This sample Taste Research Paper is published for educational and informational purposes only. If you need help writing your assignment, please use our research paper writing service and buy a paper on any topic at affordable price. Also check our tips on how to write a research paper, see the lists of psychology research paper topics, and browse research paper examples.
The evolution of life on this planet required organisms to sense chemicals suspended or dissolved in water for their survival. Some such chemicals were destructive and required avoidance, whereas others provided nourishment and sustenance. Single-celled organisms such as the bacterium Escherichia coli that inhabits our own digestive systems, developed multiple chemical receptors that determined whether they should approach or avoid a given situation. In the case of E. coli, for example, the direction of rotation of the flagella—whip-like appendages that propel them through their environment—is influenced by the type of chemical they encounter. Thus, chemicals important for sustenance produce a counterclockwise rotation of the flagella that facilitates a smooth and somewhat linear swimming path, whereas other, seemingly noxious chemicals induce a clockwise flagellar rotation that produces tumbling and turning away from the source of the stimulus.
Like these simple single-celled organisms, the ability to sense chemicals was critical for the evolution of multi-celled organisms, and most developed sophisticated chemosensory systems for that purpose. In both invertebrate and vertebrate species, the sense of taste plays a critical role in determining what materials are eaten or rejected. In mammals, the peripheral elements of this system are the taste buds, bulb-like structures located on the tongue and palate, usually on protuberances termed papillae.
The human taste bud contains multiple types of sensory receptors that mediate the basic sensations of sweet, sour, bitter, salty, metallic, and perhaps fat and umami. Umami represents taste sensations derived from some amino acids, including monosodium glutamate. The basic taste sensations ensure the energy needs of the organism (sweet, fat, umami), proper electrolyte balance (salty), and avoidance of toxic substances (bitter, sour). Combined with elements of texture, temperature, and smell during eating, such sensations produce the “flavor” of foods. It is important to note that many “tastes” such as those recognized as apple, cherry, chocolate, meat sauce, pizza, strawberry, and lemon actually depend upon the olfactory receptors, not the taste buds. During chewing and swallowing, volatiles arising from the ingested food pass from inside the oral cavity to the olfactory receptors via the opening to the rear of the nasal cavity (i.e., the nasopharynx), producing such sensations. This can be readily appreciated when one holds the nose shut during eating, minimizing the ability of air to freely move from oral cavity into the nasal cavity.
This research-paper describes the anatomy, development, and function of the human taste system. The ability to taste begins in the uterus and gradually matures or becomes modified by learning as postnatal life progresses. The appreciation of sweet seems to predominate in the taste world, likely being the first of the basic taste qualities to appear and the last to disappear during the life process.
Anatomy And Physiology
Humans possess approximately 7,500 taste buds. The sensory receptor cells within each taste bud are responsive to organic molecules, made up of mostly carbon, hydrogen, and oxygen. Such molecules, termed tastants, enter the buds through small openings, termed taste pores, located at the surface of the epithelium (Figure 22.1). Multiple buds can be present on a single papilla. Although individual papillae can vary considerably in structure and size, they are typically classified into four major types: filiform, fungiform, foliate, and circumvallate. The pointed filiform papillae contain no taste buds, serving mainly to move, and perhaps abrade, food particles during the process of eating. The mushroom-shaped fungiform papillae, most but not all of which harbor taste buds, are found largely on the tip and sides of the tongue (Figure 22.2). The foliate papillae appear as folded ridges along the tongue’s margin relatively far back on the tongue. The large circumvallate papillae, which range in number from 6 to 10, resemble flattened hills across the “chevron” of the tongue, each being surrounded by a circular trench or valley.
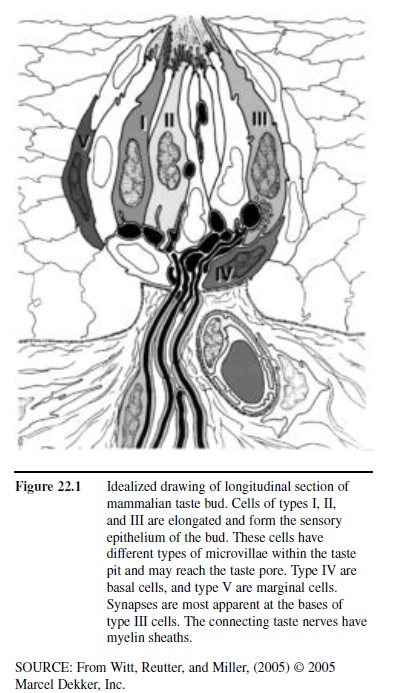
Figure 22.1 Idealized drawing of longitudinal section of mammalian taste bud. Cells of types I, II, and III are elongated and form the sensory epithelium of the bud. These cells have different types of microvillae within the taste pit and may reach the taste pore. Type IV are basal cells, and type V are marginal cells. Synapses are most apparent at the bases of type III cells. The connecting taste nerves have myelin sheaths.
