This sample Imaging Techniques and Brain Function Research Paper is published for educational and informational purposes only. If you need help writing your assignment, please use our research paper writing service and buy a paper on any topic at affordable price. Also check our tips on how to write a research paper, see the lists of psychology research paper topics, and browse research paper examples.
The incorporation of human imaging methods into the psychological sciences has been rapid and dramatic. The movement of behavioral research in this direction requires careful consideration of these methods and what they reveal about behavior and the brain. For this reason, the present chapter will highlight the basic assumptions that underlie these methodologies. The assumption that behavior is localized in the brain underlies the need for imaging methods in psychology. But these methods are only as good as the information they provide. Accordingly, we start with a review of the relation between neural activity and metabolism in the brain, and then proceed to describe the relation between blood flow and the signals measured, first with functional magnetic resonance imaging (fMRI). Because both positron emission tomography (PET) and fMRI utilize structural magnetic resonance (MR) images to identify the location of the activation, I will briefly discuss the basis of structural MRI and will illustrate its use to psychologists. The majority of the chapter will describe the basis of fMRI, which is the imaging method most widely used at the moment by psychologists. For comparison purposes, there will be a brief description of PET functional imaging. Finally, I discuss the limits of these methodologies in relation to the questions of interest in psychology.
Elemental Functions Localized Within The Brain
Behind the emergence of human imaging methods is the belief that function is localized in the brain. The belief that the mind and behavior are products of a biological machine, the brain, has emerged slowly over the last several centuries. These beliefs began with Galileo and Newton’s interpretation that the world is mechanical in nature (Schultz, 1981). Once Newton established that the laws of nature extended to the stars and planets, philosophers began to question why the body and mind should be excluded from these laws. Ultimately, the empiricists began to believe that the mind is a product of the natural world, and therefore can be studied like the natural world. The empiricists concluded that thoughts are based solely on sensory experiences and associations between sensory experiences. Eventually they reasoned that sensory impressions and associations are formed within the brain. Altogether, this progression of philosophical ideas forms the early basis of our current belief that the mind and its output, behavior, are a product of the brain.
In the field of neuroscience, there was an initial conflict as to whether specific locations within the brain sub-served specific behaviors, or whether the brain operated as a whole (the aggregate field view of Fluorens; Kandel, Schwartz, & Jessell, 2000). Over time, there developed a set of data that together pointed squarely to the concept that elemental functions, a reduction from the personality traits proposed to be localized by Gall, are localized within discrete regions of the brain. Broca and Wernicke, for example, found that deficits in language were associated with damage to specific brain locations. Fritsch and Hitzig were able to stimulate specific motor responses by electrically stimulating the motor cortex. Brodmann demonstrated that despite the six layers of the cerebral cortex, there are subtle differences in the cellular and layer architecture (cytoarchitectonics) over the cortex. Thus the potential for differential computational processing across cortical regions existed. Eventually, the field of neuroscience has adopted and framed data on the belief that function is localized within the brain.
Prior to the advent of human imaging, the field of neuropsychology utilized natural lesions to develop an understanding of the relation between brain lesions and behavioral deficits. Only rarely were there other opportunities to obtain converging data by measuring neural activity. Typically, electrophysiological recordings are carried out only in epileptics preparing for surgery, and these studies are problematic because the population may not necessarily represent the population as a whole. Furthermore, only a few brain sites can be recorded from at any one time. To avoid invasive methods, researchers have used recordings of scalp EEG widely. Although these methods, like electro-physiological recordings from implanted electrodes, have a superior ability to measure potentials over very brief time periods (ms), the EEG potentials could not be local-ized. Instead they reflect electrical activity summed over large areas. Given that function is localized, the location of the source of activity is of great interest. Human imaging methods brought about the opportunity to study location of function without the limitations of natural dysfunction or disease. Most of these methods, however, reflect signals that are collected over seconds. Accordingly, there is strong interest in combining the location information from human imaging methods and rapid electrical activity provided by EEG recordings.
The advent of human imaging methods, PET in the 1980s and fMRI in the early 1990s, provided greater opportunities to visualize activation in areas of the brain during sensory, perceptual, cognitive, social, affective, and motor processing. Furthermore, fMRI allowed the study of activation throughout the whole brain rather than in single regions at one time. The potential to image many areas at once opens up the possibility of studying how areas work together to produce complex behaviors.
Coupling Between Neural Activity, Metabolism, And Blood Flow
Although PET and fMRI are two different methods for studying human neural activation during task performance, these techniques rely on the assumption that increases in brain metabolism are reflections of an increase in neural activation. Therefore, to trust the relevance of the data from these methodologies requires an understanding of the relation between neural activity and brain metabolism. It may help to know first that the brain uses a substantial proportion of the energy consumed by the body. The brain uses 15 percent to 20 percent of the oxygen consumed despite making up only 2 percent of body weight (Clarke & Sokoloff, 1999; Roland, 1993). The glucose and oxygen used by the brain serves to produce ATP. This molecule is valuable because the free energy it stores is easily accessible. ATP is considered the currency required for the biological reactions that support life. Instead of storing ATP, the brain makes ATP as needed. This requires that neural activity stimulate increases in metabolism. In other words, as neural activity increases, it must produce signals to accelerate multiple stages of metabolism necessary to produce ATP. For example, as neuronal activity increases, it produces signals that increase local blood flow (Roland, 1993). These signals cause dilation of upstream arterioles, leading to an increase in blood, and the oxygen and glucose it carries, in local capillary beds. Capillaries, the smallest vessels, transport oxygen and glucose into brain tissue. Glucose is used for glycolysis, which produces pyruvate, lactate, and ATP. The ATP produced from glycolysis doesn’t adequately meet the needs of neural activity. The pyruvate and lactate, however, are used for a second stage of metabolism, the Kreb’s (TCA) cycle. The Kreb’s cycle, in turn, produces high-energy compounds needed for the final stage of metabolism, oxidative phosphorylation. This last stage requires oxygen and produces the greater amounts of ATP needed to support brain activity. In summary, when neural activity increases, it produces signals that increase the production of ATP. The ATP is broken down to release the free energy needed to restore the resting neuronal state.
