This sample Xenotransplantation Research Paper is published for educational and informational purposes only. Free research papers are not written by our writers, they are contributed by users, so we are not responsible for the content of this free sample paper. If you want to buy a high quality paper on argumentative research paper topics at affordable price please use custom research paper writing services.
Abstract
Xenotransplantation has a few serious bioethical issues although it is considered as a promising treatment modality for patients in diverse conditions. Described here are the current research activities in xenotransplantation conducted in the world. In addition, its bioethical and legal implications for clinical trial and clinical implication are briefly reviewed. Some representative guidelines from the International Xenotransplantation Association (IXA) and World Health Organization (WHO) are also provided.
Introduction
Xenotransplantation is defined as “any procedure that involves the transplantation, implantation, or infusion into a human recipient of either (a) live cells, tissues, or organs from a nonhuman animal source, or (b) human body fluids, cells, tissues or organs that have had ex vivo contact with live nonhuman animal cells, tissues or organs” (US Department of Health and Human Services 2003). All the procedures satisfying the definition share one peculiar thing, i.e., direct contact between living human body and living animal parts. From the perspective of healthcare regulation, the risks associated with this direct contact (especially risk of zoonotic infection) put any procedure that satisfies this definition into the criteria of xenotransplantation. However, xenotransplantation generally means the transplantation of nonhuman animal organs, tissues, or cells into human body for the purpose of curing diseases.
Organ transplantation from human donor (all transplantation) is established as an effective treatment modality for patients in diverse hopeless conditions. The developments of potent immune suppressants, improved surgical skills, and pre-/postoperation care have contributed to the success of organ all transplantation. As the number of patients in need of organ transplantation is increasing, the length of waiting list is also increasing. However, the number of source organs from brain-dead, cadaveric, or even living donors has not been increased so much to satisfy the need, which is why common animals are considered as a source of organ transplantation. The massive production of source organs for transplantation in animal stock surely will solve the global problem of organ shortage. Xenotransplantation has other merits in terms of transplantation. It could keep the source animal in the best health condition. In addition, it will screen the risk of possible harmful infection meticulously prior to transplantation. It could provide the best quality organ to the recipient any time when needed (Groth 2007). Thus, xenotransplantation has advantage over all transplantation that wholly depends on the indefinite condition of human donors.
In spite of these great merits, xenotransplantation still has many huddles to become a practical treatment modality. The hardest huddle is to moderate the immune response from the recipient that causes rejection of the xenograft. All kinds of rejection phenomena – hyperacute, acute, and chronic – could occur in xenotransplantation. However, control of the hyperacute-type rejection is the main focus of current xenotransplantation research (Ekser et al. 2012). There are two mainstreams in current xenotransplantation research. One is to modify the antigenicity of the donor organ through genetic modification of the source animal. The other is to suppress the immune responses from the recipient. Of course both methods would be applied at the same time. The second technical huddle is the requirement to make sure that no zoonotic infection would break out in xenotransplantation (Chapman et al. 1995). Many known human infectious diseases have come from other animals such as the cow, the dog, and the bird. Recently, the number of novel epidemic diseases such as AIDS, SARS, Ebola, and others are suspected of animal origin. Xenotransplantation is believed to be especially vulnerable to zoonotic infections because it enables the direct contact of living human organism with living animal cells and tissues which may have some unknown infectious agents as well as known ones. The physical vulnerability of the recipients who have been suffering from the original disease for a long time will have increased risk of zoonotic infections if they have to be treated with potent immune suppressant. Therefore, special measures are necessary to prevent the possible outbreak of zoonotic infection in xenotransplantation, which may seriously infringe basic human rights of the recipients as well as the widely accepted principles in biomedical ethics. There are also other serious issues in xenotransplantation such as the rights of the donor animals and animals used in the research, the risk-benefit evaluation of the procedure, and others. In short, xenotransplantation has raised a lot of serious and interesting issues in terms of bioethics and research ethics. It is very worthy of being discussed at the global level.
Short History Of Xenotransplantation Research
Experiments concerning xenotransplantation go back a long way historically. The pioneers of xenotransplantation realized xenotransfusions as early as the 16th century, then cell and tissue xenotransplantations in the 19th century. Most trials were failed without any knowledge of species barrier (Deschamps et al. 2005). In 1902, after the development of anastomosis, it was reported that Ulmann transplanted dogs’ kidneys into not only the same species but also in other species of animals (Carrel 1908). After that experiment, many other researchers attempted to transplant the internal organs of pigs, dogs, goats, sheep, and other animals to other species, but still with no success. However, in 1960, with the development of immunosuppressive drug, organ transplantation became possible, and, as a result, various forms of xenotransplantation were attempted. The first clinical xenotransplantation was in 1963. Reemtsma tried to transplant chimpanzee kidneys into 13 patients. However, almost all patients died in a few days after the transplantation. In the same year, there was also an attempt to transplant baboon kidneys into rhesus monkeys, but the experiment failed. In 1977, Dr. Christiaan Barnard, a South African surgeon who had performed the first human heart allotransplant in 1967, attempted to use chimpanzee and baboon hearts as bridge organs in patients who had undergone unsuccessful open heart surgery. The recipient of the baboon heart died after six hours, while the recipient of the chimpanzee heart survived for 4 days before the heart was rejected. In 1984, Bailey reported that a newborn infant with a congenital heart disease lived for 20 days after receiving a baboon kidney transplantation. In 1992, Starzl attempted to transplant a baboon’s liver into a patient with hepatitis B, but the patient died after 70 days from cerebrovascular complications. The best result of a xenotransplantation experiment happened in 1963, when one patient lived for 9 months after receiving a kidney transplant from a chimpanzee (Cooper 2012).
