This sample Chagas Disease Research Paper is published for educational and informational purposes only. If you need help writing your assignment, please use our research paper writing service and buy a paper on any topic at affordable price. Also check our tips on how to write a research paper, see the lists of health research paper topics, and browse research paper examples.
Introduction
Linguistic, paleoparasitologic, and artistic information suggests that the domiciliation of triatome bugs and the transmission of Trypanosoma cruzi have pre-Columbian antecedents in the Andean region of South America. T. cruzi infection has been detected in Peruvian and Chilean mummies (Guhl et al., 1999) of 500 to greater than 4000 years of age. Several words describe the domestic vector in the languages of the ancestral Andean cultures; ‘vinchuca’ or ‘quechua,’ both mean ‘something that falls.’ The Spanish chronicles of the sixteenth and seventeenth centuries referred to the domestic triatome, especially in Argentina, Bolivia, Chile, and Paraguay. Charles Darwin, who may have been a victim of Chagas’ disease (CD), described the domestic vector in his paper on the journey of the British ship Beagle, while visiting Mendoza, Argentina. In 1835 Darwin narrated his encounter with the benchugas (or vinchucas, i.e., Triatoma infestans) as follows:
At night we were attacked (there is no other way to call it) by Benchugas, a type of Reduvius, which is the large black bug from the Pampas. It is disgusting to feel that 1-inch long soft and wingless insect crawling on one’s own body. Before sucking, the insect is quite thin, but afterwards it gets plump and filled with blood. When we placed it on a table, and in spite of being surrounded by people, the insect immediately protrudes its ‘‘sucker’’, makes a charge against its target and, if allowed, sucks blood in. There is no pain. It is curious to see the insect’s body during the sucking act. Its body, as flat as a wafer, takes a globule-like shape. This is the party in which the benchuga got obliged to one of the officials. It was then a fat bug for several months, but within 2 weeks the insect is ready to suck again.
Chagas’ Disease
American trypanosomiasis was described by Carlos Justiniano Riveiro das Chagas (Chagas, 1909) as a human nosological entity. Twenty years later Salvador Mazza proved the endemic extension of the disease in Argentina. CD is a wild animal zoonosis caused by Trypanosoma (Schizotrypanum) cruzi. The parasite bug is passed on to domestic animals and humans largely through domesticated triatomine (Rhodnius, Triatoma, and Panstrongylus).
Man’s encroachment on the natural environment led to major ecological disbalance, triatomine adaptation to human dwellings, and genetic adaptation and simplification of the wild, peri-domestic and domestic cycles. Determinants of triatomine-man contact and transmission include intensity of household infestation, triatomine anthropophily, defecation time, evacuation time, quantity and form of evacuated parasites, and propensity of bug feces to cause allergic reactions and pruritus. Vertical, oral, blood transfusion–induced, organ transplant–induced, and lab-accident transmission have become more important as vector control programs have reduced or eliminated vectorborne transmission.
Development of T. cruzi infection as an endemic disease was a consequence primarily of the adaptation of the insect vectors to human dwellings. That process was a result of human activity during the occupancy of new territories under precarious living conditions and the need to clear forest areas to produce food during the last 300 years. Specifically, certain types of substandard, human-dwelling conditions favor triatomine colonization. Other factors are the triatomine biological characteristics, such as phototropism, hematophagy, and thigmotaxism. The pathway from the primitive infection of animals by T. cruzi to human infection was a result of vectors being chased from their wild ecotopes to artificial ecotopes having new food sources.
The T. cruzi transmission zones are located in rural, dry, and tropical climates of the Americas from Mexico to Argentian and Chile in general, with similar domestic and peridomestic buildings and landscapes, as shown in Figure 1.

Etiologic Agent
T. cruzi is a hemoflagellate protozoan parasite that alternates between a vertebrate host and an invertebrate host during its life cycle. It is classified as follows: Kingdom: Protista Subkingdom: Protozoa Phylum: Sarcomastigophora Class: Zoomastigophora Order: Kinetoplastidae Family: Trypanosomatidae Genus: Trypanosoma Subgenus: Schizotrypanum Species: T. cruzi T. cruzi has a kinetoplast, a mitochondrial DNA structure, and a flagellum. The location of the kinetoplast and flagellum varies among the different developmental forms: amastigote in the vertebrate host, epimastigote in the invertebrate host, and trypomastigotes in both the invertebrate host and vertebrate host, as shown in Figure 2.
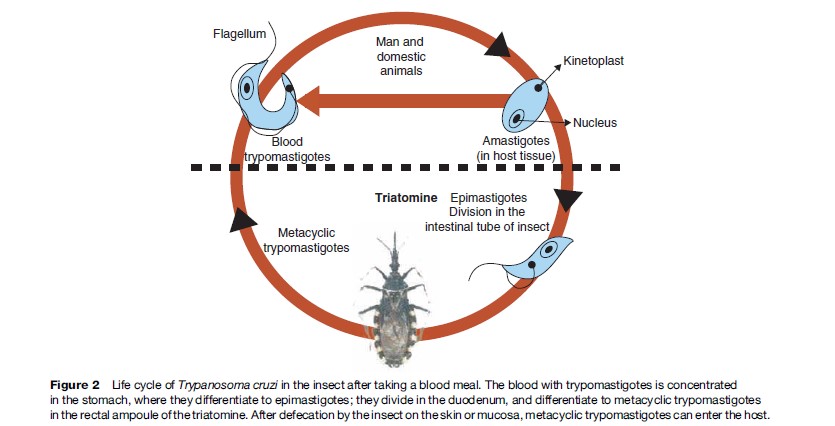
Biodiversity In The T. Cruzi Species
The study of different antigenic or biological properties in parasites isolated from different patients provided insight into the different clinical manifestations and response to treatment. Isoenzyme analysis was the method of choice for the first attempt at biochemical characterization of T. cruzi strains. Three zymodemes (Z1, Z2, and Z3) were identified, and subsequent studies showed the correlation between zymodemes Z1 and Z3 with cycles of wild transmission of T. cruzi and Z2 with domestic transmission of the parasite. Despite great variability found by isoenzyme analyses of hundreds of strains and clones of T. cruzi, populations of the parasite have been characterized as belonging to two major lineages – lineage 1 and lineage 2 – on the basis of dimorphism observed in sequences of gene 24 rRNA and of the nontranscribed spacer of the mini-exon gene. This region is identified by initiators TC, TC1, and TC2 that amplify 300 base pair (bp) (T. cruzi lineage 1) or 350 bp (T. cruzi lineage 2). Lineage 1 was shown to be identical to zymodeme 2 and lineage 2 corresponded to zymodeme 1. Most of the TC lineage 2 strains of T. cruzi were isolated in patients with chronic CD in countries of the Southern Cone of the American continent. In a group of 67 strains isolated in the Brazilian states of Amazonas, Para´ıba, Piau´ı, and Minas Gerais, 91% corresponded to T. cruzi lineage 2, and 9% to T. cruzi lineage 1. T. cruzi lineage 1 has been mainly associated with the wild transmission cycle. In Northern Argentina two specimens of T. infestans were found to be infected with TC1, 24 specimens with TC2e and one with TC2d. In one specimen there was a mixed TC1 + TC2 infection in the fecal sample, although corresponding culture only showed TC2, thus providing evidence of the advantages of direct typing of biological samples. This updated lineage identification of T. cruzi in field-collected Triatominae showed that T. cruzi lineage 2 strains are predominant in the northern region of Argentina (Marcel et al., 2006).