It has long been recognized that increased neuronal activity can increase blood flow (Roy & Sherrington, 1890), but the biological signals responsible for this “neurovascular coupling” remain a mystery (Metea & Newman, 2006; Roland, 1993). Over the years, many signals have been suspected. These include CO2, pH, extracellular potassium, purines (adenosine, AMP, ADP, and ATP), and more recently, EETs and 20-HETE, prostoglandins, and cyclooxygenase metabolites (Metea & Newman, 2006; Roland, 1993). Each has substantial support for a role in mediating neurovascular coupling, but also fails in some experimental models to cause vasodilation. The ongoing contradictions in experimental outcomes likely reflect different mechanisms operating in different regions of the brain, thus leading to different outcomes in different experimental models, and also the likelihood that several mechanisms work in concert to alter blood flow, making the effectiveness of any single signal difficult to detect. For the present purpose, it is simply important to note that within the neuroscientific community it is widely accepted that products of neuronal activity (which are yet to be agreed upon) cause vasodilation. Other experimental data support a coupling between neural activity and glucose uptake (Sokoloff et al., 1977), glycolysis (Roland, 1993), oxygen consumption (Hill & Howarth, 1958), and oxidative phosphorylation (Wong-Riley, 1989). Because of this tight coupling, the activity of multiple stages of metabolism can be used as indirect indicators of neuronal activity. This coupling is exploited by many imaging methods. fMRI measures relative changes in blood flow and volume, whereas PET imaging is often used to quantify changes in glucose and oxygen uptake. In summary, although PET and fMRI provide indirect measures of neural activity, the basis for the relation between metabolism and neural activity is well established. Furthermore, metabolic mapping based on these relationships was used in neuroscience well before the advent of human imaging methods.
Correlation Of Synaptic Potentials With Metabolism And Blood Flow
At the level of a single neuron, it is of interest to consider whether the metabolic changes being measured reflect synaptic input or action potential output. Neurons are made up of a number of compartments. The compartments include the dendrites, which serve to greatly expand the membrane available for synaptic input, and the cell body, which both serves as a site for synaptic input and stores genetic material. The axon hillock arising from the cell body integrates the synaptic input, and when that input reaches a threshold, it produces an action potential. Action potentials are conducted by the axon over long distances at a rapid rate and without amplitude loss. Action potentials arrive at the synaptic terminals, which are responsible for releasing transmitter that serves to relay chemical information to the postsynaptic cells. This chemical information causes an electrical change in the postsynaptic membrane (dendrite) in the form of a “synaptic potential.” Synaptic potentials are difficult to detect with electrical recording methods. Direct measures of neural activity (i.e., electro-physiological recordings) often represent action potentials, because the potential amplitude of action potentials is far larger than that of synaptic potentials, and therefore easier to detect. Action potentials are far shorter in duration (1-10 ms) than synaptic potentials (5 ms to 20 minutes; Kandel et al., 2000). The majority of ATP serves to maintain membrane potentials. Thus, it is relevant to ask, does the increase in metabolism, which produces ATP, support the production and conduction of action potentials, or the enormous number of synaptic potentials on the vast surface of the dendritic membrane? Said more simply, does metabolism correlate more closely with synaptic input or action potentials output?
Investigation of the location of highest metabolic demand suggests that synaptic potentials create more metabolic demand than action potentials. Capillary density increases as synaptic density increases (Dunning & Wolff, 1937; as cited by Roland, 1993), and enzymes coupled to the production of ATP, along with the organelles that house them, are also highest in dendritic fields (Borowsky & Collins, 1989; Erecinska & Wilson, 1982; Zheng, LaMantia, & Purves, 1991). The highest energy consumption occurs in regions with the highest density of synapses, not regions with cell bodies, where action potentials would originate. For example, stimulation of rat whiskers increased the metabolism of glucose in areas of the cortex that represented whiskers. The cortical layers with the highest increase in metabolism were those layers with the highest density of dendrites and synapses (Hand, Greenberg, Sylvestro, Weller, & Reivich, 1979). Similar results were found from the stimulation of monkey fingertips (Juliano, Hand, & Whitsel, 1981). Taking advantage of the gross segregation of cell bodies in the dorsal root ganglion near the spinal cord, Kadekaro, Crane, and Sokoloff (1985) first showed that baseline levels of glucose utilization differed between areas containing dendrites and synapses compared to areas containing cell bodies only. Further, as stimulation was increased over a range of 0 to 15 Hz, glucose utilization increased only in the regions with synapses, not in the region with cell bodies (Kadekaro et al., 1985). In conclusion, neurovascular coupling appears to closely represent metabolism influenced by synaptic input rather than by action potential production and conduction. Thus, to integrate single unit recordings from nonhuman primates and human imaging data requires an understanding that single units would be expected to correspond to metabolic change in the downstream dendritic fields targeted by axonal projections.
With an understanding of the physiological bases supporting the use of metabolic measures as indicators of neural activity, it is possible to proceed to a description of imaging methods that are currently applied in human subjects. Both PET and MR imaging of functional activation utilize the superior spatial information provided by MR structural images. For that reason, we will start with a brief description of the basis of MRI, and its use for the acquisition of images of brain structure.
Origin And Basis Of MRI
Prior to imaging with magnetic resonance, researchers used nuclear magnetic resonance (NMR) to study the structure of molecules. In a magnetic field, atoms will absorb radio frequencies (RF) and resonate. Each atom has its own resonant frequency that will be influenced by the magnetic field. The resonating frequency then codes which atoms are present, and in what number, in a molecule. The integration of knowledge from each of the resonant frequencies can lead to an understanding of the molecular structure. Such studies typically use pure molecules in solution, and are important in the field of organic chemistry, but less so for the field of biology.
NMR exploded into biology and medicine when methods were developed that allowed the location of the resonant frequencies to be mapped in space. This method is widely known as magnetic resonance imaging, or MRI. Because the resonating frequency of an atom is dependent in part on the strength of the magnetic field, a variation in field strength over space can be used to influence the resonating frequencies (Schild, 1990). The frequencies can, in turn, be used to decode the position in space from which they arise. By applying magnetic gradients in three different dimensions, it is possible to identify the origin of a signal in three-dimensional space. Suddenly, NMR was no longer limited to the collection of spectral frequencies from homogenous solutions, but instead could be used to collect three-dimensional images of atoms in space. MRI has been widely used to noninvasively image the human body.
