This sample History of Yellow Fever Research Paper is published for educational and informational purposes only. If you need help writing your assignment, please use our research paper writing service and buy a paper on any topic at affordable price. Also check our tips on how to write a research paper, see the lists of health research paper topics, and browse research paper examples.
The Earliest Epidemics
Although yellow fever has undoubtedly been endemic in tropical Africa for thousands of years, it was only after the arrival of the European migrants in the New World at the end of the fifteenth century that this scourge emerged in the form of devastating epidemics. The Yucatan peninsula of Mexico is credited with the first known epidemic in 1648, which affected Spanish settlements and indigenous populations. Yellow fever was therefore considered for a long time as a primarily American disease, all the more so since large outbreaks occurred in the New World during the seventeenth, eighteenth, nineteenth, and early twentieth centuries. The term ‘vomito negro’ was used in those days to describe clinical aspects of this pathological condition, because death was frequently preceded by black vomit, or partially digested blood. Other terms used to designate yellow fever included ‘Yellow Jack’ and ‘Safran scourge,’ in reference to the jaundice observed in many patients.
The Philadelphia Epidemic (1793)
Among the heaviest epidemics which struck the United States, and also which were the most thoroughly studied, were those of Philadelphia in 1793 and New Orleans in 1853. In 1793, Philadelphia, Pennsylvania, was a flourishing but quite insalubrious city with 40 000 inhabitants. Its port on the Delaware River was very active, harboring vessels from all over the world, mainly from the Caribbean. In July, the sloop Amelia from Santo Domingo moored with a cargo of coffee, even though the captain and some sailors were seriously ill. During the very hot summer of 1793, mosquitoes were abundant everywhere in the city but nobody paid attention to this nuisance.
The first cases of the disease that appeared around the harbor were accompanied by high mortality. Municipal authorities and physicians were unable to determine the true nature of the disease. By the first week of September, 300 cases had already been registered; some patients died in less than 12 hours. Various diagnoses were put forward, such as influenza, contagious catarrh, or intestinal fever. Another explanation for the disease was attributed to the alteration of the coffee freight from the Amelia, which went rotten on the wharf, thus generating ‘miasmas.’ The disease was in fact yellow fever.
During October there were 100 deaths each day, and the total mortality among patients reached 55%.
City life was paralyzed and trade entirely disrupted. By 14 November, Thomas Mafflin, the Governor of Pennsylvania, announced the end of the scourge but by then 4044 citizens – or one-tenth of the population – had died.
The New Orleans Epidemic (1853)
Between 1793 and 1853, the number of epidemics in the United States increased steadily, but the most devastating outbreak in an American town hit New Orleans during the summer and fall of 1853. At that time, the city’s population was about 150 000 persons. Its prosperity was dependent on its port activity. It was only 170 kms from the Mississippi River delta, which carried all the cotton trade from the Mississippi and Missouri basins. Docks were loaded with sugar, rice, and other goods. However, the city was built on marshes and bayous and was frequently flooded, so mosquitoes were very abundant in the city during the summer and fall of 1853.
In June 1853, the first cases of yellow fever appeared gradually around the harbor docks in the poor districts of the city, but their number quickly increased in July. Municipal authorities and newspapers minimized the situation. During August the number of deaths reached 100 and then 250 each week, and corpses accumulated along the streets. The epidemic was finally identified as yellow fever. By the end of August the number of cases had decreased, but 740 persons were still reported to have the disease during the first week of September. Finally the epidemic stopped in November, when a small outbreak of Asiatic cholera occurred. During five months, 40 000 inhabitants felt ill, among whom 11 000 died (27.5%). Trade activities gradually went back to normal, sanitation was rigorously developed, and by 1881 the population rose to 220 000. By 1905, when yellow fever broke out in the city again, important progress had been made regarding knowledge and prevention of the disease, and only 423 deaths were registered.