In Colombia, Venezuela, and Central American countries a second trypanosome, Trypanosoma rangeli, infects humans. It is considered nonpathogenic for humans and other vertebrate hosts. Previous studies showed a significant genetic difference between T. rangeli strains from Colombia, Venezuela, and Honduras, and strains isolated in the south of Brazil using kDNA probes, DNA fingerprinting, and isoenzyme and RAPD analyses.
Invertebrate Vectors Of T. Cruzi
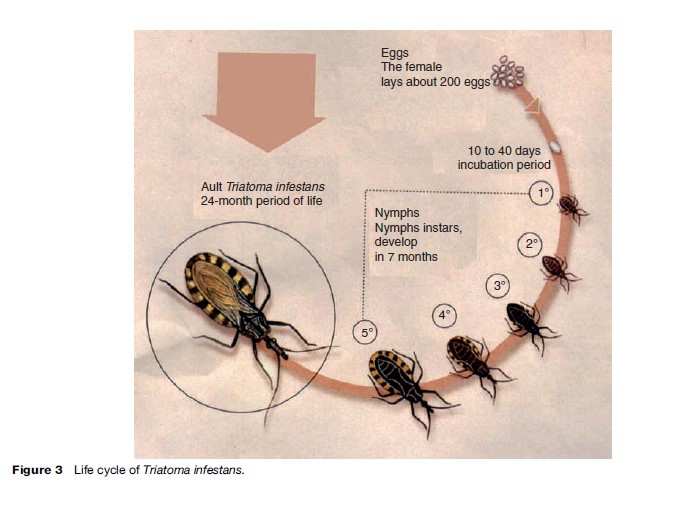
These insects belong to the order Hemiptera, family Reduviidae, and subfamily Triatominae. They are widely distributed in tropical and subtropical regions on the American continent, from south of the United States down to the northern and central regions of Chile and Argentina. Sixteen genera and more than 130 species have been described, as reported by the World Health Organization (WHO) (2002: i–vi, 1–109). These hematophagous insects lay up to 200 eggs during their lives (around 24 months), and evolve through five nymphal stages (Nymphs 1, 2, 3, 4, and 5) from egg hatching until reaching the adult stage (Figure 3). Triatomine bugs are obligate blood feeders from birth. Once infected with T. cruzi from an infected animal or human being, the insect keeps the T. cruzi in its intestine for life.
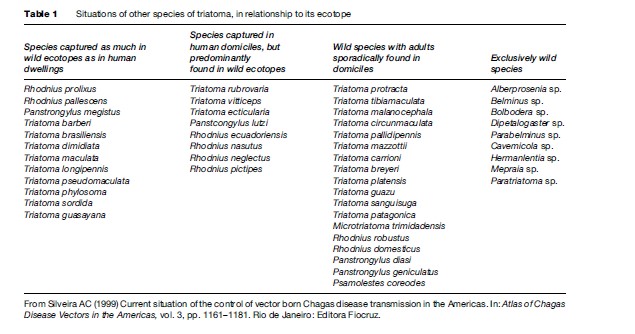
For the greater than 100 proven vectors, and potentially vector species, the ecological conditions that influence the parasite transmission pattern vary widely. Some vectors are exclusively domiciliated, such as T. infestans and Triatoma rubrofasciata. The situations of the other species of triatomae are shown in Table 1.
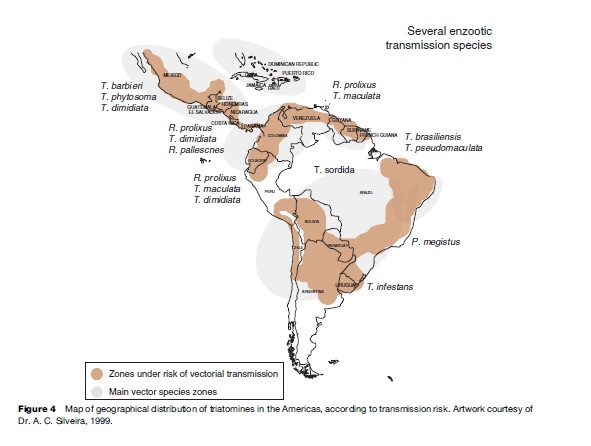
The most important species of the triatomidae transmitting T. cruzi to human beings are: T. infestans, Triatoma brasiliensis, Triatoma pseudomaculata, Triatoma sordida, Panstrongylus megistus, Triatoma dimidiata, and Rhodnius prolixus. The geographical distribution of the area of risk transmission of T. cruzi is shown in Figure 4.
Transmission Routes And Biological Cycle
In endemic zones, T. cruzi is mainly transmitted through the Triatominae vector. Other frequent transmission routes are blood transfusions due to the existence of infected donors who are unaware of their infection status. Congenital transmissions take place during gestation although it also can occur rarely during delivery. In organ transplants, transmission occurs when a donor is infected with T. cruzi; transmission is facilitated by the immunosuppression to prevent rejection of the transplanted organ. Other nonvectorial transmission is accidental transmission, which occurs when manipulating lab material infected with T. cruzi, accidents suffered by surgeons or other health workers, as well as transmission risk of injecting drug users sharing needles. Oral transmission caused by contaminated food and drink has been reported in sporadic human microepidemics in Brazil (Shikanai-Yasuda et al., 1991).