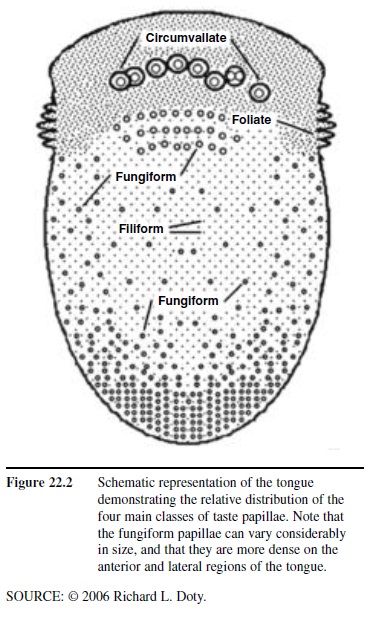
Figure 22.2 Schematic representation of the tongue demonstrating the relative distribution of the four main classes of taste papillae. Note that the fungiform papillae can vary considerably in size, and that they are more dense on the anterior and lateral regions of the tongue.
Like the olfactory receptors, and epithelial cells in general, the neural elements of a taste bud, each of which advances small extensions called microvilli toward the taste pore, die and become replaced at various intervals from basal cells. Such receptor cells vary in number from bud to bud, ranging from none at all to well over 100. Unlike the olfactory receptors, the taste receptor cells are not neurons. They form synaptic connections to projection neurons that make up the taste nerves. Depending upon their location, these cells are innervated by one of three sets of paired cranial nerves, namely the facial (CN VII), glossopharyngeal (CN IX), and vagus (CN X) nerves (Figure 22.3).
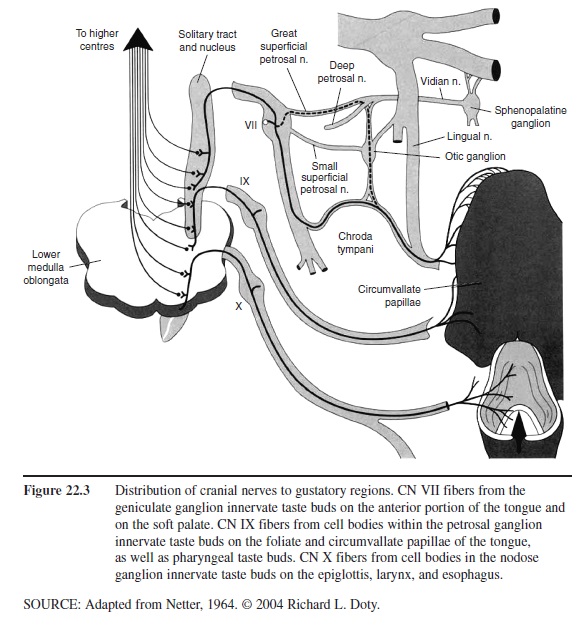 Figure 22.3 Distribution of cranial nerves to gustatory regions. CN VII fibers from the geniculate ganglion innervate taste buds on the anterior portion of the tongue and on the soft palate. CN IX fibers from cell bodies within the petrosal ganglion innervate taste buds on the foliate and circumvallate papillae of the tongue, as well as pharyngeal taste buds. CN X fibers from cell bodies in the nodose ganglion innervate taste buds on the epiglottis, larynx, and esophagus.
Figure 22.3 Distribution of cranial nerves to gustatory regions. CN VII fibers from the geniculate ganglion innervate taste buds on the anterior portion of the tongue and on the soft palate. CN IX fibers from cell bodies within the petrosal ganglion innervate taste buds on the foliate and circumvallate papillae of the tongue, as well as pharyngeal taste buds. CN X fibers from cell bodies in the nodose ganglion innervate taste buds on the epiglottis, larynx, and esophagus.
In humans and most mammals, the chorda tympani division of CN VII innervates the fungiform papillae on the anterior two-thirds of the tongue and the most anterior of the laterally located foliate papillae. Because this nerve courses near the tympanic membrane within the middle ear (hence its name), it can be damaged by various pathologies, including ear infections and otosclerosis, as well as by middle ear operations that compromise the eardrum. A common complaint of patients whose chorda tympani has been stretched or otherwise compromised is the presence of chronic “metallic” taste sensations. Damage to this nerve also results in denervation of taste buds and a reduction in the number of taste papillae on the ipsilateral twothirds of the tongue.
The palatine branch of the greater superficial petrosal division of the facial nerve innervates the taste buds within the soft palate (Figure 22.3). The taste buds on the large circumvallate papillae are innervated by CN IX, and those at the base of the tongue, by CN X. Although not involved in taste perception, per se, the trigeminal nerve (CN V) also innervates taste bud regions, as well as most areas of the nasal and oral epithelia, and the teeth. This nerve signals touch, pain, and temperature sensations, and therefore participates in the formation of flavor. Thus, the warmth of coffee and the fizziness of carbonated soft drinks are largely dependent upon the stimulation of fine branches of CN V.