Imported Yellow Fever In European Ports
During the eighteenth and nineteenth centuries, yellow fever arrived in Europe. Infected ships arriving from the Caribbean brought epidemics to Portugal, Italy, Spain, France, and England. For instance, the Gran Turco from Havana, Cuba, suspected of participating illegally in the slave trade, introduced the disease in Barcelona, Spain, during August 1821. The epidemic broke out in the poor suburbs and finally devastated the center of the city, killing about 120 000 inhabitants, or one-sixth of the total population.
Even so, by 13 July 1861, the three-master Anne-Marie from Havana, arrived at Saint-Nazaire, France, with a cargo of sugar. While crossing the Atlantic Ocean, the captain and ten sailors had been seriously ill, and two seamen died. Nevertheless, nobody among the medical or harbor authorities was worried, and unloading started. At the beginning of August, yellow fever appeared in the city of Saint-Nazaire and its surroundings; several other ships arriving or departing were then contaminated, thus spreading the disease. During the 2-month period of outbreak, 44 caught the disease, among whom 26 died.
The Discovery Of The Viral Nature Of Yellow Fever
The Earliest Studies On The Etiology Of Yellow Fever
During the last quarter of the nineteenth century, the etiology of yellow fever remained a mystery, although it was generally explained to be caused by the inhalation of ‘miasmas’ emanating from decomposition of organic matter. However, at that time, the role of several bacteria had been demonstrated in diseases such as anthrax, tuberculosis, and Asiatic cholera by Louis Pasteur in France and Robert Koch in Germany. The etiological role of molds or bacteria in yellow fever therefore appeared to be a credible hypothesis, and a number of microorganisms had been isolated from patients in South and North America. Among these, the Bacillus icteroides isolated in 1897 by Guiseppe Sanarelli in Montevideo, Uruguay, seemed a plausible candidate. Frequently isolated from patients’ blood during epidemics and inducing jaundice by inoculation to guinea pigs, it was considered by several authors as the causative agent of the disease until F. G. Novy demonstrated in 1898 that it was, in fact, the well-known agent of hog cholera, a Salmonella.
Moreover, in 1881, a Cuban physician, Carlos Finlay (Figure 1), hypothesized that ‘vomito negro’ was transmitted in nature by the bite of a specific mosquito: Culex fasciatus (¼ Stegomya fasciata ¼ Aedes aegypti L). In order to confirm his theory, Finlay carried out at least 104 experiments on human volunteers between 1884 and 1900 in order to transmit the disease from a yellow fever patient to a healthy subject via the bite of C. fasciatus that had previously engorged on the patient. Although he obtained only mild pathological episodes, he claimed success in 11 attempts. Today, it is quite evident that these experiments failed because the experimental protocol was inadequate (Chastel, 2003).

The First US Commission In Cuba (1879)
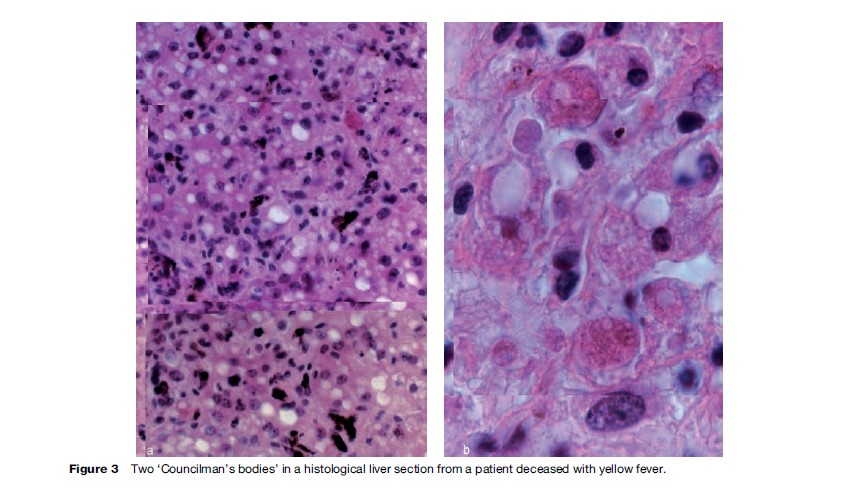
In 1879, a first US Commission was sent to Havana, Cuba, a permanent focus of yellow fever (Figure 2) that continuously threatened the southern and northern ports of the United States. This commission was headed by Standford Challie´ from New Orleans, assisted by Juan Guiteras, a Cuban physician, and George M. Sternberg of the U.S. Army Medical Service. The objective of this commission was to carefully study the infectious nature of the disease. Autopsy material was studied but no clear conclusion was suggested, and no bacteria were isolated from patients’ blood. However, the pathological material collected allowed the great pathologist W. T. Councilman in 1890 to describe the yellow-fever lesions of the liver, particularly the so-called ‘Councilman bodies,’ which are quite pathognomonic for the disease (Figure 3).