MRI works by taking advantage of signals produced by spinning protons (Pykett, 1982). Because protons have an electrical charge that is moving, they produce an electrical current that can be detected. Because electrical currents produce a magnetic field, each proton is equivalent to a small magnetic bar. In MRI, a longitudinal magnetic field is applied. The mobile protons orient the axis of their spin with that of the longitudinal magnetization, like spinning tops aligned by gravity. Rather than spin perfectly parallel to this axis, they wobble around it, much like a wobbling top. Two variables are of interest for MRI: first, the vertical orientation of the tops (which side is up) and their low angle of spin around the axis, and second, whether they wobble together on one side of the axis, or randomly around the axis (Pykett, 1982; Schild, 1990). The protons produce an RF signal that a receiver coil in the MRI can detect. For the psychologist, the three variables used for imaging the human brain include T1, T2 and T2*. To understand what is measured by the MRI, see Figure 16.1. Briefly, the spinning protons wobble randomly around a central axis. There is only a small angle between their orientation and the longitudinal axis. When a brief RF pulse is applied, their angle relative to the axis increases (they tip by 90 or 180 degrees). Furthermore, the RF pulse causes the protons to spin in synchrony from one side of the axis to another (called coherence of precession). Over time, coherence is lost, which is called dephasing. The dephasing reflects inhomogeneities in the magnetic field produced by both internal sources (e.g., tissue) and external sources (e.g., iron in blood). The time it takes to dephase is measured as T2* and T2 The time it takes for the protons to return to their low angle of spin around the longitudinal axis is referred to as T1. T1 is short for lipids (dense in white matter) and long for liquids (e.g., cerebral spinal fluid). Accordingly, the signal mapped across an image of the brain would be light for white matter, dark for gray matter (relatively more water content), and darkest for CSF. MRIs designed to image brain structure are usually weighted to measure either T1 or T2. Liquids have long T2 relaxation times (opposite T1). The T2 weighted image of the brain would have dark white matter with its dense lipids, lighter gray matter with relative less density of lipids and greater water content, and lightest for the cerebral ventricles.
Uses Of Structural MRI
The ability of T1 and T2 weighted images to distinguish between gray and white matter, based on differences in water content, offers the opportunity to study differences in relative brain region size. Size can be studied across individuals, or within individuals over time. The measurement of anatomical differences is of particular interest for understanding disorders such as depression (Videbech & Ravnkilde, 2004; Vythilingam et al., 2004), schizophrenia (Davatzikos et al., 2005; DeLisi, Sakuma, Maurizio, Relja, & Hoff, 2004; Schild, 1990), and PTSD (Pitman et al., 2006). Differences have also been studied, in normal humans with exceptional talents such as musical ability (Gaser & Schlaug, 2003). Because structural MRIs can be taken repeatedly, they are used to study whether experience can lead to changes in brain region size over time. One such study demonstrated that the brain area sensitive to visual motion (areaMT) increased in size following the acquisition of a skill for juggling (Draganski et al., 2004; Draganski et al., 2006), which presumably requires visual as well as motor learning.
Structural MRI is used in almost all fMRI and PET studies. Structural MRIs have a higher spatial resolution than functional images from MRI or PET. Thus, functional maps are overlaid onto the structural MRIs to identify the regional location of the functional activation. Whereas the coregistration is carried out on an individual basis, there is the need to average signals across subjects. Because each brain is different in absolute size and shape, the brain images have to be normalized before averaging. This is carried out by identifying landmarks on each brain, and then stretching and squeezing the images of different regions so the landmarks line up across brains. The human brain atlas that described the procedure most often used for normalization of brain structure is that of Talairach and Tournoux (1988). The simple basis of this method is to first align the brains in the same coordinate system. Once the coordinate system is established from these landmarks, the brain is divided into a proportional grid system by marking extreme points (e.g., the most anterior and posterior points). The compartments are then stretched and squeezed to scale the size to that of the brain in the atlas.
The brain in the Talairach and Tournoux (1988) atlas is, according to the authors, below average in size, which then requires considerable morphological squeezing for some subjects. To avoid this issue, some consortiums and institutes have used Talairach and Tournoux’s grid strategy, but began to scale to a different “brain.” For example, the Montreal Neurological Institutes created an average brain from 152 normal subjects, and the International Consortium for Brain Mapping created an average brain from 452 normal subjects
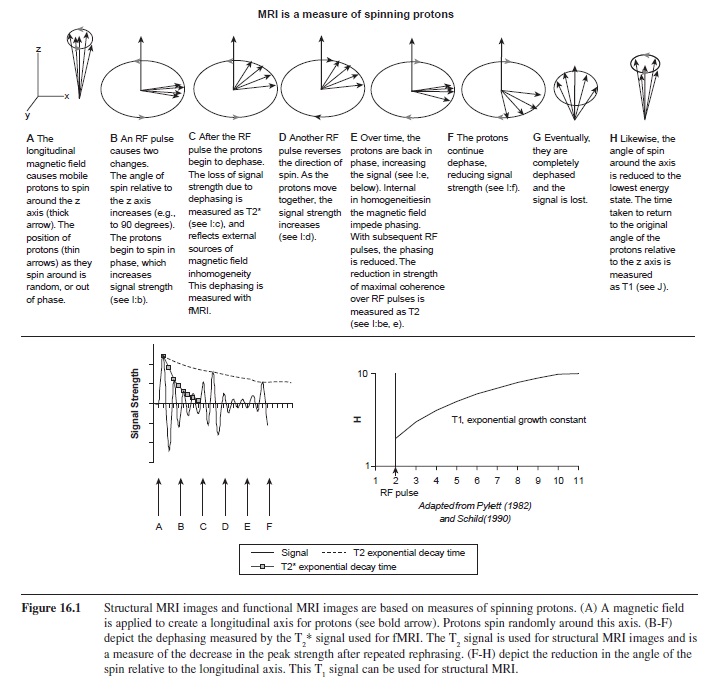 Figure 16.1 Structural MRI images and functional MRI images are based on measures of spinning protons. (A) A magnetic field is applied to create a longitudinal axis for protons (see bold arrow). Protons spin randomly around this axis. (B-F) depict the dephasing measured by the T2* signal used for fMRI. The T2 signal is used for structural MRI images and is a measure of the decrease in the peak strength after repeated rephrasing. (F-H) depict the reduction in the angle of the spin relative to the longitudinal axis. This T1 signal can be used for structural MRI.
Figure 16.1 Structural MRI images and functional MRI images are based on measures of spinning protons. (A) A magnetic field is applied to create a longitudinal axis for protons (see bold arrow). Protons spin randomly around this axis. (B-F) depict the dephasing measured by the T2* signal used for fMRI. The T2 signal is used for structural MRI images and is a measure of the decrease in the peak strength after repeated rephrasing. (F-H) depict the reduction in the angle of the spin relative to the longitudinal axis. This T1 signal can be used for structural MRI.
