This sample Neurotransmission Research Paper is published for educational and informational purposes only. If you need help writing your assignment, please use our research paper writing service and buy a paper on any topic at affordable price. Also check our tips on how to write a research paper, see the lists of psychology research paper topics, and browse research paper examples.
Neurotransmission involves the sharing of information within the nervous system from one neuron, or nerve cell, to another membrane (i.e., another neuron, muscle, or gland). This process entails a passage of electrical and chemical information from one neuron to many other neurons or organs. In order to explain the process of neurotransmission, we must first understand the key components of the neuron, the characteristics of the neuronal membrane, the process of stimulating the cell, the action potential, and neurotransmitter release.
Parts Of A Neuron
The human body contains approximately 100 billion neurons with cell bodies ranging in size from 6-8 pm to 80 pm in diameter (Kandel, 1991). Researchers have divided neurons into three main categories based on function: sensory neurons, motor neurons, and interneurons. Sensory neurons, or afferents, transmit sensory information from the sense organs (i.e., eyes, skin, etc.) to the central nervous system (CNS). Contrastingly, motor neurons, or efferents, send information away from the CNS to the muscles and glands of the body. Interneurons are tiny cells that are found only in the CNS. They only communicate information within their cell clusters (Katz, 1966).
Although there are many types of nerve cells, there are basic components of a neuron that differentiate it from other types of cells. A typical neuron has three main parts: the receiving, the transmitting, and the communicating components (see Figure 14.1). The receiving part of the cell consists of the cell body (soma) and dendrites. Typical to most animal cells, the cell body houses the structures needed for the maintenance of the cell such as the nucleus, the mitochondria, and the ribosomes. The nucleus is considered the “brain” of the cell and contains all of the genetic material (i.e., DNA and chromosomes) for cell maintenance. Metabolic activities for the cell occur in the mitochondria, the “powerhouse” of the cell. Mitochondria produce adenosine triphosphate (ATP), the fuel source for cellular activities (Thomas, 1989). Ribosomes are small structures that translate genetic information into proteins that are used both inside and outside of the cell.
Dendrites, also a receiving component of the cell, are branch-like structures that protrude from the cell body. Smaller appendages that form on the dendrites are called dendritic spines. Dendritic spines are instrumental in the communication between neurons by receiving many input signals from other cells. In fact, primates housed in enriched environments have an increased number of dendritic spines compared to those who were not housed in enriched environments (Kozorovitskiy et al., 2005). The dendrites and cell body of only one neuron can receive information from over 50,000 axon terminals from other cells.
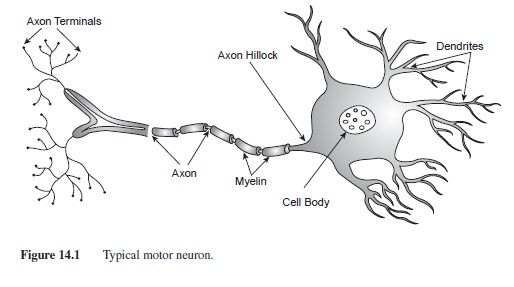 Figure 14.1 Typical motor neuron.
Figure 14.1 Typical motor neuron.
The axon is a tubular structure ranging from .2 to 20 pm diameter and up to 1 m in length (Kandel, 1991); it is the transmitting part of the cell. Axons exit the cell body at the point called the axon hillock (Figure 14.1). In many neurons the axon is insulated by segments of protein and fat called myelin. Myelin is formed by Schwann cells in the peripheral nervous system (PNS) and oligodendrocytes in the CNS. Although the function of the myelin is the same in both nervous systems, increasing the speed of the signal down the axon, the manner in which Schwann cells and oligodendrocytes form myelin is very different. In the PNS, each Schwann cell wraps around the axon to form one segment of myelin. In the CNS, oligodendrocytes develop arms that wrap around nearby axons, forming myelin segments. Each oligodendrocyte can form several myelin segments on axons of many different cells. Oligodendrocytes also provide structure and support for neurons in the CNS. Each segment of myelin is separated by tiny gaps in the insulation called Nodes of Ranvier. These breaks in insulation are very important for the propagation of the action potential down the axon (Katz, 1966; see section on action potential for additional details).