Sensitivity to sweet, sour, bitter, and salty tastants is not uniform across the regions of the tongue. Although diagrams in some children’s books and even in some physiology textbooks suggest that different regions of the tongue are responsible for the four basic taste qualities, this is an oversimplification of the facts. In general, the tongue is most sensitive to tastants at its tip, around its margins, and at its base. While it is true that the base of the tongue is more sensitive to bitter tastants, and less sensitive to sweet tastants, than the front of the tongue, both tongue regions can detect bitter and sweet tastants. The relative average sensitivity of tongue regions to the four prototypical taste qualities is shown in Figure 22.4, although significant individual differences are present. When small tongue regions are compared for their sensitivity between children and adults, children have been found to be more sensitive, presumably reflecting the greater density of receptors within the regions evaluated. However, whole-mouth testing rarely finds children more sensitive than adults. In a recent study, for example, whole-mouth detection thresholds for sucrose, sodium chloride, citric acid, and caffeine were measured for 68 children 6 to 8 years of age and for 61 young adults. Thresholds were not different between girls and either the adult men or the adult women. Boys were less sensitive, on average, than the adult women were to all of these stimuli and less sensitive than the men were to all but caffeine. They were also less sensitive than girls were to sucrose and sodium chloride.
The sensing of chemicals within taste buds is via specialized receptors located within the microvillar membranes of the taste receptor cells. Sweet and umami sensations depend upon a small family of three G-protein-coupled receptors (GPCRs) termed T1R1, T1R2, and T1R3 receptors. Bitter sensations are mediated by a family of ~30 GPCRs, the T2R receptors, which are expressed on cells different from those that express sweet and umami receptors. T2Rs, which are expressed on the same cells, recognize a wide range of bitter substances, but do not distinguish between them. The salt sensation of sodium chloride is believed to arise from the direct entrance of Na+ ions into the cells via specialized membrane channels, such as the amiloride-sensitive Na+ channel. Although a range of receptors has been suggested to mediate sour taste, PKD2L1 has been suggested to be the primary, if not sole, receptor. The deletion of the gene expressing this receptor, a member of the transient receptor potential protein (TRP) family, results in mice unable to detect sour tastants but able to detect sweet, bitter, and salty ones.
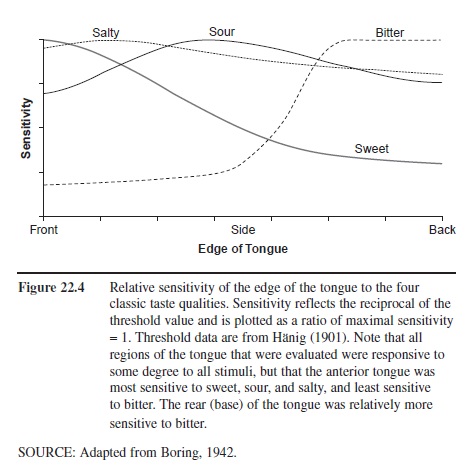 Figure 22.4 Relative sensitivity of the edge of the tongue to the four classic taste qualities. Sensitivity reflects the reciprocal of the threshold value and is plotted as a ratio of maximal sensitivity = 1. Threshold data are from Hanig (1901). Note that all regions of the tongue that were evaluated were responsive to some degree to all stimuli, but that the anterior tongue was most sensitive to sweet, sour, and salty, and least sensitive to bitter. The rear (base) of the tongue was relatively more sensitive to bitter.
Figure 22.4 Relative sensitivity of the edge of the tongue to the four classic taste qualities. Sensitivity reflects the reciprocal of the threshold value and is plotted as a ratio of maximal sensitivity = 1. Threshold data are from Hanig (1901). Note that all regions of the tongue that were evaluated were responsive to some degree to all stimuli, but that the anterior tongue was most sensitive to sweet, sour, and salty, and least sensitive to bitter. The rear (base) of the tongue was relatively more sensitive to bitter.
The peripheral taste fibers integrate information from multiple taste cells and send this information to the first relay station within the brain, termed the nucleus of the solitary tract of the brainstem (Figure 22.3). Although large species differences exist, the nerve fibers that send information from the taste bud to the brainstem can be classified into categories based upon their relative responsiveness to sweet-, sour-, bitter-, and salty-tasting agents. In the hamster, for example, sucrose-best, NaCl-best, and HCl-best fibers have been observed based upon electrophysiological responses. Although fibers are “tuned” for rather specific stimuli, they can nonetheless respond to other stimuli. For example, a few sucrose-best fibers also respond to NaCl and HCl. NaCl-best fibers and HCl-best fibers are less tightly tuned than sucrose-best fibers, with more fibers responding to multiple classes of stimuli.
Taste information is carried from the nucleus of the solitary tract to a taste center within the upper regions of the ventral posterior nuclei of the thalamus via the medial lemniscus, a pathway connecting the brainstem to the thalamus. From here, information is sent to the amygdala and several cortical regions. Two of the cortical regions are the primary somatosensory cortex and the anterior-insular cortex, a region of the frontal cortex located near the anterior temporal cortex. Neurons within these regions respond to taste, touch, and, in some cases, odors.