.The idea of averaging a brain is somewhat suspect when we intuitively understand that there will be considerable variation in the sulcal and gyral boundaries across subjects, and that we are interested in localizing function to these structures. To compensate for this problem, brains can be “smoothed” before averaging. Thus, Talairach coordinates will not directly specify locations in sulci. Likewise, human brain areas are often referred to as “Brodmann’s areas.” But Brodmann defined his areas based on cytoarchitectonics, which do not necessarily correspond to sulcal and gyral boundaries (Schleicher, Amunts, Geyer, Morosan, & Zilles, 1999). With the highest resolution MRI methods, it may be possible to use layer size differences to delineate cortical areas (Walters et al., 2007); however, full cytoarchetectonic analysis requires analysis of layer size, cell size, and cell density. With these issues in mind, caution is required with regard to references to Brodmann’s areas in human imaging studies.
Functional MRI
The development of more rapid imaging sequences for MRI was required before it was recognized that changes in blood flow and volume could be detected, and used to infer changes in neural activity. Because these more rapid imaging sequences provide a window into brain function, the general method is referred to as fMRI. There are several different MR pulse sequences that can be used to measure function. Blood oxygen level dependent (BOLD) imaging is by far the most commonly used, and will be discussed further. Briefly, the other methods, called perfusion-imaging techniques, are advantageous because they can quantify cerebral blood flow rather than reflect only relative changes in flow and volume. In these methods, an RF pulse is applied to one slice, and signal is measured from the image slice of interest above the slice receiving the pulse. This takes advantage of the fact that blood in arteries moves upward. The methods have the advantage of providing quantitative measures of cerebral blood flow (CBF) in absolute units, and a signal origin closer to the activated neurons than the BOLD signal. Because the image slice of interest is perfused with this endogenous tracer, these techniques are referred to as “perfusion imaging methods.” They include the following variations: EPI STAR, FLAIR, FLOW, FAIR, and ASL.
The most commonly used functional imaging technique is BOLD. Iron in blood hemoglobin can be a source of magnetic susceptibility, and can cause a shortening of the T2* relaxation time (Tracey, 1998). Oxygenated hemoglobin (oxy-Hb) has a small magnetic susceptibility effect, but deoxygenated hemoglobin (deoxy-Hb) has a relatively large magnetic susceptibility effect. Thus, deoxy-Hb will cause faster dephasing and a shorter T2* relaxation time. Conversely, little deoxy-Hb (proportionally more oxy-Hb) will be related to slower dephasing and longer T2* relaxation time, or for our purposes, a greater signal intensity.
At any one time, at a baseline level of neural activity, the blood will have more oxy-Hb and less deoxy-Hb in arteries than in capillary beds, where oxygen is extracted, and in veins, which collect blood from capillaries. Having said that, the veins will have relatively shorter T2* relaxation times (i.e., faster dephasing) because of the magnetic susceptibility caused by the relatively high concentrations of deoxy-Hb (Tracey, 1998). But baseline neural activity and the differences in signals between arteries and veins are of little interest to psychologists.
Psychologists are interested in what happens when neural activity increases in relation to behavior or sensory input. As discussed before, increasing neural activity increases oxygen consumption, which, in turn, causes greater oxygen extraction. With greater extraction the proportion of paramagnetic deoxy-Hb with its high magnetic susceptibility is expected to increase in capillaries and veins. However, as noted earlier, that neural activity increases blood flow from arteries, and this would lead to an increase in availability of oxy-Hb. Taking no chances, this increase in blood flow overcompensates for the oxygen extraction, which in turn leads to the counterintuitive effect of the proportion of oxy-HbO2 increasing in capillaries and veins when neural activity increases (see Figure 16.2). Conversely, the proportion of deoxy-Hb decreases, causing less magnetic susceptibility and slower dephasing. Altogether, this leads to a longer T2* relaxation time, or more simply a higher signal intensity. In summary, when neuronal activity increases in response to behavioral tasks, the relative decrease in deoxy-Hb has less influence on dephasing, and signal strength increases.
The vasculature can be compartmentalized into those vessels that carry blood into the brain (arteries), those that transport oxygen, glucose, and other chemicals into the brain tissue (capillaries), and those that passively carry blood out of tissue (veins). Arteries are capable of constricting or dilating, which influences the volume of blood in all three compartments. Capillaries are substantially smaller in diameter but higher in density than arteries, arterioles, venules, and veins. Deoxy-HB is expected to be present in capillaries and veins, rather than arteries (see Figure 16.2). At the magnetic field strengths, and with the pulse sequences typically employed for fMRI studies, the BOLD signal reflects primarily venous blood oxygenation, flow, and volume. Because veins are not immediately adjacent to the active neuronal population, the spatial resolution of the BOLD signal is limited by the distance between the site of neural activation and the veins carrying blood from that site. Thus, even when MRI methods have higher spatial resolution, that resolution is limited by the biological location of the signal being detected. The weight of signals from capillaries can be increased with high magnetic field strength and special pulse sequences (Goense & Logothetis, 2006). Nevertheless, these widely used methods have a spatial resolution that is sufficient to identify changes within regions and brain nuclei, and these are not trivial. For example, the visual representations in discrete areas of the retina can be mapped to specific locations within the primary visual cortex (DeYoe et al., 1996).
Relation Between The Bold Signal And Synaptic Potentials
When interpreting the BOLD signal it is important to understand what aspect of neural activity is represented. Because the BOLD signal reflects changes in oxygenation, blood flow, and volume, it is expected, based on the previous discussion of physiology, to reflect synaptic signals. Researchers have confirmed this predication by the simultaneous collection of electrical recordings from synaptic field potentials, action potentials, and the BOLD signal in monkeys. Single recordings can measure multiple action potentials (multiple unit recordings) and synaptic potentials (local field potentials) simultaneously. Because action potentials are short in duration, they are signals with a high-frequency waveform. In contrast, synaptic potentials last longer and therefore produce lower-frequency waveforms. By filtering either high- or low-frequency signals each separate source can be compared to the BOLD signal. From simultaneous recordings, Logothetis and Wandell (2004) have shown that the synaptic potentials correlate better with the duration of the BOLD signal than with the duration of the action potentials. The action potential duration was brief and associated only with the onset of the stimulus, whether the duration was short or long, whereas the synaptic potentials were sustained and varied in duration, like both the stimulus and the BOLD signal.