The communicating part of the neuron consists of the axon terminals. As the axon reaches the end of the neuron, it branches into smaller, thin appendages with protuberances at each end (axon terminals or synaptic buttons). Within the synaptic buttons are small spherical structures called synaptic vesicles that house the neurotransmitters until they are released (see Figure 14.1).
Types Of Neurons
Three main types of neurons can be classified by structure: monopolar neurons, bipolar neurons, and multipolar neurons. Monopolar neurons, also called unipolar neurons, have only one process that exits the cell body. These cells are most often found in the spinal cord and PNS. Bipolar neurons have one dendrite and one axon exiting the cell body and are primarily found in the PNS. The majority of neurons in the central and peripheral nervous systems are multipolar; they have an axon and many dendrites. The Purkinje cell of the cerebellum and the pyramidal cells, named for their triangular shape and found in several layers of the cerebrum, are just two examples of multipolar neurons (Figure 14.2).
Characteristics of Neuronal Membranes
Most of what we know about the characteristics of neuronal membranes, also called plasma membranes, has come from the experiments using the squid giant axon (Hodgkin & Katz, 1949). The large nerve of the squid (Loligo pealli) has an axonal-like structure that spans the animal’s length (F. O. Schmitt & O. H. Schmitt, 1940). This nerve serves as the squid’s CNS and has a similar membrane structure to those found in human cells. Although still very small, .5 pm in diameter, the axon is large enough to measure ionic changes across the membrane.
The plasma membrane is composed of phosphoglyceride molecules, each with a phosphoric acid head and glyceride tails made of fatty acids and hydrocarbons (Thompson, 1993). The lipid segments of the membrane are hydrophobic (repel water) whereas the phosphates are hydrophilic (seek water). These opposing characteristics create a two-layered phospholipid bilayer where the lipid molecules are attracted to each other, shielding it from the fluid inside and outside of the cell.
The phospholipid bilayer is approximately 6-8 nm thick (Kandel, 1991) and is selectively permeable, letting some particles flow freely through the membrane while blocking others from passing (Bean, Shepard, & Chan, 1968). Some particles need specialized protein channels to travel through the membrane (this will be discussed later).
The Resting Membrane Potential
There are many features of the plasma membrane and surrounding fluids, both inside (intracellular) and outside (extracellular) of the cell, that contribute to the resting membrane potential (Hodgkin & Huxley, 1945, 1952). A potential is calculated by measuring the difference in charge between two points. For a neuron, this resting potential is defined by the difference in electrical current between the charge inside the cell and the charge outside of the cell. At rest, a neuron’s potential is approximately -70 millivolts (mV) in that the inside of the cell is more negative than the outside of the cell. This potential is created by the distribution of ions (charged particles) across the membrane. Ions can be either positively charged (anions) or negatively charged (cations). The ions that create the resting membrane potential are sodium (Na+), potassium (K+), chloride (Cl-), and large organic anions (A-). There are much higher concentrations of Na+ and Cl- outside of the cell compared to the inside of the cell. Contrastingly, the inside of the cell has a higher concentration of K+ and large negative organic anions (A-) that cannot cross the membrane (see Table 14.1; adapted from Koester, 1991). These concentrations are maintained by characteristics of the membrane and forces that act on the ion distribution.
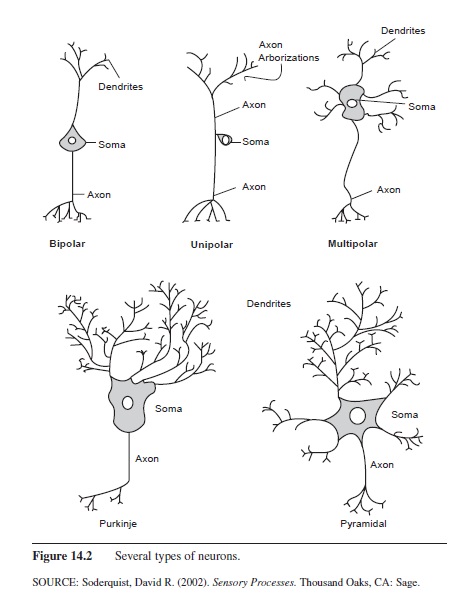 Figure 14.2 Several types of neurons.
Figure 14.2 Several types of neurons.