How does the brain identify the quality of a taste stimulus (i.e., whether it has a sweet, sour, bitter, or salty taste)? As previously noted, receptors are dedicated to chemicals known to elicit these sensations. Although there is clear evidence that at least some taste qualities are mediated by specific receptors, the “taste code” interpreted by the brain is reflected not only in information provided by the specific neurons that are activated (labeled line theory) but also in the pattern of firing that occurs across taste neurons (across-fiber pattern theory). Intensity or strength of the sensations reflects a combination of the number of taste neurons activated, their identity, and their relative firing rates. Although seemingly simple, the brain must remember what a sweet stimulus, for example, tastes like, and a comparison of information coming from the taste pathways must be made at some point with the remembered sensation to allow for its recognition or identification. Moreover, higher brain centers play a significant role in explaining taste contrasts (e.g., something tasting sweeter after prior experience with a sour stimulus), sensory fatigue, integration of multiple taste sensations, loss of awareness of taste sensations due to inattention, the degree to which a stimulus is like and disliked, and other phenomena similar to those well described for olfaction (see Chapter 24).
Taste In The Fetus
By the 10th week of gestation, the taste bud-containing papillae of the tongue, namely the fungiform, foliate, and circumvallate papillae, have developed. In general, the large circumvallate papillae develop earlier than the fungiform papillae. By 10 to 14 weeks, the taste pores are observed in fetal fungiform papillae, although synaptic connections of the early taste bud primorda to nerve fibers are observed by as early as 8 weeks. Taste buds continue to differentiate and make synaptic connections long after the opening of the taste pores.
The human fetus chews, swallows, and even regurgitates during the second half of gestation. At term, the fetus swallows 70 to 260 ml of fluid per day per kilogram of body weight. Such swallowing is important for fetal amniotic fluid resorption, recirculation of lung and urine fluid volumes, gastrointestinal development, and somatic growth and is likely the sole means by which fetal resorption of the principal amniotic fluid electrolytes is made. Whether the taste system plays a fundamental role in such swallowing is not clear, although the frequency of such swallowing is influenced by tastants introduced into the amniotic fluid of animals.
The nature of taste function in the late-term human fetus can be inferred by testing the ability of premature infants to taste. Taste-induced behavioral responses, including facial expressions seemingly reflecting pleasure or displeasure, are present in premature infants at six to nine months of gestational age. Increases in sucking vigor, reflexive salivation, and, in some cases, retching can be induced in premature babies by placing a single drop of lemon juice into their mouths. Sucking responses are inhibited by low concentrations of quinine, a bitter tastant, whereas sweet-tasting stimuli generally increase sucking frequency. Premature infants, as well as neonates, transiently cease crying when given oral solutions of sucrose or glucose, but not water. Such tastants also promote analgesic-like reactions during heel lance and other invasive procedures. Such responses are taste-bud mediated, since sweet solutions are ineffective in producing analgesia when administered directly into the stomach. The calming effects are induced within seconds of sucrose delivery, well in advance of stomach clearance or absorption.
In general, negative responsiveness to salt ingestion seems to develop between the ages of 4 and 24 months postpartum. Nonetheless, there are reports that variable facial expressions to salt stimuli occur in premature infants. For example, in one study 20 premature infants (1.2-2.9 kg) were orally presented with 0.9 percent NaCl. More than half responded with a rejecting grimace, although 4 readily accepted this solution. In another study, a 1 percent solution of NaCl produced indifference in two-thirds of the premature infants, and rejection in the other third.
In summary, taste buds are functional and capable of conveying at least some types of gustatory information to the central nervous system by the sixth gestational month. Such information is likely available to neural systems that organize salivation, sucking, facial expressions, and other affective behaviors at this early age. In adults, not all regions of the tongue are equally sensitive to tastants, with the rear of the tongue being most sensitive to bitter substances and least sensitive to sweet substances. The perceptual “code” for taste seems to involve a combination of factors, including the specific receptors that are activated and the pattern, type, and frequency of firing of nerve cells that project into the brain stem and, from there, to cortical centers.
Influence of Early Experiences with Tastes on Later Taste Preferences
Exposure of fetuses to electrolyte imbalances and accompanying dehydration appears to alter postnatal taste preferences. In one study, 16-week-old infants of mothers who had experienced morning sickness or had vomited during pregnancy exhibited stronger preferences for 0.1 and 0.2 M NaCl than infants of mothers who had no such experiences. They also ingested larger volumes of the stronger of these solutions. Moreover, the babies of the sick mothers were less likely to express aversive facial reactivity patterns, and more likely to exhibit hedonically positive responses, to the salty solutions. This altered salt preference likely continues into adulthood, since adults whose mothers experienced marked morning sickness exhibited, relative to ones whose mothers did not, (a) greater self-reported use of salt, (b) greater intake of salt in the laboratory, and (c) stronger preferences for salty snack food.