There are many successful cases of using cells from different species to treat patients. In 1994, Maribeth Cook, suffering from a brain stroke, volunteered to receive a transplant from a pig’s nerve cell. In all, thirty hundred million nerve cells were transplanted into her brain from a pig embryo. After the surgery, Cook was able to walk and even participate in a half marathon with leg braces. In 1999, 21-year-old Amanda Davis was paralyzed on her left side due to a brain stroke. Davis’ condition was improved after pig nerve cells were transplanted into her damaged brain, and she was able to walk without leg braces after the surgery (Waldman 2013).
Current Research Activities
Regarding xenogeneic islet transplantation used to cure diabetes, the LCT company in New Zealand suggests using an alginate capsule to avoid immune rejection responses. A clinical trial is ongoing in Argentina and New Zealand. In addition, this company capsularizes choroid plexus. There are also many experiments that use traditional methods such as minimum immunosuppressive drugs to prevent rejection response.
Several experiments seeking the cure for diabetes involved separating Langerhans islets from pigs and transplanting these into a monkey with diabetes. Currently, preclinical studies of primate species are testing the efficacy and safety of this method. In 2006, a research group led by Bernhard Hering at the University of Minnesota and another group by Christian P. Larsen at Emory University reported the results of these preclinical studies to the academic world. More similar studies had been followed. In 2009, to further progress the systemic clinical trial, the IXA and WHO established international guidelines about the necessity of safety and efficacy for the clinical application of pig islet cells.
In Korea, the transplantations of the islets and the cornea of pig are receiving international attention within the field of organ transplantation. Korea’s scientists have reported that five out of eight monkeys survived for more than 6 months after receiving pig islet transplants. Korea then took the lead to enact guidelines regarding the clinical application of xenotransplantation. At a 2013 conference on xenotransplantation in Osaka, Japan, a Korean research team suggested enacting and enforcing the IXA’s international standards on xenotransplantation clinical trials with porcine cornea (Kim et al. 2013).
On the other hand, solid xenotransplantation of the heart, kidney, liver, and lungs has not yet reached the capacity to last several hours or months (Ekser et al. 2012). It is necessary to control the hyper acute rejection to attain the successful solid organ xenotransplantation. The physiological differences between human and the source animal are another problem. To overcome these huddles, it is required to “humanize” the source animal with genetic engineering methods and cloning technology. Several research groups over the world are conducting xenotransplantation studies with genetically modified pigs. Though new techniques and methods are actively explored and introduced in this field, it may take some time to achieve the clinically compatible solid organ xenotransplantation.
Animal Rights And Treatment Of The Source Animal
In the past, nonhuman primates were used as source animal to provide solid organs such as the heart to human recipient. However, they are rarely used nowadays. Rather, nonhuman primates are used in preclinical trial of xenotransplantation as a recipient of the xenograft from other animals. The so-called monkey model is considered as a useful tool replacing the human subject. It is impossible to use a human subject for xenotransplantation research without prior experiment with animal models. However, research studies using nonhuman primates are widely criticized by animal activists and environmentalists in the world. Most nonhuman primates are in danger of extinction. Many people consider them as sociable species similar to human that should be protected and cared. Some nations have very strict legal system regulating research with nonhuman primates. Other research animals such as rats and mice are used in xenotransplantation research. But they are commonly used for general research purposes not limited to xenotransplantation. Many nations have established regulating body, i.e., the Institutional Animal Care and Use Committee (IACUC) or its compatible form for using animals for research purposes.
Besides nonhuman primates, another prospect source of animal for xenotransplantation is pig. As a source animal, pig has many advantages: (1) the size and physiology of their organs are similar to human, and some of their dead tissues of cardiac valve, vessels, and skin have already been used for medical purpose; (2) the diseases and the infectious agents of the pig have for a long time been investigated and well known by veterinarians; (3) their rearing and breeding is relatively easy, and they breed many offspring a litter; and (4) the porcine genome is thoroughly investigated, and it is easier to make genetically modified pig to knock out the porcine antigenicity.
