Ntsekhe M, Volmink JA, and Commerford PJ (2002) Interventions for treating tuberculous pericarditis. Cochrane Database Syst Rev CD000526.
- Medical Research Council Working Party on Tuberculosis of the Spine (1976) A five-year assessment of controlled trials of in-patient and out-patient treatment and of plaster-of-Paris jackets for tuberculosis of the spine in children on standard chemotherapy. Studies in Masan and Pusan, Korea. Fifth report of the Medical Research Council Working Party on tuberculosis of the spine. The Journal of Bone and Joint Surgery 58-B: 399–411.
- Medical Research Council Working Party on Tuberculosis of the Spine (1978) Five-year assessments of controlled trials of ambulatory treatment, debridement and anterior spinal fusion in the management of tuberculosis of the spine. Studies in bulawayo (rhodesia) and in Hong Kong. Sixth report of the medical research council working party on tuberculosis of the spine. Journal of Bone and Joint Surgery (British) 60-B: 163–177.
- Mitchison DA and Nunn AJ (1986) Influence of initial drug resistance on the response to short-course chemotherapy of pulmonary tuberculosis. American Review on Respiratory Diseases 133: 423–430.
- Mitchison DA (1993) Assessment of new sterilizing drugs for treating pulmonary tuberculosis by culture at 2 months. Am Rev Respir Dis 147: 1062–1063.
- Moss AR, Hahn JA, Tulsky JP, Daley CL, Small PM, and Hopewell PC (2000) Tuberculosis in the homeless. A prospective study. American Journal of Respiratory and Critical Care Medicine 162: 460–464.
- Nemir RL, Cardona J, Vaziri F, and Toledo R (1967) Prednisone as an adjunct in the chemotherapy of lymph node-bronchial tuberculosis in childhood: A double-blind study. Ii. Further term observation. Am Rev Respir Dis 95: 402–410.
- O’Brien RJ and Nunn PP (2001) The need for new drugs against tuberculosis. Obstacles, opportunities, and next steps. Am J Respir Crit Care Med 163: 1055–1058.
- O’Toole RD, Thornton GF, Mukherjee MK, and Nath RL (1969) Dexamethasone in tuberculous meningitis. Relationship of cerebrospinal fluid effects to therapeutic efficacy. Ann Intern Med 70: 39–48.
- Pablos-Mendez A, Raviglione MC, Laszlo A, et al. (1998) Global surveillance for antituberculosis-drug resistance, 1994–1997. World Health Organization-International Union Against Tuberculosis and Lung Disease Working Group on Anti-Tuberculosis Drug Resistance Surveillance. N Engl J Med 338: 1641–1649.
- Perrin FM, Lipman MC, McHugh TD, and Gillespie SH (2007) Biomarkers of treatment response in clinical trials of novel antituberculosis agents. Lancet Infect Dis 7: 481–490.
- Raviglione MC (2006) The global plan to stop TB, 2006–2015. Int J Tuberc Lung Dis 10: 238–239.
- Raviglione MC and Smith IM (2007) XDR tuberculosis – implications for global public health. N Engl J Med 356: 656–659.
- Rosenthal IM, Williams K, Tyagi S, et al. (2006) Potent twice-weekly rifapentine-containing regimens in murine tuberculosis. Am J Respir Crit Care Med 174: 94–101.
- Singh MM, Bhargava AN, and Jain KP (1969) Tuberculous peritonitis. An evaluation of pathogenetic mechanisms, diagnostic procedures and therapeutic measures. N Engl J Med 281: 1091–1094.
- Strang JI, Kakaza HH, Gibson DG, Girling DJ, Nunn AJ, and Fox W (1987) Controlled trial of prednisolone as adjuvant in treatment of tuberculous constrictive pericarditis in transkei. Lancet 2: 1418–1422.
- Suarez PG, Watt CJ, Alarcon E, et al. (2001) The dynamics of tuberculosis in response to 10 years of intensive control effort in Peru. J Infect Dis 184: 473–478.
- Suwanogool S, Smith SM, Smith LG, and Eng R (1984) Drug-resistance encountered in the retreatment of mycobacterium tuberculosis infections. J Chronic Dis 37: 925–931.
- Telzak EE, Sepkowitz K, Alpert P, et al. (1995) Multidrug-resistant tuberculosis in patients without HIV infection. N Engl J Med 333: 907–911.
- Thwaites GE, Nguyen DB, Nguyen HD, et al. (2004) Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N Engl J Med 351: 1741–1751.
- Volmink J and Garner P (2006) Directly observed therapy for treating tuberculosis. Cochrane Database Syst Rev CD003343.
- Watts HG and Lifeso RM (1996) Tuberculosis of bones and joints. J Bone Joint Surg Am 78: 288–298.
- Weis SE, Slocum PC, Blais FX, et al. (1994) The effect of directly observed therapy on the rates of drug resistance and relapse in tuberculosis. New England Journal of Medicine 330: 1179–1184.
- World Health Organization (2002) An expanded dots framework for effective tuberculosis control. Geneva, Switzerland: World Health Organization.
- World Health Organization (WHO) (2003) Treatment of tuberculosis: Guidelines for national programmes. Geneva, Switzerland: WHO.
- Xianyi C, Fengzeng Z, Hongjin D, et al. (2002) The dots strategy in China: Results and lessons after 10 years. Bull World Health Organ 80: 430–436.
- Zhang Y (2005) The magic bullets and tuberculosis drug targets. Annu Rev Pharmacol Toxicol 45: 529–564.
- Zhao FZ, Murray C, Spinaci S, et al. (1996) Results of directly observed short-course chemotherapy in 112,842 Chinese patients with smear-positive tuberculosis. China tuberculosis control collaboration. The Lancet 347: 358–362.
See also:
Free research papers are not written to satisfy your specific instructions. You can use our professional writing services to buy a custom research paper on any topic and get your high quality paper at affordable price.