After birth, the taste of the mother’s milk, which is usually rich in lactose and other sugars, can be influenced by her diet, thereby influencing suckling responses and, ultimately, the amount and quality of milk that is ingested. For example, some infants breast-feed longer if their mothers are on a flavor-rich diet than if they are on a bland diet. Experience with the ingestant also alters suckling behavior, and in some instances novelty of the taste of the milk will increase the amount ingested. The influences of experience can also play an important role in determining the food preferences of children and teenagers. Children increase their acceptance of a novel food, even food that is initially aversive, after repeated dietary exposure to that food. One study, for example, exposed four- to five-year-olds to either plain tofu or tofu made sweet or salty multiple times over the course of several weeks. When tested months later, the children preferred the tofu with which they had experience. This taste preference did not generalize to other foods of similar color and texture (e.g., ricotta) that were made similarly sweet or salty.
In light of such findings, it is perhaps not surprising that context and culture are important for establishing taste preferences. In a study of first- and second-generation Chinese adolescent immigrant boys to Canada, the second-generation boys and those with more acculturated patterns of language use gave higher hedonic flavor and prestige ratings to dessert, snack, and fast foods, and discriminated better among nutrient-rich and nutrient-poor foods.
Human Studies Of Bitter Taste Perception
A series of studies in the 1930s highlighted the fact that considerable variation exists in the human population in the ability to detect bitter-tasting agents. Although the whole-mouth sensitivity of humans to most tastants follows a normal distribution in the general population, there are notable exceptions. Among such exceptions are a class of approximately 40 bitter-tasting antithyroid compounds, the most widely studied of which are phenylthiourea (PTC) and 6-n-propylthiouracil (PROP). Nearly all human studies examining the genetics of tasting have focused on these two compounds, whose bitter taste is dependent upon the N – C = S structure within the molecules.
The largely bimodal distribution of sensitivity to PTC/PROP led to the classification of subjects as either “tasters” or “nontasters.” Although the inability to taste these agents was first believed to reflect a simple Mendelian recessive trait, subsequent data contradicted this genetic model, and numerous other models were proposed over the years to explain the inheritance of PTC/PROP taste sensitivity. In 1993 a small region was identified on chromosome 7q that is associated with PTC taste sensitivity. This region contained a single gene that encodes a member of the TAS2R bitter taste receptor family. Five worldwide haplotypes in this gene have been shown to largely explain the biomodal distribution of PTC taste sensitivity, accounting for the inheritance of the classically defined taste insensitivity and for 55 percent to 85 percent of the variance in PTC sensitivity. Importantly, the sensitivity of individuals to PTC/PROP is associated with the number of taste buds, as well as the number of fungi-form papillae located on the anterior tongue.
The question arises as to why insensitivity to PTC and related compounds exists in the human population, and why populations in different parts of the world have differing numbers of PTC tasters and nontasters. In one study, for example, only 7 percent of Japanese evaluated were nontasters, compared to 30 percent of Caucasians. Early researchers also found that more PTC nontasters were present in populations with nodular goiters and with cretinism. Derivatives of PTC are known to cause the formation of goiters and to block the synthesis of thyroxine within the thyroid gland. One explanation of why the gene responsible for PTC nontasting is maintained in the gene pool is that those who possess it are protected against malaria. Thus, in a study of Ecuadorian Andean communities in which goiter is endemic, PTC tasters were found not to eat a maize which contains a bitter-tasting goitrogen, and therefore they do not develop goiters. However, these individuals were found more likely to succumb to malaria, unlike their nontasting counterparts.
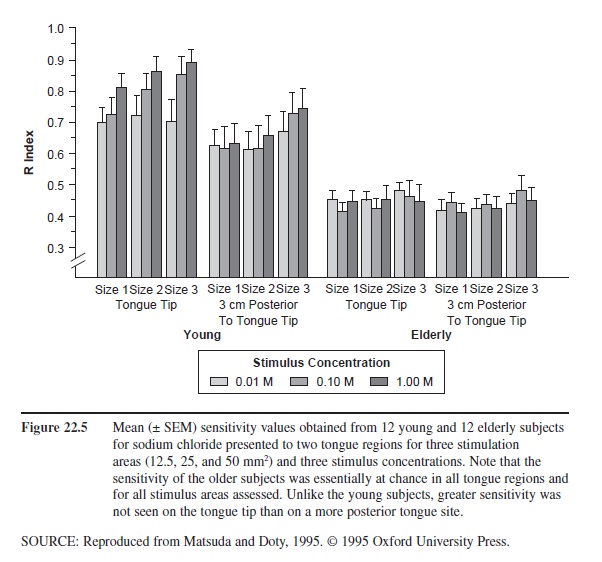 Figure 22.5 Mean (± SEM) sensitivity values obtained from 12 young and 12 elderly subjects for sodium chloride presented to two tongue regions for three stimulation areas (12.5, 25, and 50 mm2) and three stimulus concentrations. Note that the sensitivity of the older subjects was essentially at chance in all tongue regions and for all stimulus areas assessed. Unlike the young subjects, greater sensitivity was not seen on the tongue tip than on a more posterior tongue site.