There are three sorts of pigs to be considered for the purpose of xenotransplantation: common farm pigs, gnotobiotic or specific pathogen-free (SPF) pigs, and genetically modified pigs. Common farm pigs are generally considered as inappropriate as a source animal for human transplantation because they have many infectious agents. In addition, it is hard to control their quality consistently. Therefore, common farm pigs are usually employed in animal to animal transplantation research only. Some tissues from porcine neonates or fetuses treated in aseptic condition may be used as xenografts to human recipients, but further microbiology study is needed. Gnotobiotic or SPF (or “know pathogen-free”) pigs are specially inbred and reared in a very specific aseptic condition from birth to death. They could be candidate source animal for xenotransplantation to human because the risk of zoonotic infection would be controlled. However, the special raising condition to make SPF animals would be very stressful to the animals themselves. They have to be raised in limited space under isolated condition. Their feeding is strictly controlled. Their health condition is regularly checked up. “Pathogen-free” or “gnotobiotic” should be a mandatory condition for source animal raised for xenotransplantation. But the necessary procedures mean substantial sufferings for the animals. For genetically modified pigs, the issue of animal rights and welfare would be much more complicated. It needs sacrifices of many other animals as surrogate mother to produce a genetically modified pig through gene knockout and cloning technology. In addition, the success rate of the production of genetically modified (GM) pig is substantially low. The number of neonates would be suffered from many congenital anomalies and other problems. To be used as source animal, GM pig has to be raised in specific pathogen-free condition where the feeding, drinking, breeding, and social activities are strictly controlled. The mandatory special conditions would raise a difficult question: Can xenotransplantation be ethically justified in front of the considerable sufferings of the source animals, not to mention the animals used in the research? The answer should depend on the attitude toward animals and animal rights in a given culture and society.
Concern For Zoonosis In Xenotransplantation
The risk of outbreak of zoonotic infection or novel infection from the xenotransplantation procedure is a great concern and the major hurdle in the realization of xenotransplantation as an effective treatment modality for certain diseases. The current knowledge and understanding of the zoonotic infection is considerably limited. There are two kinds of zoonotic infection which may be associated with xenotransplantation: known infection and unknown (novel) infection. Known infections include the viruses, bacteria, fungi, and parasites normally found in the source animal (generally pig) or other agents contaminated through the xenotransplantation procedure. Most agents would not infect human under normal condition. However, the prospect recipients might be seriously ill and immunologically fragile that they are too sensitive to many infectious agents. The use of immune suppressants to prevent the rejection of the xenograft will compromise the condition. Thus, very strict cautionary process to prevent the possible “known” infection has to be prepared in xenotransplantation, including the use of gnotobiotic source animals, prior screening of known possible pathogens of the source animals and xenografts, GMP level handling of the xenografts, aseptic maneuver in the transplantation procedure, and postoperative screening and monitoring of possible infections. Most known infectious agents such as bacteria and fungi could be screened and controlled with the current knowledge and technology, although it requires high cost. But the most concerning infectious agent is “porcine endogenous retrovirus (PERV).” PERV is an RNA retrovirus that inhabits in the nucleus of the porcine cell. Therefore, it is impossible to remove them with any means. PERV may infect the cells of human host through xenotransplantation. They might be integrated into the human genome. Nobody knows the long-term effect of PERV infection. Besides the PERV issue, there may be other unknown infectious agents that could not be identified with the current technology. Furthermore, a well-known nonpathogenic agent may be mutated into a pathogenic form through the xenotransplantation procedure that permits the interaction of living cells from different species and the use of highly potent immunosuppressive drugs. Any kind of zoonotic infection would be potent to become epidemic or even pandemic disease. The consequence is much more serious in the case of novel zoonotic infection. Therefore, the risk of zoonotic infection from xenotransplantation poses a global public health issue beyond one nation’s border. In general, the so-called precautionary principle should be required to address the risk issue. In this regard, the problem is that the risk cannot be exactly evaluated with currently available scientific methods. Since the late 1980s, the wave of novel epidemics including AIDS, SARS, avian flu, and Ebola today has assaulted the global society, causing great concern to the humankind. The expectation and support of xenotransplantation research for clinical application has been occasionally discouraged by the break out of novel pandemic. Numerous measures such as rigorous quarantine and screening of the source animal as well as contamination-free procedures from the procurement to transplantation must be taken to prevent possible outbreak of zoonotic infections. The most peculiar thing to prevent an outbreak is “lifelong surveillance” of the human recipient receiving the animal cells, tissues, or organs.
Lifelong Surveillance
Lifelong surveillance (LLS) has been required as an essential regulatory element to monitor and prevent the outbreak of any zoonotic infection or other adverse events from xenotransplantation. LLS should not be limited to only the recipient but also be extended to the “close contacts” of the recipient. There is no general consensus on who should be considered as close contacts of the xenotransplantation recipient or the nature of “close contact.” But a realistic view based on other novel infections would define the close contacts as the living person who could exchange body fluid with the recipient through sexual intercourse, pregnancy, or blood transfusion. However, the current Ebola epidemic shows that simple contact with the body fluid of the infected could transmit the virus. There are many debates on how to define the close contacts in xenotransplantation.