Epidemiology
In the Americas, there are approximately 16 million patients with chronic CD. This is a zoonosis that constitutes the fourth largest tropical disease burden only after malaria, tuberculosis, and schistosomiasis, as reported by Moncayo (2003).
The vectors of T. cruzi are much more widely spread than human cases of infection. Triatominae distribution is not limited to the American continent, where the presence of vectors, either actual or potential, was delimited between latitude 40 north and latitude 45 south. Specimens have been found at altitudes of up to 3000 meters above sea level. Natural human transmission of the disease has been observed from south of the United States, where several cases have been reported, down to the province of Chubut, Argentina. Vectorborne transmission in different areas is related to insect feeding and defecation habits, insect genetics and population dynamics, and infectivity of parasites.
The limits of the areas where the disease occurs, or where its transmission is endemic, are determined by or dependent on natural – primarily economic – variables. In Carlos Chagas’ own words, ‘‘this sanitary problem offers practical difficulties, all of economic order. The problem is linked to development, work, agriculture prosperity, soil settlement’’ (Chagas, 1909). As a consequence, disease distribution is often focal, although there can be extensive geographic areas with risk of transmission in homes, especially in areas of widespread, low income and limited social services.
Since the 1950s and 1960s, studies have shown a close correlation between infestation with triatomine bugs, the prevalence of T. cruzi infection, and heart disease, which can be attributed to CD, as described by Rosenbaum and Cerisola (1961). Additionally, because of increasing migration, no region is exempt from the occurrence of local transmission caused by blood transfusion, congenital transmission, organ transplantation, or by the use of needles shared by injecting drug users.
Cardiomyopathy secondary to T. cruzi infection is the most common form of nonischemic cardiomyopathy worldwide. The most recent figures provided by the WHO indicate that 40 million persons are exposed to infection, approximately 200 000 new cases occur per year, 16 million persons are currently infected, 20 to 30% of infected individuals will eventually develop cardiomyopathy, and 21 000 persons will die each year from CD.
Transmission of T. cruzi by blood transfusion results in the presence of trypomastigotes, albeit in low numbers, in a large number of persons in the chronic stage of CD. Transmission is possible through transfusion of whole blood as well as through blood components, such as platelets, leukocytes, frozen plasma, and cryoprecipitates. In the Southern Cone countries, approximately 6 million persons donate blood every year. According to Schmunis et al. (1998), in countries participating in the Southern Cone Initiative, the risk of transmission via donation of blood products varied from 219 out of 10 000 in Bolivia, 49 out of 10 000 in Peru, and 2–24 out of 10 000 in other countries. Donor selection and quality assurance in diagnostic tests used for screening donors account for varying numbers of transfusion-associated cases (Cura and Segura, 1998).
Due to an increase in the number of serologically screened blood donors and deferral of infected donors, as well as in the number of homes treated with insecticides in Argentina, Brazil, Chile, Uruguay, and Paraguay, there has been a steady decrease in the prevalence of positive serology for T. cruzi in blood collected in blood banks. In these countries, there also has been a decline in the number of infected pregnant women when compared to historical data. In other endemic countries, the prevalence of T. cruzi in pregnant women varies from 5 to 40%, depending on the geographical area. Migrations of persons from rural areas to areas surrounding the cities, where triatomines are not found, have facilitated detection of congenital T. cruzi infection because transmission by vectors can be ruled out. Although presumptive diagnosis of congenital infection can be made on clinical and epidemiological grounds, a case can be confirmed as congenital only if: (1) the baby is born from a mother with T.cruzi infection, and (2) either parasites are identified at birth, or specific antibodies are detected at birth, and such antibodies persist after 6–10 months of life or otherwise can be shown to originate in the infant and not the mother (Blanco et al., 2000). When antibodies are detected in a child born in a nonendemic area of a seronegative mother, transfusion-associated transmission must be ruled out.
In the Southern Cone countries the transmission rate of congenital T. cruzi infection, that is, the number of congenital cases/mothers infected with T. cruzi, varies widely, ranging from 1 to 12%. These differences have been discussed at length and attributed to possible special characteristics of the infecting parasites; differences in the host’s immunological, genetic, or nutritional status; specific epidemiological situations; or the different methodologies used for detection of congenital cases. PCR (polymerase chain reaction) is an efficient tool for diagnosis of infection in a newborn from a T. cruzi-infected mother, as demonstrated in Paraguay by Russomando et al. (2005).
In most studies of the last 20 years, congenital infection in most cases was asymptomatic. However, the Bolivian report of Torrico et al. (2004) indicates that symptomatic cases accounted for about 50% of overall congenital cases, and infected infants have a mortality rate of 6%.
Immunity To T. Cruzi
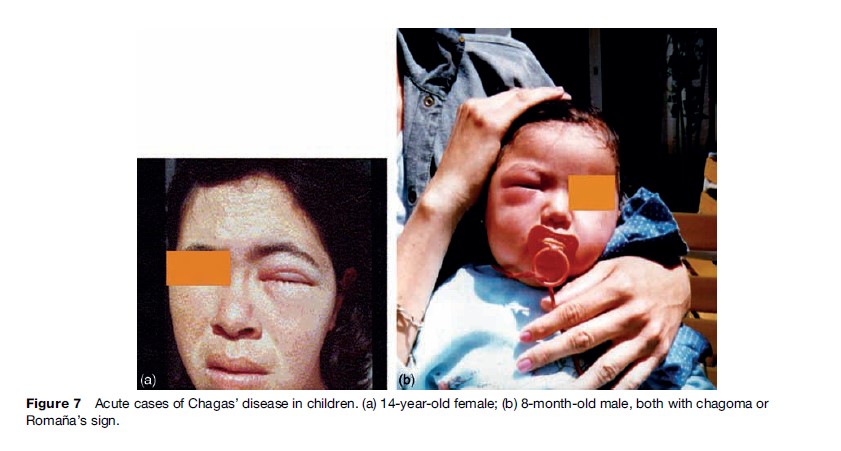
At the beginning of the infection, trypomastigotes deposited on the injured skin or on the mucous membranes enter macrophages and stimulate a local inflammatory reaction, the inoculation chagoma and/or Roman˜ a’s sign. Both the inoculation chagoma and Roman˜ a’s sign are specific to CD in its acute stage, although they occur in less than 5%.