The Second US Commission In Cuba (1900)
In 1900, Surgeon General G. M. Sternberg, appointed director of the U.S. Army Medical Service, recruited Major Walter Reed (Figure 4), a fine pathologist and microbiologist from the same service, in order to run a second scientific commission to Cuba, together with James Carroll, Jesse Lazear, and Aristides Agramonte, a Cuban physician. The first aim of this new commission was to answer the question: Was yellow fever contagious?

By 25 June 1900, the staff was at work at ‘Columbia Barracks.’ Serendipitously, a new yellow fever outbreak was starting in Quemados, near Havana. Reed and his colleagues quickly established that the disease was not a contagious disease, that is to say, directly transmitted by contact between humans such as in measles or scarlet fever. Moreover, as had been recently demonstrated by H. P. Carter in Missouri (1900), at the beginning of an outbreak, the period from the first (infecting) case to the secondary cases generally ranged from 2 to 3 weeks, thus indicating an indirect mode of transmission of the disease. Hence the mosquito-vector theory regained great interest (Chaves-Carballo, 2005).
As no laboratory animal was sensitive to yellow fever, Reed decided to repeat Finlay’s experiments, that is, the inoculation of soldiers and other volunteers via mosquito bites. These new experiments were successful, but two accidental infections occurred among the staff. James Carroll and Jesse Lazear were infected by mosquitoes previously allowed to feed on yellow fever patients. Carroll recovered but Lazear died. This disaster greatly affected the members of the commission, and Leonard Wood, military governor of Cuba, decided that the human experiments would be continued only when the Columbia Barracks could be sufficiently secured, for instance after covering windows and doors with mosquito-proof wire mesh. In these better conditions, the experiments started again with success, and by the end of 1900 it was demonstrated that the causative agent present in patients’ blood during the early phase of yellow fever, was transmitted from infected blood by Aedes aegypti mosquitoes, and was a filterable virus. Reed and his colleagues also concluded that the viral infection was not life-threatening or hereditary in the mosquito vector. In addition, an interval of about 12 days was necessary after engorgement on a patient before the mosquito would be able to transmit the virus during the next bite.
Confirmation Of Walter Reed’s Results By Other Searchers
In 1900, Juan Guiteras, who had been a member of the first US Commission, performed further experiments of mosquito transmission in human volunteers in Cuba. Three persons died, including an American nurse, Clara Maass, a real heroine in the United States (Figure 5). Moreover, all these experimental conclusions were entirely confirmed in Rio de Janeiro, Brazil, from 1901 to 1905, by a French team consisting of P. L. Simond, E. Marchoux, and A. Salimbeni. However, unlike Reed’s conclusions, Marchoux and Simond discovered that the infection was hereditary in female Ae. aegypti, an observation first denied by other scientists but confirmed at the end of the 1970s by several American entomologists. The experimental results obtained by the French team contributed to the success of the yellow fever campaign initiated later by Oswaldo Cruz in Rio.

Sanitation In Cuba And Panama
At the beginning of the twentieth century, the solution to control and eventually eradicate yellow fever appeared quite evident. By exterminating the mosquito vector, Aedes aegypti, the disease would quickly disappear.