Regular checkups for the possibility of any infection or other health events in the recipient and his or her close contacts are needed for LLS. There is no established consensus among xenotransplantation researchers regarding the period of the checkups, the admitted methods, and the kinds of suspicious agents in case of xenotransplantation (WHO 2011). However, it is evident that LLS should be applied to the xenotransplantation recipient and his or her close contacts through their lifetime regardless of the form of xenotransplantation. In addition to LLS, autopsy after the death of the recipient (and perhaps the close contacts) should be mandatorily conducted. Some of the tissues from major organs and body parts should be stored for further research and epidemic study in case of outbreak of any zoonotic infection due to xenotransplantation (WHO 2008). These things compromise the basic human rights of the recipient and his or her close contacts. In ordinary clinical trials and clinical researches, some of the rights of the human subject could be temporarily reserved by the human subject’s own will through informed consent. But LLS is beyond the ordinary practice required for common clinical trials or clinical research. Could a man make a clinical decision at a certain time in his or her life which would have influence on his or her whole lifetime as well as his or her close contacts? LLS should never be withdrawn once the xenotransplantation is conducted to the recipient to protect the whole society in terms of public health. If the recipient regrets his or her decision to get xenotransplantation, nothing would help him or her to escape from the LLS.
To execute LLS, the very private lifetime events of the recipient such as marriage, moving, bearing children, getting ill, and death should be monitored by any group in the xenotransplantation. Considering the human right issue in this matter, only the legal authoritative agency of a nation-state could conduct LLS. Moreover, the execution of the LLS should be assured though any legislation in a nation. Some form of global collaboration among nations is necessary when a xenotransplantation recipient decides to immigrate to other country to guarantee the LLS after the emigration. Supposing a country decides to provide a xenotransplantation treatment for any health condition and receives the patients from other countries, LLS will be a very complicated public health issue at the global level.
Informed Consent
Informed consent (IC) is the hardest ethical issue in xenotransplantation. First, IC could never been withdrawn once it is signed in the case of xenotransplantation. If the xenograft failed and it was removed from the recipient, he or she will never drop out from the xenotransplantation program. Any precautionary procedures including LLS and autopsy should be maintained through his or her lifetime in spite of his or her dropout. It is also true for his or her close contacts as well. Second, IC must be taken from the close contacts in addition to the recipient himself. Without the participation of the close contacts, the clinical trial (and clinical application) of xenotransplantation is impossible. Unless the safety of the xenotransplantation is fully ensured, LLS to the recipient and his or her close contacts must be maintained. Only the full agreement of the recipient and the close contacts on the whole issue of xenotransplantation could make the procedure go on as planned. Third, it is almost impossible to list up the whole necessary information and to explain them to potential recipient and his or her close contacts before the xenotransplantation begins for there are so many uncertainties regarding the procedure. In addition, some major changes in follow-up schedules and in certain preventive strategy would happen with the development of related scientific knowledge or with the change of the attitude of the society toward xenotransplantation and the risk of zoonosis. These specific requirements in IC for xenotransplantation are so different from the ethical principles ordinarily accepted in clinical trials and other clinical applications. For example, the World Medical Association’s Declaration of Helsinki article 26 reads as follows:
“In medical research involving human subjects capable of giving informed consent, each potential subject must be adequately informed of the aims, methods, sources of funding, any possible conflicts of interest, institutional affiliations of the researcher, the anticipated benefits and potential risks of the study and the discomfort it may entail, post-study provisions and any other relevant aspects of the study. The potential subject must be informed of the right to refuse to participate in the study or to withdraw consent to participate at any time without reprisal. Special attention should be given to the specific information needs of individual potential subjects as well as to the methods used to deliver the information.”
After ensuring that the potential subject has understood the information, the physician or another appropriately qualified individual must then seek the potential subject’s freely given informed consent, preferably in writing. If the consent cannot be expressed in writing, the non-written consent must be formally documented and witnessed.
All medical research subjects should be given the option of being informed about the general outcome and results of the study (WMA 2013).
The requirements for IC in xenotransplantation could not wholly satisfy this entry. In addition, the interdependent relationship between the potential recipient and his or her close contacts may compromise the genuine judgment on this matter from both sides.
For the peculiarity of IC in xenotransplantation, clinical trial or application should be limited to the patient and his or her family who are competent and mature to take responsibilities associated with the enrollment. It is better to exclude the ones that are too young or too old, mentally retarded or mentally ill, or less competent human subjects for any reason as a potential recipient for xenotransplantation. The character and the dynamic of the family should be considered as potential close contacts. They should have enough time of deliberation on the matter required by the enrollment in xenotransplantation before making the decision. All necessary information currently available should be provided to them from the very experts in this field. A scientific expert in neutral position unrelated to the xenotransplantation research team may be assigned to help the potential recipient and his or her family to make the decision. Sometimes mental status exam or interview with a psychiatrist is necessary to confirm the mental competence of all people involved and the family dynamics. Enough discussion and enough deliberation time may be essential to get IC for xenotransplantation because the decision could never be withdrawn.