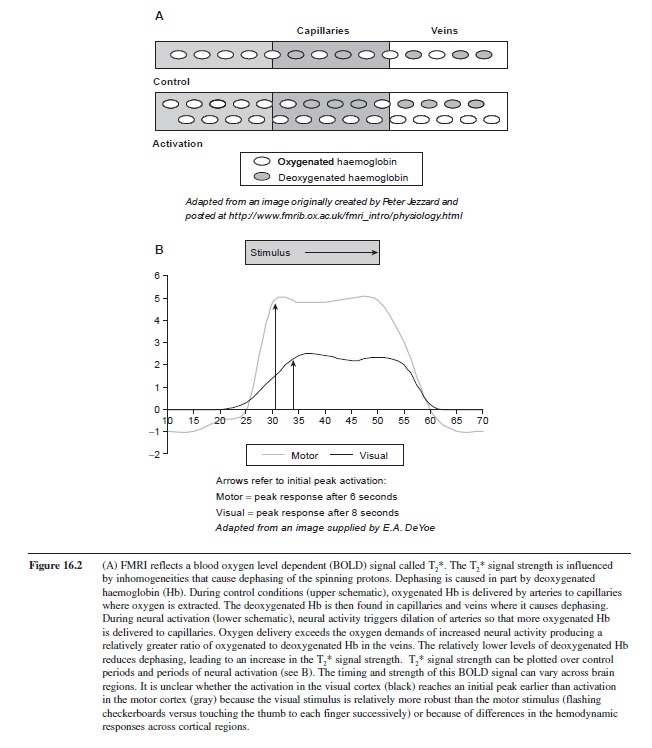 Figure 16.2 (A) FMRI reflects a blood oxygen level dependent (BOLD) signal called T2*. The T2* signal strength is influenced by inhomogeneities that cause dephasing of the spinning protons. Dephasing is caused in part by deoxygenated haemoglobin (Hb). During control conditions (upper schematic), oxygenated Hb is delivered by arteries to capillaries where oxygen is extracted. The deoxygenated Hb is then found in capillaries and veins where it causes dephasing. During neural activation (lower schematic), neural activity triggers dilation of arteries so that more oxygenated Hb is delivered to capillaries. Oxygen delivery exceeds the oxygen demands of increased neural activity producing a relatively greater ratio of oxygenated to deoxygenated Hb in the veins. The relatively lower levels of deoxygenated Hb reduces dephasing, leading to an increase in the T2* signal strength. T2* signal strength can be plotted over control periods and periods of neural activation (see B). The timing and strength of this BOLD signal can vary across brain regions. It is unclear whether the activation in the visual cortex (black) reaches an initial peak earlier than activation in the motor cortex (gray) because the visual stimulus is relatively more robust than the motor stimulus (flashing checkerboards versus touching the thumb to each finger successively) or because of differences in the hemodynamic responses across cortical regions.
Figure 16.2 (A) FMRI reflects a blood oxygen level dependent (BOLD) signal called T2*. The T2* signal strength is influenced by inhomogeneities that cause dephasing of the spinning protons. Dephasing is caused in part by deoxygenated haemoglobin (Hb). During control conditions (upper schematic), oxygenated Hb is delivered by arteries to capillaries where oxygen is extracted. The deoxygenated Hb is then found in capillaries and veins where it causes dephasing. During neural activation (lower schematic), neural activity triggers dilation of arteries so that more oxygenated Hb is delivered to capillaries. Oxygen delivery exceeds the oxygen demands of increased neural activity producing a relatively greater ratio of oxygenated to deoxygenated Hb in the veins. The relatively lower levels of deoxygenated Hb reduces dephasing, leading to an increase in the T2* signal strength. T2* signal strength can be plotted over control periods and periods of neural activation (see B). The timing and strength of this BOLD signal can vary across brain regions. It is unclear whether the activation in the visual cortex (black) reaches an initial peak earlier than activation in the motor cortex (gray) because the visual stimulus is relatively more robust than the motor stimulus (flashing checkerboards versus touching the thumb to each finger successively) or because of differences in the hemodynamic responses across cortical regions.
The relation between the BOLD signal and synaptic signals is important to keep in mind as we compare studies using electrophysiological recordings and studies using fMRI. Invasive electrophysiological recordings often reflect single or multiple units, which reflect action potentials. From that traditional perspective of measuring neuronal activity, the BOLD signal may be a better reflection of action potentials from structures projecting into the structure imaged with fMRI. For example, the first CNS structure that receives visual information is the lateral geniculate nucleus of the thalamus (LGN), which in turn projects to the primary visual cortex. Action potentials in the LGN would produce synaptic potentials in the primary visual cortex that in turn drive the BOLD signal there. Thus, action potential recordings from the LGN rather than the primary visual cortex would be expected to correspond more closely to the BOLD signal. Unlike invasive recording methods, scalp electrode recordings reflect potentials from dendritic fields. Thus, EEG and fMRI data are compatible because both predominantly reflect postsynaptic potentials.
Originally, BOLD imaging was considered to detect only increases in signal, whereas studies using PET imaging reported decreases in glucose and oxygen uptake in regions. Eventually, negative signals were also observed with BOLD imaging. Simultaneous fMRI and electrical recordings suggest that decreases in the BOLD signal reflect a decrease in both local field potentials and multiple unit activity (Shmuel, Augath, Oeltermann, & Logothetis, 2006). However, the negative BOLD signal was 7 mm away from the stimulated positive BOLD signal, suggesting that the negative signal was not selectively coupled to the increase in neural activity.
Timing Of The Bold Signal
After increases in neural activity, the BOLD signal can take as long as 6 to 8 seconds to reach is maximal change (DeYoe, personal communication, see Figure 16.2). To take advantage of the maximal signal change, many researchers use a block design. In this design subjects perform a task repeatedly over a period of 20 to 30 seconds, and then have an equal length of time over which they perform a control task, or no task at all. Knowing the timing of signal change for brain areas, it is possible for the experimenter to design the expected signal change that should occur in regions active during task performance. This expected signal is then correlated with the observed brain signal measured with fMRI. The smallest unit from which a signal arises is called a “voxel,” which is the three-dimensional version of the more familiar word “pixel.” The expected signal can be correlated with the signal from every voxel measured, which can be a large number when the whole brain is imaged. With such a large number of voxels, and therefore statistical comparisons, substantial statistical correction for the number of comparisons must be carried out. Alternatively, some investigators apply a mask, which excludes voxels from regions that are not of interest or expected to be active during the task. This reduces the number of comparisons, or family wise error, so that lower correlation values, or t-scores, are necessary to reach significance.