The Leading Part Of William C. Gorgas
Putting into practice this concept, Major William C. Gorgas (Figure 6), chief sanitary officer in Havana and a very strong personality, initiated sanitation programs in Cuba and later in Panama with the objective to eradicate yellow fever. With the active support of Walter Reed, a drastic program was implemented in Havana in February 1901; the main measures were as follows: (1) any case of yellow fever would be registered and quickly admitted to a specialized ward in a secured hospital; (2) the patient’s house and surroundings would be fumigated in order to exterminate adult mosquitoes; and (3) each inhabitant would carefully look for any domestic breeding site for mosquito larvae, and any container filled with water would be covered with a lid or a wire netting.

These very simple measures were strictly applied, and the results were immediate and spectacular. Although during previous years hundreds of deaths occurred each year, the last case to be registered was in September 1901, and not a single case was observed from 1902 to 1904. Thus, the mosquito-control campaign undertaken by Gorgas and Reed eliminated the pestilence from one of the unhealthiest areas of the New World.
However, a greater challenge was waiting for William Gorgas: the Panama isthmus, an area of considerable strategic and economical interest for the United States, also quite insalubrious. ‘‘One of the hottest, wettest and most feverish regions in existence’’ reported W. C. Gorgas in 1912.
For instance, in 1893, the Fourth Infantry of the United States Army crossed the isthmus from the eastern coast to California. General Grant was the quarter master of this regiment. The troops were transported half way by railroad and the other half by marching through the jungle. Eighty men out of 810 died from yellow fever between Colo´ n and San Francisco.
At the same time, the building company imported foreign workers to finish the railroad across the isthmus, first 1000 men from West Africa and then 1000 from China. All died within six months after their arrival at the building sites.
The Panama Canal Crisis
However, the most dramatic episode occurred during the French initial phase of the construction of the Panama Canal. In 1881, Ferdinand de Lesseps (Figure 7), a French diplomat and businessman, confident in his success after the opening of the Suez Canal in 1865, began operations through the isthmus with a staff of French engineers training nonacclimated foreign workers. Field difficulties and health risks were largely underestimated by de Lesseps. Panama rocks were not sandy grounds, and Central American jungle was more insalubrious than the Egyptian desert.

By 1889, the Panama operation was a financial and health disaster and had stopped. Gorgas estimated that 22 189 deaths had occurred among managers, families, and workers between 1881 and 1889, mainly due to yellow fever or malaria. At that time, the ‘Panama crisis’ exploded in Paris, causing the ruin of many investors.
When the United States took charge of the Canal in 1904, Gorgas was colonel, appointed chief sanitary officer, and responsible for sanitation in the Canal zone. According to him, the health conditions there were very bad, but considerable efforts were displayed to control diseases. Finally, the Panama Canal was ready to pass ships during fall 1913; it officially opened 1 January 1915. During the American period, only 4000 workers were lost, essentially due to malaria, but ‘‘most important of all, yellow fever has been entirely banished’’ (Gorgas, 1912).
The Theory Of Permanent Foci Of Infection
Following these brilliant successes in Cuba and Panama, William Gorgas, together with H.R. Carter, developed a theory concerning the ‘permanent foci of infection.’ In areas such as Panama, Vera Cruz, Brazil, or West Indies harbors where yellow fever was endemic and transmitted by Ae. aegypti (the only vector known at that time), mosquitocontrol programs should quickly eliminate the disease and lead to its later eradication.
Unfortunately, this theory was quickly refuted; yellow fever reappeared in Latin America, for instance in Bucaramago, Colombia, by 1923, despite the US$13 000 000 spent by the Rockefeller Foundation in various sanitation campaigns. At the beginning of 1928, the northern part of Brazil was struck by a severe outbreak. The permanent foci of infection theory was called into question.
Isolation Of The Virus From West Africa
The failure of the Gorgas’ theory and campaigns implemented to eradicate the mosquito vector Ae. aegypti was a very serious matter, as there was no alternative to fight against yellow fever.