Forces Involved in the Resting Membrane Potential
Three forces involved in the maintenance of the resting potential are electrostatic pressure, diffusion, and the sodium potassium pump. Electrostatic pressure and diffusion are passive forces, requiring no energy from the cell. Electrostatic pressure involves the idea that particles of opposite charges are attracted to each other. For example, if you put Na+ and Cl- in a medium, perhaps water, the Na+ particles would be attracted to and bind to the Cl- ions, forming sodium chloride or table salt. Diffusion is a similar concept in that like particles will diffuse somewhat evenly throughout a medium. The nature of diffusion is based on the concept of electrostatic pressure in that like particles have the same charge and, thus, repel each other.
The third force that regulates the resting membrane potential is the Na+/K+ pump. This structure is a protein channel that actively pumps Na+ out of the cell and brings K+ back into the cell. For every three Na+ ions pumped out of the cell, two K+ ions are pumped back into the cell. These channels are located on all parts of the plasma membrane and require a significant amount of energy (ATP) from the cell (Rakowski, Gadsby, & De Weer, 1989; Sagar & Rakowski, 1994).
Ion concentrations in the Resting Membrane Potential
At rest, there is a high concentration of K+ inside of the cell. The force of diffusion acts to push it out of the cell; however, the outside of the cell is more positive than the inside. Thus the force of electrostatic pressure helps keep K+ inside the cell. A similar situation occurs with Cl-. Cl- is in a high concentration outside of the cell with diffusion pushing it in; however, the negative charge on the inside of the cell works to keep it out of the cell. This is not the case with Na+. Na+ is in abundance outside of the cell and both diffusion and electrostatic pressure (Na+ is positive and the inside of the cell is negative compared to the outside) are drawing it to the inside of the cell. In order to keep the resting membrane potential at -70 mV, the Na+/ K+ pump is needed to pump out Na+ and bring in K+ (see Figure 14.3).
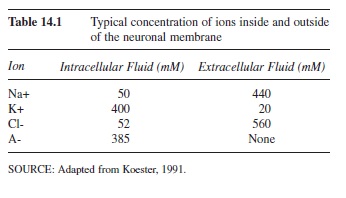 Table 14.1 Typical concentration of ions inside and outside of the neuronal membrane
Table 14.1 Typical concentration of ions inside and outside of the neuronal membrane
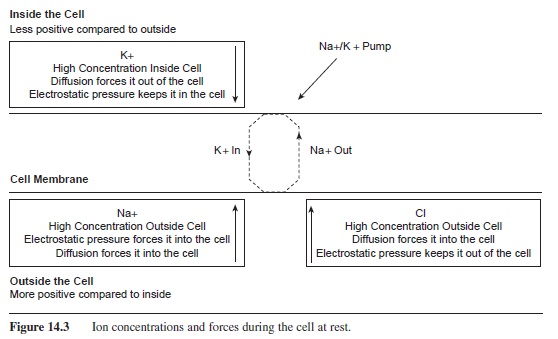 Figure 14.3 Ion concentrations and forces during the cell at rest.
Figure 14.3 Ion concentrations and forces during the cell at rest.
Ion Channels
Although the plasma membrane is permeable to certain molecules, specialized protein channels are needed for many ions to pass through the membrane. These channels are in abundance throughout the membrane on all parts of the cell. Simply, the channels are proteins that are embedded through the membrane, acting as conduits for passing ions across the membrane. These pores are selective to certain ions, some letting only one particular ion through and others letting many positive ions pass. The selective nature of the channels is related to the size and charge of the ion, and the hydration of the ion passing through the membrane (Hille, 1967, 1971). Protein channels can be nongated or gated. Nongated channels are always open. For example, K+ is 20 times more permeable to the membrane because it is continuously leaking out of the cell through a non-gated protein channel (Kandel & Siegelbaum, 1991).
Gated channels must have a mechanism that opens them, letting certain ions in or out of the cell. When the channel is open, ions can pass freely through the membrane. Contrastingly, when it is closed, ions cannot pass through. Gated channels can be ligand-gated, voltage-gated, or concentration-gated.