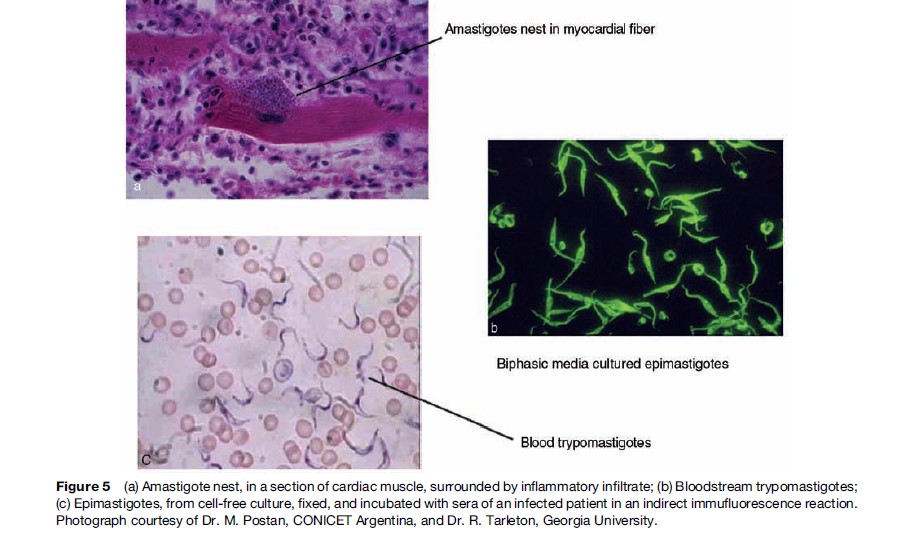
Parasites invade macrophages at the site of inoculation, replicate as amastigotes, transform to trypomastigotes, and are released into the bloodstream from where they invade cells in the liver, spleen, lymph nodes, and skeletal and cardiac muscle (Figure 5). Replication of amastigotes in the myocardium leads to acute myocarditis with lymphocyte-monocyte infiltration, mediated by T CD4+ and CD8+ cells and IL2 and IL4 cytokines. The chronic inflammatory reaction leads to muscular and neuronal destruction, and is maintained by the presence of T. cruzi or T. cruzi fragments. The inflammatory reaction, damage to the microcirculation, and fibrosis cause chronic myocardial dilation, congestive heart failure arrhythmias, dysperistalsis, megaesophagus, and megacolon. Prevalence of these two last clinical conditions is lower in Argentina and Uruguay than elsewhere in the Southern Cone, but neither occurs in infections acquired north of the Amazon.
The presence of two developmental stages of T. cruzi in mammalian hosts provides two anatomically and antigenically distinct targets of immune detection: the trypomastigotes in the bloodstream and the amastigotes in the cytoplasm of infected cells. Thus, it would be expected that immune control of T. cruzi might require a combination of different immune responses effective against these stages, and experimental data provide strong evidence for this. At a minimum, the generation of a substantial antibody response, and the activation of both CD4+ and CD8+ T cell compartments are needed to prevent death from an overwhelming acute parasitemia as demonstrated by Tarleton et al. (1996). Additionally, these same responses are probably required to maintain control of T. cruzi during the chronic phase of the infection. The evidence for the latter comes from experimental models and natural human infections in which targeted depletions of immune cells, immunosuppressive treatments, or infection-induced immunosuppression result in an exacerbation of a chronic infection.
Three factors are likewise associated with the development (or not) of severe disease: (1) parasite burden, (2) effectiveness of the host immune response in controlling parasites in specific tissues, and (3) effectiveness of the host immune response in controlling damage to tissues. Parasite burden is, in turn, largely dependent on how effective the host immune response is in killing parasites or limiting parasite replication. When immune control is inefficient, parasite load and also inflammation – and therefore the potential for tissue damage – increase.
In the past, many investigators believed that Chagas’ heart disease was the consequence of an autoimmune process. However, the results of immunosuppression on CD are contrary to what would be predicted if CD were an autoimmune disease; immunosuppression should reduce the disease severity, but paradoxically, inflammation increases despite immunosuppression. It is possible that the effects of induced immunodeficiency regarding the development of CD may represent an accelerated and intensified version of what happens in the minority of T. cruzi–infected patients who develop severe acute CD; when one effector arm of the immune response is blocked or even suboptimal in efficiency, a compensatory but less effective and therefore potentially more damaging immune response takes its place.
There are no data that can absolutely rule out autoimmunity as having a role in disease development and being a primary cause of pathology. One area of agreement reached in recent years is that parasite persistence is required for the damage to occur. Whatever its triggering mechanism, one goal seems clear: patients will benefit from a reduced parasite burden. It is clear that this cannot be achieved by targeting autoimmunity as the problem in CD. A reduction in parasite burden can be achieved not only through the discovery and implementation of better chemotherapeutics, but also with the use of immunologicals, including therapeutic vaccines, to enhance host immune control of the infection.
Chronic T. cruzi infection results in tissue-specific damage focused primarily in the gut and heart and associated with the persistence of parasites in these disease sites. Tissue-specific control of T. cruzi infection is evidenced by Postan et al. (1983), early in the acute infection stage and is even more obvious in the chronic stage, with persistence of parasites most commonly restricted to muscle and, to a lesser extent, the central nervous system (CNS). It is not at all clear what factors allow for parasite clearance from some tissues but not from others. Parasite strain-specific factors almost certainly contribute. But it is unlikely that tissue restriction is due solely to differential tropism of distinct strains as parasites are widely distributed in most if not all tissues and organs early in the infection, before the full development of B and T cell responses. More likely, the tissue-specific persistence of T. cruzi in the chronic infection is related to qualitative and quantitative aspects of the local immune responses in these tissues.
Better knowledge of the immune response to the parasite will provide a basis for diverse approaches to vaccine development including: identification of the target molecules as potential vaccine target candidates, a therapeutic vaccine for T. cruzi, and/or transmission blocking vaccine for T. cruzi.
Pathophysiology
Organ damage arising during the acute phase is closely related to high-grade parasitemia and parasite presence in target organs (i.e., gastrointestinal tract, CNS, and heart). As the parasitemia abates and the systemic inflammatory reaction subsides, silent, relentless focal myocarditis ensues during the indeterminate form of CD. In predisposed hosts, encompassing approximately 20–30% of the infected population, this chronic myocarditis evolves to cumulative destruction of cardiac fibers and marked reparative fibrosis.