Figure 22.5 Mean (± SEM) sensitivity values obtained from 12 young and 12 elderly subjects for sodium chloride presented to two tongue regions for three stimulation areas (12.5, 25, and 50 mm2) and three stimulus concentrations. Note that the sensitivity of the older subjects was essentially at chance in all tongue regions and for all stimulus areas assessed. Unlike the young subjects, greater sensitivity was not seen on the tongue tip than on a more posterior tongue site.
Changes In Taste With Advancing Age
Age-related influences on taste function are well documented. The degree to which age-related effects are observed depends upon the type of testing involved. For example, it is greater when small regions of the tongue are evaluated than when the whole mouth is tested using, for example, “sip and spit” methods. Age-related decreases in taste threshold sensitivity have been reported for such tastants as caffeine, citric acid, hydrochloric acid, magnesium sulfate, propylthiourea, quinine, sodium chloride, sucrose, tartaric acid, and a large number of amino acids. Not all taste qualities exhibit the same degree of age-related loss. Thus, sweet sensations are less ravaged by age than are salty, bitter, and sour sensations. The marked age-related decrement to NaCl seen in small regions of the tongue is shown in Figure 22.5.
As with other sensory systems, a relationship between the number of functioning receptor elements and the system’s sensitivity is present for the sense of taste. Thus, the perceived intensity of tastants presented to localized regions of the anterior tongue is correlated with the number of fungiform papillae and, hence, the number of taste buds within the stimulated regions. Individuals with higher densities of taste buds and fungiform papillae rate the intensity of a number of tastants as more strong than do ones with lower densities of such structures. Although it would seem to follow that age-related declines in the taste function of older persons would relate to decreased numbers of taste buds, there is some controversy as to whether the number of taste buds meaningfully decrease with age. Although age-related losses of taste buds have been reported for human cirmuvallate papillae, most studies of rodent, monkey, and human tongues suggest that taste bud numbers in the anterior and medial lingual regions are little influenced by age. For example, the average percentage of fungiform papillae-containing taste buds in Fischer 344 rats aged 4 to 6 months, 20 to 24 months, and 30 to 37 months was found in one study to be 99.6 percent, 99.3 percent, and 94.7 percent, respectively, differences that were not statistically significant. A human study found no meaningful relationship between age and taste bud densities on either the tip or the mid-region of tongues from young adults (22-36 years, N = 5), middle-aged adults (50-63 years, N = 7), and old adults (70-90 years, N = 6). Given the small sample sizes, however, this research could stand replication with larger numbers of subjects.
Despite the fact that taste bud numbers appear not to be markedly decreased in old rats, electrical responsiveness of the chorda tympani nerve is decreased in such rats to some salts, acids, and sugars. Among the possible explanations of this phenomena are decreased intrinsic reactivity of taste buds to taste solutions, decreased multiple neural innervation of taste buds by some taste fibers, alterations in the general structure of the epithelium (which, for example, might impair the movement of stimulus solution into the taste bud pore), and decreased taste nerve responsiveness, per se. It is also possible that some taste buds, although anatomically present, are not fully functional because of altered turnover time or related metabolic events. Since taste buds function in a complex ionic milieu and are bathed with saliva and other secretory products, changes in such products may also undergo age-related changes. There is suggestion that heightened taste threshold values and flattened suprathreshold psychophysical functions observed in many elderly reflect background tastes noticeable at low, but not at moderate or high, stimulus concentrations. Both neural and oral environment changes (e.g., salivary amount and constituents) could contribute to this noise. Evidence that improved oral hygiene improves taste sensitivity in some elderly persons is in accord with this hypothesis.
Food preferences expressed during childhood, as well as in later life, also reflect genetic determinants that produce considerable variability in acceptance. The best-documented examples of this for children are from studies involving PROP. For example, in one study, five- to seven-year-old children were tested for their sensitivity to PROP Relative to adults, proportionately fewer “nontasters,” defined by threshold sensitivity and suprathreshold ratings of intensity, were found, suggesting that PROP thresholds may rise with age and may partially account for the greater food finickiness observed in many children.
Influences Of Medications On Taste Perception
According to the Physician’s Desk Reference (PDR), hundreds of medications are associated with taste-related side effects. Terms used to describe such side effects in the PDR include “loss of taste,” “altered taste,” “ageusia,” “taste loss,” “dysgeusia,” “bad taste,” “hypogeusia,” “bitter taste,” “metallic taste,” “unpleasant taste,” and “salty taste.” Among such medications are widely prescribed ones, including antimicrobials, antifungals, antihypertensives, antihyperlipidemics, and antidepressants. Unfortunately, the true incidence and prevalence of such taste side effects are not known. Most literature reports are anecdotal case reports and only rarely has taste function been measured quantitatively. In some cases, it is unclear if it is the medication or the underlying disorder that is responsible for the taste symptoms, and confounding and interactive influences from other medications may be present, a common situation in the elderly. Since many persons confuse loss of flavor sensations from olfaction with loss of taste, a number of literature reports likely mistake smell losses with taste losses.