The block design in fMRI was a natural transition from previous functional PET studies, which required long task performance intervals. The block design has obvious drawbacks, and did not allow for localization in tasks used for event-related potentials studies. Over time, the potential for event-related fMRI was recognized. It was already known that cerebral blood flow increased within two seconds of activation of sensory cortex (Dalkara, Irikura, Huang, Panahian, & Moskowitz, 1995; Woolsey et al., 1996), and the BOLD signal is capable of detecting this increase (Blamire et al., 1992). Eventually, detectable signal change was demonstrated after a stimulus lasting only 34 msec (Rosen, Buckner, & Dale, 1998). By averaging signals across trials, as carried out in event-related potential studies, the cancellation of baseline activity increased the detectability of the event-related BOLD signal change (Rosen et al., 1998).
Signals elicited by sensory stimulation or motor output have a higher signal-to-noise ratio than signals related to cognitive processing. However, event-related fMRI has more potential for the study of cognitive processing than sensory input or motor output. The possibility of using event-related fMRI for cognitive tasks was realized once it was demonstrated that a word generation task could evoke a fMRI signal change in both sensory and higher-order processing areas (Buckner et al., 1996). At high magnetic field strengths, averaging is no longer necessary; signal change related to a single trial can be detected.
Diffusion Tensor Imaging
In the human brain, white matter makes up nearly 50 percent of the volume in comparison to only 14 percent of volume in rodents (Goldberg & Ransom, 2003). It is now recognized that damage in white matter substantially contributes to dysfunction following stroke (Goldberg & Ransom, 2003). White matter in humans plays a key role in transmitting information across cortical areas as well as connecting cortical and subcortical areas. Accordingly, there is substantial interest in the use of MRI to study white matter and regional connectivity.
The direction orientation of axonal fiber tracts can be measured with diffusion tensor imaging. All MRI pulse sequences are sensitive to the spin of mobile protons, which are primarily protons in water. The movement of water in the brain is restricted by barriers such as membranes, organelles, proteins, cytoskeleton, and so on. Within cell bodies or dendritic fields, these barriers will have a relatively random orientation (perhaps with the exception of the cerebellar cortex). Within fiber tracts, however, there is a limited direction to the movement of water because axon diameters are relatively small and the parallel membranes form barriers to movement. Diffusion tensor imaging exploits this directional limitation for movement to identify the direction of axonal tracts. By applying gradient pulses in only one axis, the signal can detect diffusion relative to that axis. By applying diffusion gradients in at least six directions, it is possible to calculate the direction of diffusion for each voxel. (For an example of a DTI map, see http://www.dtiatlas.org/.) The reader should note that colors in DTI maps correspond to orientation of the fiber tracts.
Clinically, this method is very useful for detecting ischemia and disease-related changes in white matter (e.g., in MS or epilepsy). The method has also been applied to psychiatric populations. For example, using DTI, it was reported that schizophrenics had a shorter path length for the anterior limb of the internal capsule connecting to pre-frontal cortex (Buchsbaum et al., 2006). This connectivity difference could help to explain the proposed disruption of the frontal-striatal-thalamic circuit. Although the most rigorous application of DTI involves efforts to map fiber tracts, which are particularly useful in combination with fMRI, it is important to remember that there is already a plethora of data on anatomical connectivity collected over the last century by neuroanatomists. The extent to which the relatively crude method of DTI adds to this knowledge remains to be seen. DTI is susceptible to a number of artifacts, leaving histological analysis the superior method (Miller, 2005). Histological analysis, however, is restricted to a single time point. DTI has the ability to measure path length in vivo, and therefore over time. Thus, anatomical changes in clinical populations could potentially be correlated with behavioral symptoms to aid in our further understanding disease processes and behavioral symptoms.
Artifacts In fMRI
It is beyond the scope of the present chapter to cover and explain all possible artifacts that can occur in fMRI studies. The user, properly trained, can prevent artifacts arising from improperly chosen fields of view, and can avoid regions of interest susceptible to chemical shifts that arise from differences in adjacent tissue. Likewise, artifacts arising from the movement of body fluids through the tissue can be compensated for faster image acquisition, and subjects can be trained not to move in the scanner. Other artifacts can be avoided only by frequent quality checks to detect circuit failure, failure in the shielding, and errors in the magnetic gradients used to localize the source of the signal (Hornak, 1996). Whereas service contracts provide repair for such problems, they are not yet ideal in practice because fMRI is not widely used for clinical purposes. Many psychologists have found that the success of their imaging research depends on the support of an MR physicist.
Pet Imaging
Before the advent of PET and fMRI, metabolic mapping studies were carried out by injecting glucose labeled with 14C (2-deoxyglucose, 2-DG; Kadekaro et al., 1985). This form of glucose was not metabolized and therefore 45 minutes, active neurons accumulated more 2-DG than inactive neurons. Localization of the radioactive label by film exposed to histological sections provides the ability to identify the active sites. As PET instrumentation was being developed, it became clear that imaging in humans was possible, but would require a safer tracer. The substitute was F labeled glucose (2-fluoro-2-deoxy-D-glucose ( F), FDG), developed at Brookhaven National Labs. Eventually, other safe tracers were developed. PET studies utilize radiolabeled FDG and radiolabeled oxygen, to provide a quantitative measure of cerebral metabolic rate of glucose (rCMRgl), regional cerebral metabolic rate of oxygen (rCMR02), regional cerebral blood flow (fCBF), and regional cerebral blood volume (rCBV; Roland, 1993). All of the tracer detection methods measure the isotope concentration in the brain in nCicc (nanoCuries per cc) as a function of time. These absolute measures are superior to the relative measures provided by BOLD fMRI. PET is not restricted to metabolic mapping. It is also possible to label transmitter analogues for the purpose of studying receptor and transporter density.
Despite its greater versatility, PET imaging is not as widely available as MRI. The requirement of injection or inhalation of radioactive tracers is a disadvantage, but the tracers have a relatively short half-life and are relatively safe. More restrictive is the need to synthesize tracers and use them quickly because of the short half-lives (2 to 120 minutes; Roland, 1993). Because of the short half-lives of the tracers, many facilities have their own cyclotron for local production of tracers. Some standard tracers can be shipped to a PET facility without a cyclotron.