Apart from the possible ancillary role of neuronal depopulation and microvascular derangements as mechanisms of myocarditis, evidence gathered from physiopathological studies in animal models and in humans is consistent with two prevailing hypotheses to explain the pathogenesis of chronic CD: (1) T. cruzi infection induces immune responses that are targeted at host tissues and are independent from the parasite persistence (the so-called autoimmune hypothesis), and (2) parasite persistence at specific sites in tissues of the infected host results in chronic inflammatory reactions (the parasite persistence hypothesis).
The use for diagnostic techniques such as PCR and monoclonal antibodies provides evidence of the presence of the parasite (or fragments of it) or of T. cruzi antigens in human tissues in inflammatory foci with higher concentration of lymphocytes.
Clinical Evolution Of CD And Diagnosis
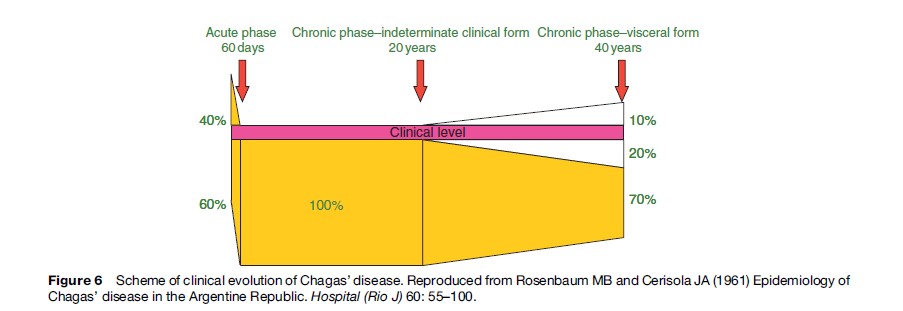
CD has two clinical phases: acute and chronic. The acute phase is undiagnosed in more than 90% of vectorborne cases. It can be symptomatic, oligosymptomatic, or asymptomatic. Some of the most frequent symptoms are febrile syndrome of long duration and lymphadenopathy. Some typical, though less frequent, symptoms are: Romana’s sign (unilateral bipalpebral edema), inoculation chagoma, hematogenous chagoma, and lipochagoma. Clinically overt, acute myocarditis or meningoencephalitis develops in approximately 1% of cases, mainly in children, and about one-tenth of cases are fatal (Lugones, 2002) (Figure 6).
Acute Phase
During the acute phase, diagnostic methods for T. cruzi infection focus on detecting the presence of the parasite or of specific T. cruzi antibodies. Some parasitological methods are concentration methods such as capillary microhematocrit, Strout, and other less sensitive methods, such as examination of a drop of fresh blood. These are useful during the acute phase of the infection, when parasitemia level is high (Figure 7).
Immunodiagnosis consists of serological reactions used to detect circulating antibodies to T. cruzi, primarily immunoglobulin G (IgG). IgG becomes detectable about 30 days after infection, and reaches maximum levels in the third month. Immunoglobulin M (IgM) is generated earlier, but is not always detectable. The absence of IgM antibodies against T. cruzi, epimastigote-derived antigens does not exclude infection. ELISA (enzyme-linked immunosorbent assay), IFA (indirect immunofluorescence), IHA (indirect hemoagglutination), gel particle agglutination, and – more recently – immunochromatography tests are used for IgG detection.
Following the acute phase, the great majority of infected people remain asymptomatic and with no clinical evidence of structural disease during the so-called indeterminate stage of CD. Most patients remain in this stage of CD for life, but antibodies to T. cruzi and low-grade parasitemia persist unless they are treated.
Approximately, 10–30% of infected patients after 2 or more decades of infection develop symptoms and signs of cardiac and digestive disease (Rosenbaum, 1961). To determine the presence of Chagas’ heart disease, an electrocardiogram (ECG) should be performed, and when abnormal, a chest radiograph or echocardiogram should be performed as well. Cardiac manifestation of CD includes abnormalities of the ventricular conduction system, ventricular arrhythmias, sinus node dysfunction, cardiac enlargement and dysfunction, heart failure, and left ventricular aneurysm. Of note, there is evidence from autopsy and biopsy studies indicating that subclinical, parasite-related myocarditis is present in 30% or more of subjects in this stage of CD who will never develop symptomatic disease.
Chronic Phase
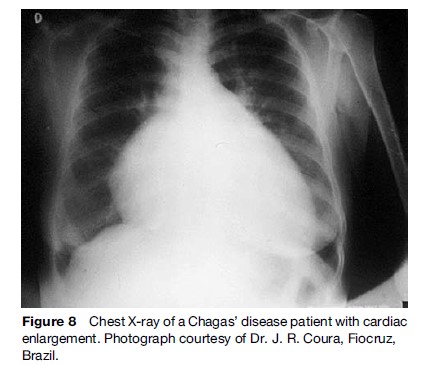
The major complications of chronic Chagas’ heart disease include signs of heart failure (usually with prominent systemic congestion and cardiac enlargement (Figure 8)), ventricular arrhythmias, and atrioventricular block. Chest pain – felt by 15–20% of patients – is usually atypical for myocardial ischemia, but in a subgroup of chagasic patients it may mimic acute coronary syndrome. However, epicardial coronary arteries are angiographically normal.
Typical ECG abnormalities (Figure 9) include right bundle branch with left anterior fascicular block and ventricular extrasystoles. Episodes of nonsustained ventricular tachycardia (VT) are present on 24-hour ambulatory ECG monitoring in approximately 40% of patients with wall motion abnormalities, and in 90% of patients with heart failure (Rassi et al., 1995). Sustained VT can be induced through programmed ventricular stimulation in a substantial proportion of patients. Not infrequently, complex ventricular rhythm disturbances coexist with bradyarrhythmias, as described by Chiale et al. (1982), and when associated with impaired left ventricular function, constitute a major risk factor for sudden cardiac death.