That being said, there is convincing evidence that a number of medications alter taste function, most notably ones related to cardiovascular function or its protection. Adverse chemosensory side effects are noted for 70 percent of the antihyperlipidemic drugs listed in the PDR. In a placebo-controlled study of Lipitor (atorvastatin calcium), side effects of altered taste and loss of taste were not uncommon. Similar side effects were found in clinical trials of Baycol, Lescol (fluvastatin), Provachol (pravastatin), Mevacor (lovastatin), and Zocor (simvastatin). Over a third of the antihypertensive drugs listed in the PDR reportedly have adverse taste side effects, including calcium channel blockers, diuretics (e.g., amiloride), and angiotensin-converting enzyme (ACE) inhibitors. ACE inhibitors block the enzyme that converts angiotensin I to angiotensin II, a potent vasoconstrictor that raises blood pressure, and decrease inactivation of bradykinin, a potent vasodilator. Captopril, the first orally active ACE inhibitor, is more frequently associated than any other ACE inhibitor with complaints of ageusia, metallic taste, and taste distortion. This drug can make sweet-tasting foods taste salty, and can produce chronic bitter or salty sensations, presumably by directly altering ion channels. Drug discontinuance usually reverses the taste disturbance within a few months.
Another well-documented drug that severely alters taste function is the antifungal agent, terbinifine (Lamisil). According to PDR, 2.8 percent of 465 individuals who were taking this drug in clinical trials experienced adverse taste effects, as compared with 0.7 percent of a group of 137 persons taking a placebo. Since quantitative assessment of taste function has only rarely been performed, it is likely that a much larger number of persons taking this medication have taste deficits. The subjective taste problems resolve over the course of a few weeks to several months after discontinuation of terbinafine, although long-lasting cases have been reported and no long-term study has been performed employing quantitative taste tests. The reason for the loss of taste is unclear, although inhibition of cytochrome p-450-dependent enzymes at the level of the receptors has been suggested as one mechanism. As shown in Figure 22.6, this medication reduces, in patients presenting to our center with complaints of terbinifine-induced altered taste function, the ability to identify sweet-, sour-, bitter-, and salty-tasting stimuli, with a greater influence on sour- and bitter-tasting ones.
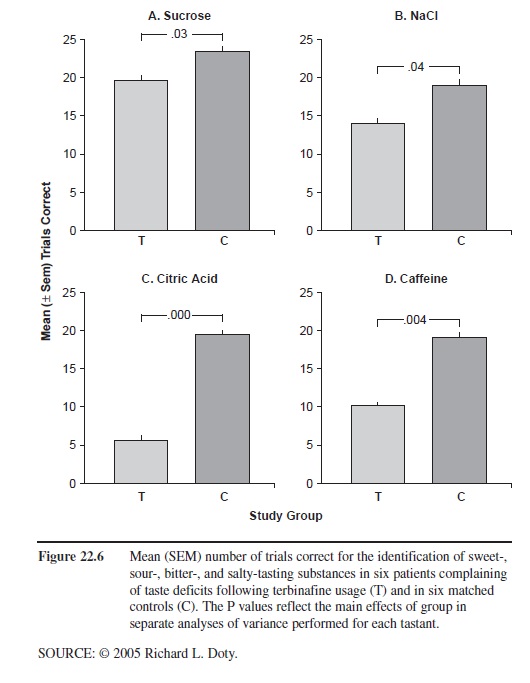 Figure 22.6 Mean (SEM) number of trials correct for the identification of sweet-, sour-, bitter-, and salty-tasting substances in six patients complaining of taste deficits following terbinafine usage (T) and in six matched controls (C). The P values reflect the main effects of group in separate analyses of variance performed for each tastant.
Figure 22.6 Mean (SEM) number of trials correct for the identification of sweet-, sour-, bitter-, and salty-tasting substances in six patients complaining of taste deficits following terbinafine usage (T) and in six matched controls (C). The P values reflect the main effects of group in separate analyses of variance performed for each tastant.
Summary
The sense of taste largely evolved to provide organisms with a means to determine the safety and nutritional value B. NaCl of substances they ingest. In humans, taste preferences, which appear to have innate elements, can be greatly modified by experience, even prior to birth. As a result of such experience and underlying differences in genetic makeup, adults exhibit a wide range of taste preferences and predilections. Human taste buds, the frontline sensors of the taste system, contain multiple types of sensory receptors that mediate basic taste sensations and largely determine, along with the receptors of the olfactory system, the flavor of foods. Unlike the olfactory system, multiple paired cranial nerves subserve taste—namely, the facial (CN VII), glossopharyngeal (CN IX), and vagus (CN X) nerves.
The sensitivity of the tongue to sweet, sour, bitter, salty, and other taste qualities is not uniform across its surface. It is most sensitive to sweet and salty substances in the front, and bitter and sour ones in the back and sides, although all three (front, side, and back) tongue regions can detect, to some degree, all of the major sensory qualities. The middle of the tongue has the least number of taste buds and, therefore, is least sensitive to tastants. Individual taste buds have varying numbers of receptor cells sensitive to the basic taste qualities, with some being sensitive to one, others to only a few, and still others to all basic taste qualities. Sweet and umami tastes are mediated by a small family of G-protein-coupled receptors, whereas bitter sensations are mediated by a family of approximately 30 such receptors. The latter receptors are not found on the same cells as the sweet and umami receptors. Responses to most salty-tasting agents likely reflect the entry of Na+ ions into the cells via specialized membrane channels. One type of receptor has been recently identified that seems to mediate most, if not all, sour tastes.