To come up with the development of the science and the environmental change, additional “updated” consent may be periodically necessary from the recipient and his or her close contacts. If the recipient would marry, the new spouse should be enrolled into the xenotransplantation program as a close contact. Additional IC should be taken from her side. The whole thing makes the IC a continuous process rather than a single event. The whole process should be recorded and reserved for later use. Mutual trust and responsibility is more important for IC in xenotransplantation research than “free and autonomous decision” of the human subject in common clinical trials. The sort of IC has rarely been explored in the bioethical field. However, the character of xenotransplantation research will boldly require it.
Public Health Risk And Social Consensus
The use of xenotransplantation, if it succeeds in clinical trials, will not only influence those who need organ transplants but also the whole society. This type of influence reflects infectious diseases that can begin with animals and spread to organ receptors, or else a completely new kind of disease may develop as a result of xenotransplantation. Scientists, doctors, and beneficiaries should not limit the risks of xenotransplantation only to that of the family, but also assume that these dangers can happen to any citizen. Citizens as well as beneficiaries carry the burden of xenotransplantation’s potential dangers. Additionally, because xenotransplantation from pigs to humans or other primates involves crossing the species boundary, research procedure could go against several people’s moral and ethical standards.
Xenotransplantation research demands social consensus and appropriate regulation because it involves many complicated issues such as the possible outbreak of novel/zoonotic infections, infringement upon privacy, and the abuse of animals, especially when nonhuman primates are used in experiments. Therefore, countries that carry out xenotransplantation research have coordinated various programs to create a social consensus on xenotransplantation research.
The first inquiry on public feelings toward xenotransplantation began in the early 1990s when the idea of initiating clinical trials became realistic and a Gallup poll reported public enthusiasm. As risks of infections started to represent an obstacle for xenotransplantation in the late 1990s, the number of quantitative analyses of xenotransplantation rapidly grew in scientific journals, where sociological literature earned growing respect with an increasingly wider pace (Sobbrio and Jorqui 2014).
In the European Union, a Eurobarometer investigation deliberately established a clause about xenotransplantation between 1996 and 2002. In the United States, the National Kidney Foundation sponsored an opinion poll on the matter in 1997. Also, between 1997 and 2000, Japan released an unfavorable opinion poll on xenotransplantation. In contrast to passive forms of opinion polls, there were many ways that citizens actively expressed their opinions. In 1999, the WHO launched the Electronic Discussion Group on International Xenotransplantation Policy Considerations to report information on the current state of affairs concerning xenotransplantation and receive opinions from regular citizens. From 1996 to 1998, the United States worked on the progress of opinion collection when they wrote the first draft of “the guidelines about the matter of infectious disease through xenotransplantation.” This collection created considerable changes in guidelines on xenotransplantation. There was a consensus conference where people could set forth their own views. In November of 2000, Switzerland opened a transplantation medicine center where a citizen panel discussed issues concerning xenotransplantation.
The public initiatives launched by the Canadian and Australian governments have gone well beyond public images produced by xenotransplantation surveys. The Canadian government still seems to be willing to explore new deliberative procedures as part of their policy activities. The Australian government has simply endorsed the US and Europe’s positive attitude toward innovation after having adopted a 5-year moratorium at the end of 2009. They are apparently encouraged by existing stringent regulatory frameworks for cells and tissues as well as the promising new developments in xenocell therapies (Sobbrio and Jorqui 2014).
In recent years, at least one important initiative of public consultation has taken place, namely, New Zealand between 2006 and 2008 (National Health Committee 2008), and the European Union (EU) has systematically begun to consult European citizens and organizations through the Web in several policy matters while preparing legislations (European Commission 2013).
In Korea, xenotransplantation research has been conducted since 2004 with the implementation of various programs aiming to reach a social consensus on xenotransplantation. The consensus conference on xenotransplantation was held from April to September in 2007. A total of 14 civil panels discussed various issues of xenotransplantation through 3 preparation meetings and 1 final conference. Although the necessity of research on xenotransplantation has received enough attention, civil panels expressed their concern that the relevant regulations should be prepared prior to the adoption of xenotransplantation (Mo and Kwon 2009).
Risk-Benefit Evaluation
There is no conventional treatment yet in the category of xenotransplantation. Every trial of xenotransplantation so far has been considered as clinical research, most of which has used animal models except in a few cases. Currently, the main target of xenotransplantation research seems to be the cell or tissue transplantation rather than solid organ transplantation. From the results of animal research studies, the most prospect solid organ for xenotransplantation might be the heart. Although there is no case of human trial yet, animal (porcine) heart might be considered as “bridging solution” for very grave heart failure patient in the waiting list for human heart from brain-dead donor. For this case, the benefit is high if it is successful. But the risk is also high. If the transplanted heart fails, the patient dies. In xenotransplantation, risk-benefit evaluation in societal dimension as well as individual ones should be considered. If the risk is increased in individual dimension (as if the earlier death is expected), the risk to society (the risk of outbreak of novel infection) might be decreased.