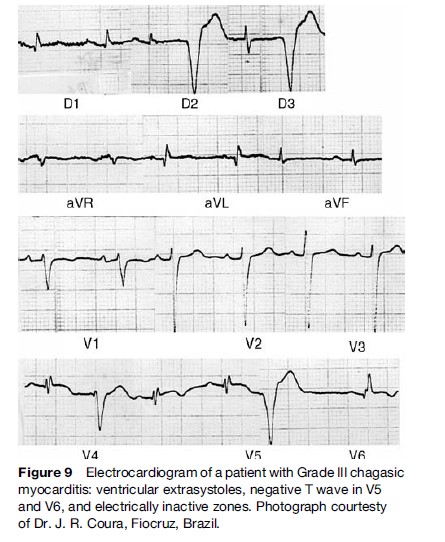
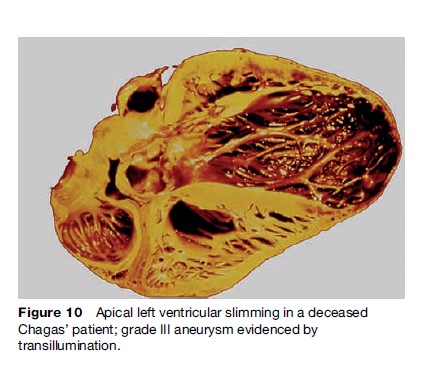
Striking segmental wall motion abnormalities in both ventricles occur early in the development of CD. The most characteristic lesion is the apical aneurysm, but it is the posterior basal dysynergy that best correlates with the occurrence of malignant ventricular arrhythmia. The aneurysms are also sources of emboli (Figure 10).
Mortality in patients with cardiac CD is primarily ascribed to sudden cardiac death, progressive heart failure, and thromboembolic events. Sudden cardiac death occurs in 55 to 65% of cases, progressive heart failure in 20 to 25%, and stroke in 10 to 15%. Sudden death is more frequent in young patients. The estimated total annual mortality caused by CD is 21 000 cases.
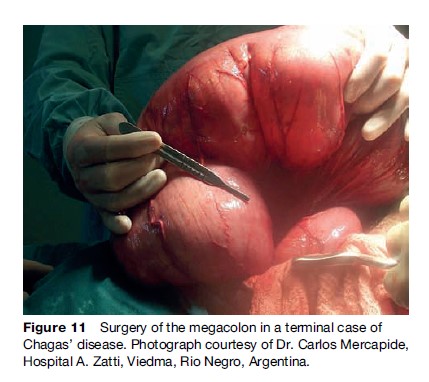
The gastrointestinal manifestations are a progressive enlargement of the esophagus or the colon, caused by chronic inflammation and destruction of parasympathetic neurons. The main gastrointestinal symptoms are dysphagia and severe constipation. Esophageal disease can appear as early as the acute phase of infection in children, while colonic disease evolves more slowly Figure 11.
Damage to the nervous system and striated muscle in the chronic stage, although not very clear in regard to its clinical significance, is expressed through motor cell loss or degeneration in various muscles, with infiltration and demyelination areas in the nerves. In the CNS, circumscribed or necrotizing inflammatory injuries can be found in the gray matter.
The diagnosis of T. cruzi infection during the chronic phase is best made by serological tests that detect circulating IgG antibody. There are useful amplification methods to establish the presence of parasites such as blood culture and xenodiagnosis, although the methods are significantly less sensitive during the chronic phase. The xenodiagnosis method used for many decades is now practically restricted to isolation work in the research area.
Assays based on PCR, which detect T. cruzi nucleic acids, have proved to be useful for verification of the parasite persistence in the host, and particularly useful for assessing the effectiveness of antiparasitic treatment.
Antiparasitic Treatment
So far it has been shown that treatment is effective for acute and congenital infections, reactivated infections in persons with HIV infection or receiving immunosuppressive therapy, and chronic infections of less than 15 years’ duration.
The effectiveness of treatment during the acute phase is easily demonstrated by the rapid drop of parasitemia and the disappearance of symptoms, signs, and circulating antibodies. Evaluation of the effectiveness of treatment is difficult in the chronic phase in which the disappearance of specific antibodies takes years, and this is the only accepted criterion of cure, as was established by a meeting of experts of the Pan American Health Organization (PAHO) (1999).
Several drugs have been tested for activity against T. cruzi, but only nifurtimox (1972) and benznidazole (1974), have demonstrated efficacy in human beings. These agents have been accepted by almost all Latin American countries’ Ministries of Health as treatment for T. cruzi infection. The objectives of treatment are to eliminate the parasite from infected persons, to diminish the probability of developing the disease, and to prevent T. cruzi transmission to others from infected persons (Sosa-Estani et al., 1998).
When trypanocidal treatment is used in patients in the late chronic phase, antibody titters may require 5 or more years to show a decline (Viotti et al., 1994). ‘‘Cure’’ rates in the late chronic phase, as determined by negative serology, are between 8 and 70%, and are higher for children than adults. Other assays that may be able to document cure sooner than conventional serology include serological tests employing recombinant antigen or purified, mucinlike glycoconjugate from cell-cultured trypomastigotes (De Andrade et al., 1996), xenodiagnosis, and PCR. Measurement of serum levels of the adhesion molecule p-selectin assays for interferon-gamma-producing T cells specific for a T. cruzi lysate group.
Several authors found that the results of treatment were more encouraging when ECG changes rather than serology were the outcome (Viotti et al., 1994; De Andrade et al., 1996). However, several other authors did not observe differences between treated and untreated patients. Based on the hypothesis that Chagas’ cardiomyopathy may indeed be triggered by persistent parasitic infection, it seems plausible that trypanocidal therapy may delay, reduce, or prevent the disease progress. This hypothesis must be tested by means of randomized clinical trials.
During treatment, patients must be under medical supervision to monitor for side effects, including cutaneous reactions, gastrointestinal intolerance, and, less frequently, central or peripheral neurotoxicity. Laboratory tests rarely show elevation of transaminases or leukopenia. Side effects are more frequently observed in adolescents and adults than in children and babies. In all cases, side effects disappear when the dose is diminished or the treatment is suspended.
It is necessary to search for better drugs than the ones available at this time to find a solution for the 16 million people infected with T. cruzi. Meanwhile, benznidazole and nifurtimox continue to be the only currently available drugs to treat persons with T. cruzi infection.
Control Of T. Cruzi Transmission
The main control strategies include surveillance and control of vectors, improvement of household construction, serological screening of blood and organ donors, and detection and treatment of congenitally infected infants.