Ligand-gated channels have a receptor cite on the outside segment of the channel. A molecule, such as a neurotransmitter, must bind to this receptor site in order for the channel to open. Ionotropic channels are one type of ligand-gated channels. When a ligand binds to the channel, the channel opens, letting ions pass through the membrane. Ionotropic channels are fast-acting, completing ion transport in less than a millisecond. Metabotropic channels also have binding sites; however, their mechanism of action is more complicated. The channel consists of a long strand of protein that winds through the membrane seven times. When a ligand binds to the receptor site, the channel goes through a conformational change, bending and activating a second messenger inside of the cell (Kandel & Swartz, 1991). This activation causes a cascade of changes within the cell including indirectly opening ion channels from the inside of the cell and causing long-term genetic changes within the cell.
Voltage-gated channels have gates that open when the intracellular fluid has become more positive and the cell is depolarized. Upon opening, ions can cross the membrane. The gates are only open for ms and then they close, blocking the passage of ions. These channels are essential for the action potential to occur. Concentration-gated channels work on the premise of concentration gradients. When the concentration of a particular ion, either inside or outside of the cell, gets high enough, concentration-gated channels open and let ions pass across the gradient (from high concentrations to lower concentrations).
Communication Between Neurons
The synapse consists of the axon terminal of one cell and the cell body or dendrite of another cell. The sending neuron is called the presynaptic cell and the receiving neuron is called the postsynaptic cell. The axon terminal does not make physical contact with the postsynaptic cell; rather, there is a small gap between the presynaptic and postsynaptic cells called the synaptic gap (Figure 14.4). When the action potential occurs in the presynaptic cell, a neurotransmitter is released from the axon terminal. A list of some common neurotransmitters and their effects can be seen in Table 14.2 (Julien, 2001). The neurotransmitter then binds to the receptor sites on the ligand-gated channels, letting ions pass through the cell membrane. The flow of ions through the membrane causes a change in the electric potential of the cell. This alteration in charge is called a postsynaptic potential because the alteration occurs in the receiving, or postsynaptic, cell.
Postsynaptic potentials can be either excitatory or inhibitory. An excitatory postsynaptic potential (EPSP) occurs when an ion channel opens and Na+ enters the cell. The positive charge of this ion brings the cell’s potential closer to threshold, creating a depolarization in the cell. Contrastingly, inhibitory postsynaptic potentials (IPSPs) occur when Cl- enters the cell or K+ leaves the cell. IPSPs move the cell farther away from threshold, or hyperpolarize the cell (Kandel & Siegelbaum, 1991).
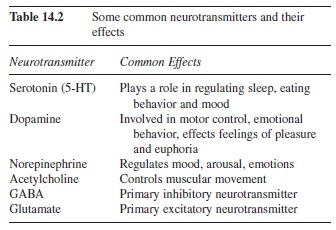 Table 14.2 Some common neurotransmitters and their effects
Table 14.2 Some common neurotransmitters and their effects
Many graded potentials, EPSPs and IPSPs, can influence a cell simultaneously. These potentials sum together to create the potential of the cell at that moment. Postsynaptic potentials can sum over space and time. Spatial summation occurs when two or more postsynaptic potentials affect the cell at two different receptor sites at the same time. Temporal summation occurs when one receptor site is repeatedly stimulated by the same presynaptic cell. Because the threshold of the cell is measured at the axon hillock, the location of the postsynaptic potential is critical. An EPSP near the axon hillock will have a greater effect on driving the cell toward threshold than an EPSP that is far from the axon hillock. Contrastingly, an IPSP close to the axon hillock will have a similar effect by preventing the cell from reaching threshold.
Once threshold has been reached at the axon hillock, voltage-gated Na+ channels open along the initial part of the axon, changing the permeability of the cell to Na+ ions (Figure 14.5). The forces of diffusion and electrostatic pressure drive Na+ into the cell. Remember that diffusion is a factor affected by the concentration gradient. The extracellular fluid has a very high concentration of Na+ ions, therefore Na+ is pushed into the cell. Additionally, Na+ has a positive charge and is attracted to the negative environment of the intracellular fluid. With these two forces working together to push Na+ into the cell, when the voltage-gated channels open Na+ is rapidly forced into the cell. The Na+ channels are open for less than a millisecond; however, enough Na+ ions enter the cell to shoot the potential to +55 mV. This increase in potential causes voltage-gated K+ channels to open. As with Na+, diffusion and electrostatic pressure affect the flow of K+ through the membrane; however, K+ is forced out of the cell. The concentration of K+ is higher inside of the cell, causing K+ to exit the cell. Also, the influx of Na+ into the cell has changed the membrane potential to approximately +55 mV. K+ is positive, therefore the electrostatic pressure pushes K+ out of the cell. As K+ exits the cell, the membrane potential is lowered, resulting in the closing of K+ voltage-gated channels. Because the K+ channels close after the Na+ channels close, the efflux of K+ ions continues even though the membrane potential has been reached. This creates a brief period of hyperpolarization of the membrane. When these K+ ions diffuse into the extracellular fluid, the resting membrane potential is regained (Kuffler & Nichols, 1976 ).