After activating taste receptor cells, the taste information is carried from the taste buds to higher brain regions via pathways that are dedicated, to a large but not total degree, to a given class of taste stimuli (e.g., sweet-tasting agents). Some such cells are more tightly tuned to a given class of stimuli than other cells, implying that both “labeled line” and “cross-fiber” processes are involved in the coding of information to be sent to higher brain regions for interpretation. The brain identifies tastes in cortical regions where the incoming sensation can be compared to memory stores, thereby allowing a given taste quality to be recognized.
A number of factors influence the ability to taste, including genetics, age, experience, health, and medications that control microbes, hypertension, depression, and cholesterol metabolism. Sensitivity to a number of bitter-tasting compounds is genetically determined and varies considerably among human populations. Studies of Ecuadorian Andean communities find that persons sensitive to bitter tastants are less likely to ingest toxicants that cause thyroid problems than ones who are insensitive to such tastants. However, such persons are more likely than nontasters to succumb to malaria, likely explaining why this genetic dimorphism is maintained in the gene pool.
References:
- Boring, E. G. (1942). Sensation and perception in the history of experimental psychology. New York: Appleton-Century-Crofts.
- Chandrashekar, J., Hoon, M. A., Ryba, N. J. P., & Zuker, C. S. (2006). The receptors and cells for mammalian taste. Nature, 444, 288-298.
- De Graaf, C., & Zandstra, E. H. (1999). Sweetness, intensity, and pleasantness in children, adolescents, and adults. Physiology & Behavior, 67, 513-520.
- Doty, R. L., Bagla, R., Morgenson, M., & Mirza, N. (2001). NaCl thresholds: Relationship to anterior tongue locus, area of stimulation, and number of fungiform papillae. Physiology & Behavior, 72, 373-378.
- Doty, R. L., & Haxel, B. R. (2005). Objective assessment of terbinafine-induced taste loss. Laryngoscope, 115, 2035-2037.
- Ganchrow, J. R., & Mennella, J. A. (2003). The ontogeny of human flavor perception. In R. L. Doty (Ed.), Handbook of olfaction and gustation (2nd ed., pp. 823-846). New York: Marcel Dekker.
- Green, L. S. (1974). Physical growth and development, neurological maturation, and behavioral functioning in two Ecuadorian Andean communities in which goiter is endemic. II. PTC taste sensitivity and neurological maturation. American Journal of Physical Anthropology, 41, 139-151.
- Hanig, D. P. (1901). Zur Psychophysik des Geschmachssinns. Philosophische Studien, 17, 576-623.
- James, C. E., Laing, D. G., & Oram, N. (1997). A comparison of the ability of 8-9-year-old children and adults to detect taste stimuli. Physiology & Behavior, 62, 193-197.
- Kim, U.-K., Jorgenson, E., Coon, H., Leppert, M., Risch, N., & Drayna, D. (2003). Positional cloning of the human quantitative trait locus underlying taste sensitivity to phenylthio-carbamide. Science, 299, 1221-1225.
- Matsuda, T., & Doty, R. L. (1995). Age-related taste sensitivity to NaCl: Relationship to tongue locus and stimulation area. Chemical Senses, 20, 283-290.
- Mennella, J. A., Pepino, M. Y., & Reed, D. R. (2005). Genetic and environmental determinants of bitter perception and sweet preferences. Pediatrics, 115, 216-222.
- Mistretta, C. M., & Hill, D. L. (2003). Development of the taste system: Basic neurobiology. In R. L. Doty (Ed.), Handbook of olfaction and gustation (2nd ed., pp. 759-782). New York: Marcel Dekker.
- Netter, F. H. (1964). The CIBA collection of medical illustrations, Vol. 1 Nervous system. New York: Ciba Pharmaceutical Co.
- Physicians’ desk reference. (2006). Montvale, NJ: Medical Economics Company.
- Ross, M. G., & Nijland, M. J. M. (1998). Development of ingestive behavior. American Journal of Physiology (Regulatory Integrative Comparative Physiology, 43), 274, R879-R893.
- Temple, E. C., Hutchinson, I., Laing, D. G., & Jinks, A. L. (2002). Taste development: differential growth rates of tongue regions in humans. Developmental Brain Research, 135, 65-70.
- Witt, M., Reutter, K., & Miller, I. J., Jr. (2005). Morphology of the peripheral taste system. In R. L. Doty (Ed.), Handbook of olfaction and gustation (2nd ed., pp. 651-677). New York: Marcel Dekker.
See also:
Free research papers are not written to satisfy your specific instructions. You can use our professional writing services to order a custom research paper on any topic and get your high quality paper at affordable price.