Work contributed by Latin American researchers established the scientific basis for vector control and demonstrated its effectiveness in interrupting transmission. In 1948, Dias and Pellegrino described the successful use of the residual insecticide HCH (hexaclorocyclohexane) when applied to the walls of houses and peri-domestic structures infested with T. infestans in a rural area of Brazil. This vector has several attributes that facilitate its elimination: complete domiciliation in most endemic areas, and lack of sylvatic populations that could reinfest houses; low reproductive potential; and little capacity for active dispersion. In populations of Argentina (Segura, 2002), Brazil (D´ıas, 2002), and Venezuela having infestation levels higher than 15%, dwelling units have been treated with insecticides since the 1950s. More recently, regional control initiatives have extended vector control activities to other countries (see the section ‘Regional initiatives for control of transmission of T. cruzi ’).
A second approach to vector control is improvement of houses to eliminate the conditions necessary for colonization by triatomine bugs. These measures include plastering of walls and replacing thatch roofs to destroy potential hiding places for bugs, and providing the residents of rural areas with new, well-constructed houses. Such modifications or replacements were carried out in Venezuela in the 1970s and 1980s with excellent results, as well as in certain geographical areas in Bolivia and Paraguay with community participation. Reinfestation by Triatoma infestans after insecticide spraying has caused elimination efforts in all the regions to fail repeatedly (Cecere et al., 2006). Although house improvement and application of insecticide can be complementary, economic constraints have prevented more widespread implementation of measures to improve dwellings.
Regional Initiatives For Control Of Transmission Of T. Cruzi
Control of CD began to be institutionalized in the 1960s in the form of national campaigns implemented in Venezuela and several countries in the Southern Cone. In 1991 the Southern Cone Initiative, initiated by the Health Ministers from Argentina, Bolivia, Brazil, Chile, Paraguay, and Uruguay, allowed national control activities to be maintained, expanded, or instituted on a regular basis. The goal was to eliminate transmission by T. infestans and prevent transmission by transfusion of blood products throughout the region. The PAHO/WHO, which played a key role as technical secretariat of the Initiative, clearly defined the objectives, monitored the quality of work, and oversaw shared activities in border areas. After 15 years, the progress achieved is undeniable. Transmission by T. infestans has been shown to be interrupted in Chile, Uruguay, Brazil, and parts of Argentina and Paraguay. In many areas, it appears that the vector itself has been eliminated, although this has not been demonstrated with absolute certainty so far.
Other initiatives followed that of the Southern Cone, including the Central American countries in 1997, the Andean countries in 1999, Mexico in 2003, and the Amazonian region in 2004. The WHO reports have demonstrated the impact of the programs: decrease in the case incidence, lower prevalence of infections as measured by serological screening of populations, and a decrease in mortality.
Domiciliated species of bugs such as T. infestans and R. prolixus, especially in areas in which the last one was introduced, are susceptible to elimination by spraying of houses and buildings in the peri-domestic areas. Other species live mainly in natural environments, but have the capacity to sporadically invade and colonize human dwellings. Thus, T. brasiliensis, T. pseudomaculata, T. sordida, T. dimidiata, and P. megistus tend to persist following application of insecticide to dwellings, and control of these species requires constant monitoring and periodic spraying (D´ıas, 2002).
Currently, in the Southern Cone countries, the major concerns regarding control are sustainability of the accomplished results and implementation of strong antitriatomine actions in countries still infested by T. infestans, mainly Bolivia, southern Peru, and areas of Argentina. There is also an urgent need for research on appropriate techniques of epidemiological surveillance and promoting community involvement in control activities in coordination with the health system.
Control Of The Other Transmission Routes
Control of blood for transfusion through serological testing of blood donors has not been completely implemented in endemic countries. Considering the high risk of infection through this route, it is mandatory that a screening strategy be uniformly adopted and sustained. Some countries are increasing efforts to screen pregnant women to facilitate detection of congenital infections. In the future, when complete control of vectorial and transfusion-associated transmission is achieved, congenital transmission will be the only source of new cases. In some nonendemic countries such as the United States, which have large numbers of immigrants from endemic areas, programs to screen blood donors are being implemented. An additional challenge for these countries is to identify and provide proper medical care for infected persons, including congenitally infected infants.
Needs For The Future
To improve CD control perspectives, we are faced with numerous challenges: in the short term, to continue entomologic surveillance, to complete activities to prevent new acute cases, to do research to improve existing interventions, and to facilitate better diagnosis and follow-up of clinical cases. Midand long-term challenges include development of a vaccine that prevents the disease from developing in persons already infected or lessens the risk of infection, and improvement of the economic, social, and educational conditions of populations at risk.
Bibliography:
- Blanco SB, Segura EL, Cura EN, et al. (2000) Congenital transmission of Trypanosoma cruzi, an operational outline for detecting and treating infected infants in northwestern Argentina. Tropical Medicine and Internal Health 5(4): 293–301.
- Cecere MC, Vasquez-Prokopec GM, Gurtler RE, and Kitron U (2006) Reinfestation sources for Chagas disease vector, Triatoma infestans, Argentina. Emerging Infectious Diseases 12(7): 1096–1102.
- Chagas C (1909) Nova espe´ cie mo´ rbida do homem produzida por um trypanosoma (Trypanosoma cruzi). Nota pre´ via. Brazil Me´dico 23(16): 161.
- Chiale PA, Halpern MS, Nau GJ, et al. (1982) Malignant ventricular arrhythmias in chronic chagasic myocarditis. Pacing Clinical Electrophysiology 5(2): 162–172.
- Cura EN and Segura EL (1998) Quality assurance of the seological diagnosis of Chagas’ disease. Am J Public Health 3(4): 242–248.
- Darwin CR (1839) Narrative of the surveying voyages of His Majesty’s Ships Adventure and Beagle between the years 1826 and 1836, describing their examination of the southern shores of South America, and the Beagle’s circumnavigation of the globe. Journal and remarks. 1832–1836. Volume III, pp. 403. London: Henry Colburn.
- De Andrade ALS, Zicker F, de Oliveira RM, et al. (1996) Randomised trial of efficacy of benznidazole in treatment of early Trypanosoma cruzi infection. The Lancet 348: 1407–1413.
- Dias JCP (2002) O controle da Doenc¸ a de Chagas no Brasil. In: Silveira AC (ed.) O controle da doenc¸ a de Chagas nos paisesdo Cone Sul da America. Historia de uma Iniciativa Internacional: 1909–2001; pp. 145–250. Pan American Health Organization, Uberaba, Impresso Facultade de Medicina do Triangulo Mineiro, Fundac¸ ao de Pesquiza de Uberaba.