PET is based on the emission of a positron from the tracer. The positron travels a short distance (0.2-7mm) and stops when it meets a negatively charged electron (Roland, 1993). The collision of the positron and electron causes them to “annihilate” and be converted to high-energy photons called gamma rays. The pair of gamma rays move in opposite directions, and hit sensors surrounding the head. The scanning device itself is like a scintillation counter divided into parts. When the gamma rays hit the scintillation material, they emit light, which is detected by a photomultiplier. By analyzing the coincident detection of gamma rays, it is possible to calculate the spatial location of the tracer within the brain, and therefore the tracer’s location.
Tracer kinetic models describe the behavior of the tracer in different tissue compartments, such as the vasculature, extracellular space, cytosol, or subcellular space, or functional compartments such as precursor pools or metabolites.
Limits Of fMRI For The Study Of Brain Function
Functional and structural imaging methods have opened windows into the localization of function in the human brain. The ability to correlate persistent hiccups to a small lesion in the medulla in a single patient with multiple sclerosis (Chang, W. H. Chen, Liu, Shih, & S. S. Chen, 1993) provides an example of the power of structural MRI for studying localization of function. To confirm brain activation during task performance through fMRI or PET provides the opportunity to search for convergence of data from studies of dysfunction and activation.
Whereas fMRI can localize active brain regions, it is limited in answering how information is processed in these areas. fMRI represents summed activity in dendritic fields. But the summed activity may not reflect more specifically how information is processed. For example, the contrast response function of the BOLD fMRI signal in the primary visual cortex approximates the perceptual contrast response function (unpublished data). But it seems unlikely that the elemental sensory features that contribute to the perceptual contrast response function would be recombined at this relatively early level visual processing. Data from electrophysiological recordings within the primary visual cortex suggest that within single cells the action potential frequency does not match the perceptual contrast response function. Instead, cells vary in their range of sensitivity. Some cells vary action potential frequency considerably over the limited range of 3 to 10 percent contrast, but not above or below. Others vary action potential frequency over the range of 10 to 30 percent contrast. Therefore, contrast in this early processing area is reflected by multiple channels (Albrecht & Hamilton, 1982). Albrecht and Hamilton proposed that the sum of activity over those multiple channels leads to the perceptual contrast response function, and the data from fMRI, which are essentially the summed input, support that hypothesis. The processing of contrast within single channels may be important for explaining why sensitivity to changes within a narrow range of contrast can be altered over time. Together this example reminds us that human imaging methods provide a summed response, and that there is a plethora of data collected in animals that can provide answers to the question of how information is processed. In summary, human functional imaging best answers questions about where active cells are located, but it is more limited in answering questions about how cells process information.
References:
- Albrecht, D. G., & Hamilton, D. B. (1982). Striate cortex of monkey and cat: contrast response function. Journal of Neurophysiology, 48(1), 217-237.
- Blamire, A. M., Ogawa, S., Ugurbil, K., Rothman, D., McCarthy, G., Ellermann, J. M., et al. (1992). Dynamic mapping of the human visual cortex by high-speed magnetic resonance imaging. Proceeding of the National Academy of Science, 89(22), 11069-11073.
- Borowsky, I. W., & Collins, R. C. (1989). Metabolic anatomy of brain: a comparison of regional capillary density, glucose metabolism, and enzyme activities. Journal of Comparative Neurology, 288(3), 401-413.
- Buchsbaum, M. S., Schoenknecht, P., Torosjan, Y., Newmark, R., Chu, K. W., Mitelman, S., et al. (2006). Diffusion tensor imaging of frontal lobe white matter tracts in schizophrenia. Annals of General Psychiatry, 5, 19.
- Buckner, R. L., Bandettini, P. A., O’Craven, K. M., Savoy, R. L., Petersen, S. E., Raichle, M. E., et al. (1996). Detection of cortical activation during averaged single trials of a cognitive task using functional magnetic resonance imaging. Proceeding of the National Academy of Science, 93(25), 14878-14883.
- Chang, Y. Y., Chen, W. H., Liu, J. S., Shih, P. Y., & Chen, S. S. (1993). Intractable hiccup caused by medulla oblongata lesions. Journal of the Formosan Medical Association, 92(10), 926-928.
- Clarke, D. D., & Sokoloff, L. (1999). Circulation and energy metabolism of the brain. In G. J. Siegel, B. W. Agranoff, R. W. Albers, S. K. Fisher, & M. D. Uhler (Eds.), Basic Neurochemistry: Molecular, Cellular and Medical Aspects (6th ed.). Philadelphia: Lippincott-Raven.
- Dalkara, T., Irikura, K., Huang, Z., Panahian, N., & Moskowitz, M. A. (1995). Cerebrovascular responses under controlled and monitored physiological conditions in the anesthetized mouse. Journal of Cerebral Blood Flow and Metabolism, 15(4), 631-638.
- Davatzikos, C., Shen, D., Gur, R. C., Wu, X., Liu, D., Fan, Y., et al. (2005). Whole-brain morphometric study of schizophrenia revealing a spatially complex set of focal abnormalities. Archive of General Psychology, 62(11), 1218-1227.
- DeLisi, L. E., Sakuma, M., Maurizio, A. M., Relja, M., & Hoff, A. L. (2004). Cerebral ventricular change over the first 10 years after the onset of schizophrenia. Psychiatry Research, 136(1), 57-70.
- DeYoe, E. A., Carman, G. J., Bandettini, P., Glickman, S., Wieser, J., Cox, R., et al. (1996). Mapping striate and extrastriate visual areas in human cerebral cortex. Proceeding of the National Academy of Science, 93(6), 2382-2386.
- Draganski, B., Gaser, C., Busch, V., Schuierer, G., Bogdahn, U., & May, A. (2004). Neuroplasticity: changes in grey matter induced by training. Nature, 427(6972), 311-312.
- Draganski, B., Gaser, C., Kempermann, G., Kuhn, H. G., Winkler, J., Buchel, C., et al. (2006). Temporal and spatial dynamics of brain structure changes during extensive learning. Journal of Neuroscience, 26(23), 6314-6317.