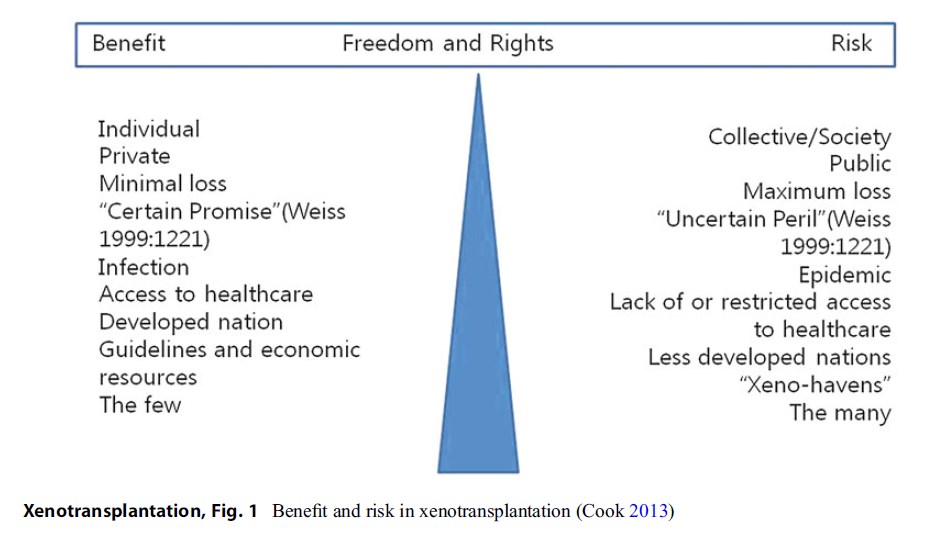 Figure 1 Benefit and risk in xenotransplantation (Cook 2013)
Figure 1 Benefit and risk in xenotransplantation (Cook 2013)
For the case of cell or tissue xenotransplantation, the recipient would experience relatively low risk. If the xenograft fails after the transplantation, the recipient could depend on alternative treatments. Diabetes itself is seldom a life-threatening condition. The recipient experiencing xenograft failure survives as in other diabetes patients in few research studies with porcine islet cell graft (Groth 2007). A serious risk will be posed when a novel infection breaks out. Such case is threatening to the whole society. It is impossible to evaluate the risk-benefit only in individual dimension in xenotransplantation. The effect to the society must be considered. That is why it is difficult to conduct risk-benefit evaluation in xenotransplantation. Cook described the benefit and risk associated with xenotransplantation in Fig. 1 to show the difficult tension arising on an individual and community basis (Cook 2013).
Ordinarily, one of the basic responsibilities of the researcher who is conducting clinical trials with human subjects and the IRB is to make sure that the ethical proceeding of the research evaluates the risk-benefit to “the individuals” enrolled in that research. However, in xenotransplantation trials, the risk-benefit evaluation on the societal dimension is mandatory for its nature, which is very different from other clinical trials. The question is: Is it really possible? If possible, who will do it? To answer these questions, public engagement is necessary. A decision at global level might influence all people on earth. Yet the public engagement on such global scale in the field of medicine and bioethics is hardly available.
Other Issues
-
Human Identity Problem
Some critics are concerned about the identity issue that might be raised from the xenotransplantation procedure, especially in solid organ xenotransplantation. For example, the heart has been thought as a very privileged organ in the human body from the past. Many people still think so. In all transplantation of the heart, some recipients are reported to experience certain personality change after the transplantation. The thought that an animal (porcine organ) functions in one’s body may have influence on self-image and identity. If other people know the fact, their view on one’s human identity might be somewhat changed, which might cause social discrimination.
Of course human identity is a complicated phenomenon, not just limited to the physical composition of the body. It is widely witnessed that many disabled and handicapped people live well by depending on many kinds of prosthesis and medical aids, including people who received all transplantation of other’s organ. It may be true for recipients who receive xenotransplantation. But the possibility of the distortion of the self-image and self-repugnance resulted from the xenotransplantation procedure should be considered. Therefore, it is necessary to discuss this thoroughly with the potential recipient and his or her family prior to the procedure. Psychiatric evaluation may be helpful for that purpose.
In the case of cellular or tissue xenotransplantation, the concern about the distortion of self-image would be little in comparison with solid organ xenotransplantation. Porcine insulin and other tissue grafts from nonhuman animal have been successfully applied to human patients without any psychological trouble. LLS and other strict regulations after the xenotransplantation procedure are much likely to trigger psychological stressful condition and social discrimination rather than the procedure itself in cellular or tissue xenotransplantation.
-
Xenotourism
The public attitude toward xenotransplantation is different from country to country. It is very hard to expect the development of any uniform regulatory framework for xenotransplantation at a global level considering the nature of legislation in each country. For the time being, the regulatory condition is different from country to country. Some countries permit it, while others prohibit it for many reasons. Actually the situation is more problematic in many developing countries, where unproven and unscientific “therapies” arbitrarily implant living animal cells or tissues into patient body in the name of traditional or folk medicine. Such procedures should be dismissed immediately. But a few governments have no will or power to do so.