- Guhl F, Jaramillo C, Vallejo GA, et al. (1999) Isolation of Trypanosoma cruzi DNA in 4000-year-old mummified human tissue from northern Chile. American Journal Physiology Anthropology 108(4): 401–407.
- Lugones HS (1999) Formas con puerta de entrada aparente en ‘‘Chagas Agudo, Situacio´ n actual’’ de Lugones H. Salta, Argentina: Universidad Cato´ lica de Salta.
- Moncayo A (2003) Chagas disease. The Burden of Disease and Mortality by Condition: Data, Methods, and Results for 2003. Boston: World Health Organization, World Bank, Harvard University.
- Marcel PL, Duffy T, Cardinal MV, et al. (2006) PCR-based screening and lineage identification of Trypanosoma cruzi directly from faecal samples of triatomine bugs from northwestern Argentina. Parasitology 132(Pt. 1): 57–65.
- Pan American Health Organization (PAHO) (1999) Tratamiento Etiolo´ gico de la Enfermedad de Chagas. Conclusiones de una Consulta Te´ cnica. OPS/HCP/HCT/140/99.
- Postan M, Dvorak JA, and McDaniel JP (1983) Studies of Trypanosoma cruzi clones in inbred mice. I. A comparison of the course of infection of C3H/HEN-mice with two clones isolated from a common source. Am J Trop Med Hyg 32(3): 497–506.
- Rassi A Jr., Rassi AG, Rassi SG, Rassi L Jr., and Rassi A (1995) Arritmias ventriculares na doenc¸ a de Chagas. Particularidades diagno´ sticas, progno´ sticas e terapeˆ uticas. Arquives Brasil Cardiologia 65(4): 377–387.
- Rosebaum M (1964) Chagasic myocardiopathy. Prog Cordiovasc 7: 199–225.
- Rosenbaum MB and Cerisola JA (1961) Epidemiology of Chagas’ disease in the Argentine Republic. Hospital (Rio J) 60: 55–100.
- Russomando G, Almiron M, Candia N, et al. (2005) Implementacio´ n y evaluacio´ n de un sistema localmente sustentable de diagno´ stico prenatal que permite detectar casos de transmisio´ n conge´ nita de la enfermedad de Chagas en zonas ende´ micas del Paraguay. Revista da Sociedade Brasileira de Medicina Tropical 38(supplement 2).
- Schmunis GA, Zicker F, Pinheiro F, et al. (1998) Risk for transfusion- transmitted infectious diseases in Central and South America. Emerging Infectious Diseases 4(1): 5–11.
- Segura EL (2002) Historia del control de la enfermedad de Chagas en Argentina. In: Silveira AC (ed.) O controle da doenc¸ a de Chagas nos paı´ses do Cone Sul da Ame´rica. Historia de uma Iniciativa intemacional. 1991–2001, pp. 42–109.
- Shikanai-Yasuda MA, Marcondes CB, et al. (1991) Possible oral transmission of acute Chagas’ disease in Brazil. Revista do Instituto de Medicina Tropical de Sao Paulo 33(5): 351–357.
- Silveira AC (1999) Current situation of the control of vector born Chagas disease transmission in the Americas. In: Atlas of Chagas Disease Vectors in the Americas, vol. 3, pp. 1161–1181. Rio de Janeiro: Editora Fiocruz.
- Sosa-Estani S, Segura EL, Ruiz AM, et al. (1998) Chemotherapy with benznidazole in children in undetermined phase of Chagas disease. American Journal of Tropical Medicine and Hygiene 59(4): 526–529.
- Tarleton RL, Grusby MJ, Postan M, et al. (1996) Trypanosoma cruzi infection in MHC-deficient mice: Further evidence for the role of both class Iand class II-restricted T cells in immune resistance and disease. International Immunology 8(1): 13–22.
- Torrico F, Alonso-Vega C, Suarez E, et al. (2004) Maternal Trypanosoma cruzi infection, pregnancy outcome, morbidity, and mortality of congenitally infected and non-infected newborns in Bolivia. American Journal of Tropical Medicine and Hygiene 70(2): 201–209.
- Viotti R, Vigliano C, Armenti A, and Segura EL (1994) Treatment of chronic Chagas’ disease with benznidazole: Clinical and serologic evolution of patients with long-term follow-up. American Heart Journal 127(1): 151–161.
- WHO Expert Committee (2002) Control of Chagas’ disease. WHO Technical Report Series 905.
- Carlier I, Dias CJ, Ostermayer Luquetti A, Hontebeyrie M, Torrico F, and Truyens C (2002) Trypanosomiase Ame´ricaine ou Maladie de Chagas, Encyclope´die Me´dico-Chirurgicale, Editions Scientifiques et Medicales. Paris: Elsevier SAS.
- Cerisola JA (1977) Chemotherapy of Chagas’ infection in man. Scientific Publication, PAHO # 347.
- Dı´as JCP and Coura JR (eds.) (1996) Clı´nica e Terapeˆutica da Doenc¸a de Chagas. Rio de Janeiro: Editora Fiocruz.
- Prata A, Andrade Z, and Guimaraes AC (1974) Chagas’ heart disease. In: Rezende JM and Moreira H (1974) Forma digestiva da doenc¸a de Chagas. In: Brener Z, Andrade ZA, and Barral-Neto M (eds.) Tripanosoma cruzi e doenc¸ a de Chagas, 2nd edn., pp. 297–343. Rio de Janeiro: Editora Guanabara Koogan.
- Rodrigues Coura J (ed.) (2005) Dinaˆmica das Doenc¸ as Infecciosas e Parasita´rias. Rio de Janeiro: Editora Guanabara Koogan.
- Shaper AG, Hutt MSR, Fejfar Z (eds.) Cardiovascular disease in the tropics. pp. 264–281. London: British Medical Association.
- Sica R (1994) Compromiso del sistema nervioso. In: Storino R and Milei J (eds.) Enfermedad de Chagas, pp. 303–320. Buenos Aires: Mosby.
- Tentory MC, Segura EL, and Hayes DL (eds.) (2000) Arrhythmia Management in Chagas’ Disease. Armonk, NY: Futura.
See also:
Free research papers are not written to satisfy your specific instructions. You can use our professional writing services to buy a custom research paper on any topic and get your high quality paper at affordable price.