- Dunning, H. S., & Wolff, H. G. (1937). The relative vascularity of various parts of the central and peripheral nervous system of the cat and its relation to function. Journal of Comparative Neurology, 67, 433-450.
- Erecinska, M., & Wilson, D. F. (1982). Regulation of cellular energy metabolism. Journal of Membrane Biology, 76(1), 1-14.
- Gaser, C., & Schlaug, G. (2003). Gray matter differences between musicians and nonmusicians. Annals of New York Academy of Science, 999, 514-517.
- Goense, J. B., & Logothetis, N. K. (2006). Laminar specificity in monkey V1 using high-resolution SE-fMRI. Magnetic Resonance, 24(4), 381-392.
- Goldberg, M. P., & Ransom, B. R. (2003). New light on white matter. Stroke, 34(2), 330-332.
- Hand, P. J., Greenberg, J. H., Sylvestro, A., Weller, L., & Reivich, M. (1979). A normal and developmentally local glucose utilization with natural stimulation of a single receptor organ. Acta Neurologica Scandinavica Supplementum, 72, 60, 46-47.
- Hill, A. V., & Howarth, J. V. (1958). The initial heat production of stimulated nerve. Proceedings of the Royal Society of London, Series B, Biological Sciences, 149(935), 167-175.
- Hornak, J. P. (1996, 2004). The basics of MRI. Retrieved December 15, 2006, from http://www.cis.rit.edu/htbooks/ mri/index.html
- Huettel, S. A., Song, A. W., & McCarthy, G. (2004). Functional magnetic resonance imaging. Sunderland, MA: Sinauer Press.
- Juliano, S. L., Hand, P. J., & Whitsel, B. L. (1981). Patterns of increased metabolic activity in somatosensory cortex of monkeys Macaca fascicularis, subjected to controlled cutaneous stimulation: a 2-deoxyglucose study. Journal of Neurophysiology, 46(6), 1260-1284.
- Kadekaro, M., Crane, A. M., & Sokoloff, L. (1985). Differential effects of electrical stimulation of sciatic nerve on metabolic activity in spinal cord and dorsal root ganglion in the rat. Proceeding of the National Academy of Science, 82(17), 6010-6013.
- Kandel, E. R., Schwartz, J. H., & Jessell, T. M. (2000). Principles of neural science (4th ed.). New York: McGraw-Hill.
- Logothetis, N. K., & Wandell, B. A. (2004). Interpreting the BOLD signal. Annual Review of Physiology, 66, 735-769.
- Metea, M. R., & Newman, E. A. (2006). Glial cells dilate and constrict blood vessels: a mechanism of neurovascular coupling. Journal of Neuroscience, 26(11), 2862-2870.
- Miller, N. R. (2005). Diffusion tensor imaging of the visual sensory pathway: are we there yet? American Journal of Ophthalmology, 140(5), 896-897.
- Pitman, R. K., Gilbertson, M. W., Gurvits, T. V., May, F. S., Lasko, N. B., Metzger, L. J., et al. (2006). Clarifying the origin of biological abnormalities in PTSD through the study of identical twins discordant for combat exposure. Annals of New York Academy of Science, 1071, 242-254.
- Pykett, I. L. (1982). NMR imaging in medicine. Scientific American, 246(5), 78-88.
- Roland, P. E. (1993). Brain activation. New York: Wiley-Liss.
- Rosen, B. R., Buckner, R. L., & Dale, A. M. (1998). Event-related functional MRI: past, present, and future. Proceedings of the National Academy of Science, 95(3), 773-780.
- Roy, C. S., & Sherrington, C. S. (1890). On the regulation of the blood-supply of the brain. Journal of Physiology, 11(1-2), 85-158.
- Schild, H. H. (1990). MRI made easy…(well almost). Berlin: Schering.
- Schleicher, A., Amunts, K., Geyer, S., Morosan, P., & Zilles, K. (1999). Observer-independent method for microstructural parcellation of cerebral cortex: A quantitative approach to cytoarchitectonics. Neuroimage, 9(1), 165-177.
- Schultz, D. (1981). A history of modern psychology (3rd ed.). New York: Academic Press. Shmuel, A., Augath, M., Oeltermann, A., & Logothetis, N. K. (2006). Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area V1. Nature Neuroscience, 9(4), 569-577.
- Sokoloff, L., Reivich, M., Kennedy, C., DesRosiers, M. H., Patlak, C. S., Pettigrew, K. S., et al. (1977). [14C] deoxyglucose method for the measurement of local cerebral glucose utilization: Theory, procedure, and normal values in the conscious and anesthetized albino rat. Journal of Neurochemistry, 28, 897-916.
- Talairach, J., & Tournoux, P. (1988). Co-planar stereotaxic atlas of the human brain. New York: Thieme Medical Publishers.
- Tracey, I. (1998). Brief introduction to FMRI—Physiology. Retrieved from http://www.fmrib.ox.ac.uk/fmri_intro/ physiology.html
- Videbech, P., & Ravnkilde, B. (2004). Hippocampal volume and depression: a meta-analysis of MRI studies. American Journal of Psychiatry, 161(11), 1957-1966.
- Vythilingam, M., Vermetten, E., Anderson, G. M., Luckenbaugh, D., Anderson, E. R., Snow, J., et al. (2004). Hippocampal volume, memory, and cortisol status in major depressive disorder: effects of treatment. Biological Psychiatry, 56(2), 101-112.
- Walters, N. B., Eickhoff, S. B., Schleicher, A., Zilles, K., Amunts, K., Egan, G. F., et al. (2007). Observer-independent analysis of high-resolution MR images of the human cerebral cortex: In vivo delineation of cortical areas. Human Brain Mapping, 28(1), 1-8.
- Wong-Riley, M. T. (1989). Cytochrome oxidase: an endogenous metabolic marker for neuronal activity. Trends in Neuroscience, 12(3), 94-101.
- Woolsey, T. A., Rovainen, C. M., Cox, S. B., Henegar, M. H., Liang, G. E., Liu, D., et al. (1996). Neuronal units linked to microvascular modules in cerebral cortex: response elements for imaging the brain. Cerebral Cortex, 6(5), 647-660.
- Zheng, D., LaMantia, A. S., & Purves, D. (1991). Specialized vascularization of the primate visual cortex. Journal of Neuroscience, 11(8), 2622-2629.
See also:
Free research papers are not written to satisfy your specific instructions. You can use our professional writing services to order a custom research paper on any topic and get your high quality paper at affordable price.