The propagation of the action potential down the axon is mediated by the myelination of the axon (Katz, 1966). Under the segments of myelin, the axon is not permeable and no ions can pass through the membrane. As shown in Figure 14.6, the Na+ ions diffuse under the myelin segment. Because the ions are more spread out over a larger space, the potential is lowered. Although the charge on the inside of the cell has dropped, it is still high enough to trigger voltage-gated channels to open at the next node. Na+ channels are opened, and sodium rushes into the cell, creating the voltage spike. This influx of Na+ occurs at each node of Ranvier down the length of the axon as the action potential is rejuvenated at each node. This process is called saltatory conduction, as the charge looks as if it is jumping down the axon (saltare means “leap” or “jump” in Latin).
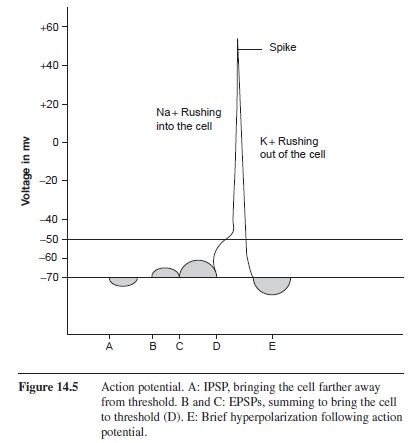 Figure 14.5 Action potential. A: IPSP, bringing the cell farther away
Figure 14.5 Action potential. A: IPSP, bringing the cell farther away
Once an action potential has been initiated, it will continue until completion. The number of action potentials, or rate of firing of the cell, is partly dependent on the characteristics of the Na+ channels. The absolute refractory period occurs when the Na+ channels have been reset and they cannot be reopened for a brief (1 ms) period of time (Kalat, 2001). During the absolute refractory period, no stimulus, however great the strength, can initiate an action potential. The relative refractory period occurs because the K+ channels stay open briefly after the Na+ channels have closed, creating a 2-4 ms period of hyperpolarization as K+ still exits the cell (Kalat, 2001). At this point the inside of the cell is more negative than it is at rest (below -70 mV). A stimulus can initiate an action potential during the relative refractory period, but it must be strong enough to drive the cell to threshold.
Neurotransmitter Release
As the action potential reaches the axon terminals, the increase in potential initiates the opening of voltagegated channels and calcium (Ca +) enters the cell. The influx of Ca + causes the synaptic vesicles to migrate to the periphery of the axon terminals and bind to the membrane. The vesicle bound to the terminal membrane forms an omega-shaped complex, and the membrane opens as the neurotransmitter spills into the synaptic gap. This process is called exocytosis.
Neurotransmitters excised into the gap migrate across the gap, binding to the receptor sites on the postsynaptic cell. After binding, the neurotransmitter separates from the receptor and returns to the fluid of the cleft. Neurotransmitters can be removed from the synaptic gap in a number of ways. The fate of the neurotransmitter in the gap depends on the neurotransmitter itself. For example, 5-hydroxi-tryptamine (5-HT), better known as serotonin, is taken back up into the cell and repackaged into synaptic vesicles to be released again. In contrast, acetylcholine is broken down by degrading enzymes into choline and acetate. The choline is taken back up into the cell by specialized protein channels and the acetate is left to disseminate.
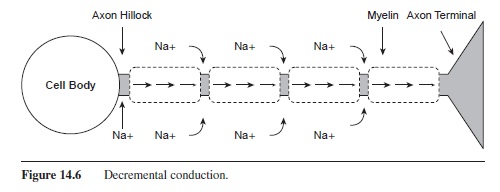 Figure 14.6 Decremental conduction.
Figure 14.6 Decremental conduction.