When a xenotransplantation procedure is established as an effective therapeutic modality in a country while other countries ban it for public health reasons, some patients from the latter country may pursue xenotransplantation treatment in the country that permits it. There is no effective tool to prohibit the “xenotourism” when the xenotransplantation treatment is really effective or better than other alternatives. There are already many examples. Commercial trade of human organs is illegal in many countries, but global organ trafficking is still prevalent. Some patients visit other countries to receive “stem cell treatment” which is banned in their own country. However, xenotourism is much more alarming because it might cause confusion in the global communicable disease control system. The quarantine agency of each country should give attention to their citizens who receive xenotransplantation procedure when xenotourism is realized. Global inequality to access an effective medical treatment is also considered in the issue. However, the problem here is not only healthcare resources but also money.
-
Cost-Effectiveness of Xenotransplantation
Although xenotransplantation may become technologically feasible someday, it is hard to say that the treatment is clinically feasible or ordinarily accessible. The exact cost associated with the xenotransplantation procedures could hardly be assessed now. It may cost a lot to produce and raise the source animal (pigs) in the gnotobiotic environment. The cost should also include the money to make genetically modified pigs. The screening and monitoring to prevent outbreak of novel infections would require high cost, not to mention the money necessary for the transplantation itself as well as for immune suppression. The demand of LLS makes the situation more complicated. Alternatives seem to be less expensive. For example, the price of a heart from human brain-dead donor is principally free. The recipient only pays the cost for transplantation and postoperation care. For islet cell transplantation, the alternative is the conventional insulin treatment. Of course, xenoislet cell transplantation is expected to have some merit to enable the normoglycemia of the recipient for some period of time. But considering the necessary cost, xenoislet transplantation would be a much more expensive treatment that not so many people can afford it. Many countries are facing financial problem due to rapidly increasing healthcare cost. Cost-effectiveness of a treatment is becoming the primary concern of policy makers. Therefore, xenotransplantation may not be a good clinical option from the view of healthcare system runners besides its issue of zoonotic infection.
Conclusion
Xenotransplantation promises a fantastic treatment of changing any morbid organs without any serious risk or ethical issues associated with the all transplantation. Organ trafficking and transplant tourism are uncomfortable reality due to the shortage of human organs for transplantation. Compared to other alternatives such as stem cell therapy or tissue-engineered organs, xenotransplantation is expected to “succeed” technologically in the near future. Some promising technology such as induced pluripotent stem cell would produce better result when combined with xenotransplantation. However, the concern of its influence on the third party in the form of zoonotic infection casts a shadow on xenotransplantation research. Partly for this reason, xenotransplantation is still in a preclinical stage using animal models in most countries. The Changsha Communique declared at the first WHO Global Consultation on Regulatory Requirements for Xenotransplantation Clinical trials (November 2008) stipulated the basic ethical principles for xenotransplantation clinical trials. The communique lists up 10 basic principles and makes some recommendations to the WHO, the member states of the WHO, and investigators of xenotransplantation. For conclusion, the principles of the Changsha Communique are included.
- Successful xenotransplantation has the potential to treat a wide range of serious diseases such as diabetes and heart and kidney disease. Successful xenotransplantation could provide transplants for people who currently would not get a transplant.
- Potentially animals could provide a plentiful supply of readily available, high-quality cells, tissues, and organs for transplantation. Genetic modification of the animals may improve the effectiveness of such xenotransplant material. Animals used in xenotransplantation should be from a closed herd bred for the purpose and housed in a well-controlled, pathogen-free environment with high standards of animal welfare.
Source animals should be extensively tested to ensure freedom from known pathogens with appropriate biosecurity and surveillance in place to ensure continued freedom from infectious disease.
- Xenotransplantation is a complex process which carries risks, including graft rejection, inadequate graft function, and transmission of recognized or unrecognized infectious diseases to the recipient. There is the risk of developing serious or novel infections which could infect not just the transplant recipient but also close contacts or the wider human or animal populations.
- Because of these wider community risks, xenotransplantation clinical trials and procedures need to be effectively regulated. There should be no xenotransplantation in the absence of effective regulation by the government of the country. Regulation should have a legal basis with powers to ban unregulated procedures and enforce compliance with regulatory requirements. The regulatory system should be transparent, must include scientific and ethical assessment, and should involve the public.
- Because of the community risk, in proposed clinical trials of xenotransplantation, there should be a high expectation of benefit to balance the risk. The level of this expectation should be in proportion to the level of the risk. The level of safety and efficacy should conform to recommendations from the international scientific community, when available, and requires rigorous preclinical studies using the most relevant animal models. Proposers of trials must provide all the information required by the regulatory authority to assess the risks and determine how the risks can be minimized.
- Proposers of xenotransplantation clinical trials must be able to clearly justify carrying out a particular trial on a specific patient population. Patient selection should be on the basis of informed consent from motivated patients willing to accept the special conditions that will be required by the trial. Patients and close contacts should be effectively educated about their treatment to encourage compliance and to minimize risks for themselves and for society.