Summary
Many different types of neurons all have the same purpose of communicating information from one location (e.g., neuron, muscle, organ) to another. This exchange of information is a complex process called neurotransmission.
Neurotransmission involves the passing of ions across cell membranes. These ions change the membrane potential, creating EPSPs and IPSPs and bringing the cell closer or farther from an action potential (AP). Once an AP is triggered, a chain reaction occurs, with Na+ entering the cell and K+ leaving the cell. The AP travels down the axon of the neuron signaling the release of neurotransmitters (NT). As the NTs are released into the extracellular space, they open channels on the receiving membrane, letting ions move into or out of the cell and changing the membrane potential.
References:
- Bean, R. C., Shepard, W. C., & Chan, H. (1968). Permeability of lipid bilayer membranes to organic solutes. Journal of Physiology, 52, 495-508.
- Hille, B. (1967). The selective inhibition of delayed potassium currents in nerve by tetraethylammonium ions. Journal of Physiology, 50, 1287-1302.
- Hille, B. (1971). The hydration of sodium ions crossing the nerve membrane. Proceedings of the National Academy of Sciences, 68, 280-282.
- Hodgkin, A. L., & Huxley, A. F. (1945). Resting and action potentials in single nerve fibres. Journal of Physiology, 104, 176-195.
- Hodgkin, A. L., & Huxley, A. F. (1952). A quantitative description of membrane current and its application of conduction and excitation in nerve. Journal of Physiology, 117, 500-544.
- Hodgkin, A. L., & Katz, B. (1949). The effect of sodium ions on the electrical activity in the giant axon of the squid. Journal of Physiology, 108, 37-77.
- Julien, R. M. (2001). A primer of drug action (9th ed.). New York: Worth Publishers.
- Kalat, J. W. (2001). Biological psychology (7th ed.). Belmont, CA: Wadsworth/Thompson Learning.
- Kandel, E. R. (1991). Nerve cells and behavior. In E. R. Kandel, J. H. Schwartz, & T. M. Jessel (Eds.), Principles of neural science (pp. 18-32). Norwalk, CT: Appleton & Lange.
- Kandel, E. R., & Schwartz, J. H. (1991). Synaptic transmission mediated by second messengers. In E. R. Kandel, J. H. Schwartz, & T. M. Jessel (Eds.), Principles of neural science (pp. 173-193). Norwalk, CT: Appleton & Lange.
- Kandel, E. R., & Siegelbaum, S. A. (1991). Directly gated transmission at the nerve-muscle synapses. In E. R. Kandel, J. H. Schwartz, & T. M. Jessel (Eds.), Principles of neural science (pp. 135-152). Norwalk, CT: Appleton & Lange.
- Katz, B. (1966). Nerve, muscle, and synapse. New York: McGraw-Hill.
- Koester, J. (1991). Membrane potential. In E. R. Kandel, J. H. Schwartz, & T. M. Jessel (Eds.), Principles of neural science (pp. 81-94). Norwalk, CT: Appleton & Lange.
- Kozorovitskiy, Y., Gross, C. G., Kopil, C., Battaglia, L., McBreen, M., Stranahan, A. M., et al. (2005). Experience induces structural and biochemical changes in the adult primate brain. Proceedings of the National Academy of Sciences, 102, 17479-17482.
- Rakowski, R. F., Gadsby, D. C., & De Weer, P. (1989). Stoichiometry and voltage dependence of the sodium pump in voltage clamped, internally dialyzed squid giant axon. Journal of General Physiology, 93, 904-941.
- Sagar, A., & Rakowski, R. F. (1994). Access channel model for the voltage dependence of the forward-running Na+/K+ pump. Journal of General Physiology, 104, 869-894.
- Schmitt, F. O., & Schmitt, O. H. (1940). Partial excitation and variable conduction in the squid giant axon. Journal of Physiology, 98, 26-46.
- Thomas, C. L. (Ed.). (1989). Taber’s cyclopedic medical dictionary (16th ed.). Philadelphia: F. A. Davis Company
- Thompson, R. F. (1993). The brain: A neuroscience primer (2nd ed.). New York: W. H. Freeman and Company.
See also:
Free research papers are not written to satisfy your specific instructions. You can use our professional writing services to order a custom research paper on any topic and get your high quality paper at affordable price.