- Participation in xenotransplantation will usually require the long-term storage of animal and patient samples, pre and post-treatment, as well as records. It will require lifelong follow-up of recipients and possibly their close contacts. There must be rigorous analysis of trial outcomes. Xenotransplant product recipients must be registered in an appropriate database with traceability to the donor animal while ensuring that patient privacy is protected. If anything happens to prevent the proposers from continuing the trial, there must be an adequate provision for all records, data, and archived samples such as their transfer to the regulatory authority or other designated organizations.
- Medical teams must have appropriate expertise and understand the risks to the patients, themselves, and the community. Because of the risk of infectious disease for the community, there must be a system in place for vigilance and surveillance with contingency plans to identify and respond to any indication of xenotransplantation-related infection in a timely manner.
- There needs to be a global system for exchanging information, preventing unregulated xenotransplantation, providing support for states, and coordinating xenotransplantation vigilance, surveillance, and response to suspected infections.
- Because of the potential benefits of successful xenotransplantation, consideration should be given from the beginning to future equitable access to this therapy, and the public sector should be encouraged to support xenotransplantation research and development.
Bibliography :
- Carrel, A. (1908). Transplantation in mass of the kidneys. The Journal of Experimental Medicine, 10(1), 98–140.
- Chapman, L. E., Folks, T. M., Salomon, D. R., Patterson, A. P., Eggerman, T. E., & Noguchi, P. D. (1995). Xenotransplantation and xenogeneic infections. New England Journal of Medicine, 333(22), 1498–1501.
- Cook, P. (2013). The social aspects of xenotransplantation. Sociology Compass, 7(3), 237–254.
- Cooper, D. K. (2012). A brief history of cross-species organ transplantation. Proceedings (Baylor University. Medical Center), 25(1), 49–57.
- Deschamps, J. Y., Roux, F. A., et al. (2005). History of xenotransplantation. Xenotransplantation, 12(2), 91–109.
- Ekser, B., Ezzelarab, M., Hara, H., van der Windt, D. J., Wijkstrom, M., Bottino, R., Trucco, M., & Coope, D. K. C. (2012). Clinical xenotransplantation: The next medical revolution? Lancet, 379(9816), 672–683.
- European Commission, Public Health. (2013). Medicinal products for human use. Advanced therapies. Available at: http://ec.europa.eu/health/human-use/advancedtherapies/developments/index_en.htm. Accessed 19 July 2013.
- Groth, C. (2007). The potential advantages of transplanting organs from pig to man: A transplant surgeon’s view. Indian Journal of Urology, 23(3), 305–309.
- Kim, M. K., Lee, J. J., et al. (2013). Ethical and regulatory guidelines in clinical trials of xenocorneal transplantation in Korea; the Korean xenocorneal transplantation consensus statement. Xenotransplantation, 20(4), 209–218.
- Mo, H., & Kwon, I. (2009). Citizen consensus conference as a way of collecting social opinions on xenotransplantation issues. Bioethics Policy Studies, 3(1), 91–109.
- National Health Committee’s Advice on Living Cell Technologies Application for Xenotransplantation Clinical Trials in New Zealand. (2008). Available at: http://nhc. health.govt.nz/system/files/documents/publications/nhcliving-cell-technologies-report-oct082.pdf. Accessed 5 May 2013.
- Sobbrio, P., & Jorqui, M. (2014). An overview of the role of society and risk in xenotransplantation. Xenotransplantation, 21(6), 523–532.
- US Department of Health and Human Services. (2003).
- Guidance for industry: Source animal, product, preclinical, and clinical issues concerning the use of xenotransplantation products in humans. Available at: https://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Xenotransplantation/UCM533036.pdf Accessed 10 Oct 2014.
- Waldman, J. P. (2013). Mechanisms of Human Erythrocyte Clearance During Human-to-PorcineLiver Xenoperfusion and the Assessment of Current Pre-clinical Models. The University of Toledo, Dissertation. Available at: http://gateway.proquest.com/open url?url_ver=Z39.88-2004&res_dat=xri:pqdiss&rft_val_ fmt=info:ofi/fmt:kev:mtx:dissertation&rft_dat=xri:pqdi ss:3613257. Accessed 10 Oct 2014.
- (2008). The Changsha Communique. First WHO Global consultation on regulatory requirements for xenotransplantation clinical trials. Changsha, 19–21 Nov 2008.
- (2011). Second WHO Global consultation on regulatory requirements for xenotransplantation clinical trials. Geneva, 17–19 Oct 2011.
- (2013). Declaration of Helsinki-Ethical Principles for medical research involving human subjects. (Last amendment at 64th WMA General Assembly, Fortaleza, Oct 2013).
- Fovargue, S. (2012). Xenotransplantation and risk: Regulating a developing biotechnology. New York: Cambridge University Press.
- Nuffield Council on Bioethics. (1996). Animal-to-human transplants: The ethics of xenotransplantation. London: Nuffield Council on Bioethics.
See also:
Free research papers are not written to satisfy your specific instructions. You can use our professional writing services to buy a custom research paper on any topic and get your high quality paper at affordable price.