The Error Of Hideyo Noguchi (1919)
In addition to the confusion, the results of experiments carried out by Reed, Simond, and Marchoux and their colleagues showing that the etiologic agent of yellow fever was of filterable (viral) nature were denied by a Japanese bacteriologist, Hideyo Noguchi. At this time, Noguchi was an influential member of the commission sent by the Rockefeller Foundation to Guyaquil, Ecuador, to investigate on yellow fever (Flexner, 1929). Between 1918 and 1924, Noguchi studied a number of patients presenting with feverish jaundice (erroneously diagnosed as yellow fever), from whom he isolated in culture a spiral organism which he named Leptospira icteroides. This leptospira reproduced symptoms and pathological changes in guinea pig that resembled those of yellow fever in man. This was a tragic double error: the clinical experts confused hemorrhagic jaundice (Weil’s disease or leptospirosis) with yellow fever, and Noguchi confused Leptospira icterohaemorrhagiae, the true agent of Weil’s disease, with the so-called L. icteroides. Other bacteriologists using the Noguchi’s technique of culture isolated L.icteroides, although not consistently, whereas other bacteriologists did not. Noguchi proposed a vaccine prepared with L. icteroides cultures that he considered efficient and injected it into himself.
Although Noguchi was very creditable among bacteriologists for his previous discoveries, the (true) thesis of a filter-passing virus as the cause of yellow fever was abandoned for years. This regressive option caused an irreparable delay in achieving an understanding of yellow fever etiology and pathogenesis, as well as the possibility of developing a vaccine. It is quite ‘‘difficult to understand how Noguchi’s claims became so widely accepted, but they unquestionably affected the whole of the yellow fever programme in the Americas and Africa’’ (Porterfield, 1989).
Confirmation Of The Viral Nature Of Yellow Fever In Africa (1927)
Fortunately, the Rockefeller Foundation’s programs focused on West Africa, where many outbreaks occurred at the end of the nineteenth and the beginning of the twentieth centuries. A laboratory was established near Lagos, Nigeria, under the authority of the West African Yellow Fever Commission. Between 1925 and 1927, many competent researchers from this institution failed to isolate L. icteroides from yellow fever patients. Guinea pigs did not react when inoculated with the blood of patients, and no immune response to L. icteroides developed among the convalescents. So, uncertainty about the Noguchi’s theory increased more and more.
Suddenly, at the beginning of 1927, yellow fever appeared in force along the coasts of West Africa, from the mouth of the Senegal river to Angola.
As guinea pigs and also African monkeys were not susceptible to yellow fever, the quest for a suitable laboratory animal was the main concern among the researchers there. On the initiative of Henri E. Beewkes, the director of the West African Yellow Fever Commission, Asian monkeys, including rhesus monkeys (Macaca mulatta), were imported from Europe during spring 1927. These primates debarked on May 25, 1927, in Accra, Gold Coast (presently Ghana), where A. F. Mahaffy and J. H. Bauer were studying a small outbreak of yellow fever. After several inconclusive attempts, they obtained a clear experimental infection after inoculating to rhesus monkeys the blood of a mildly ill, 27-year-old, African man named Asibi. The symptoms and severe pathology induced in rhesus reproduced those of the human disease. The Asibi strain was then studied by Bauer and Adrian Stokes in the Lagos laboratory, Nigeria. It was ‘‘a period of feverish and fruitful research activity’’ (Hudson, 1966).
Experimental Results From The Lagos Laboratory
In this laboratory, Stokes, Bauer, and Hudson began a series of experiments on rhesus monkeys and succeeded in transmitting the disease from monkey to monkey by using Ae. aegypti. Moreover, Stokes established that the rhesus monkey was not susceptible to L. icteroides, and immune serum prepared against this leptospire did not protect rhesus monkeys from the inoculation of the Asibi strain. Thus, the earlier experiments carried out by the Reed commission (1900) were at last validated, but 27 precious years had been lost. Tragically, Adrian Stokes was infected by handling infected monkeys without gloves. He died on 19 December 1927. The experiments on rhesus monkeys inoculated with the Asibi strain were in fact extremely hazardous, and a number of other researchers in West Africa died from laboratory-acquired yellow fever: Edward Haynes and William A. Young, as well as Hideyo Noguichi, who arrived in Accra on 17 November 1927 in order to set up his own laboratory there. Before his death in Accra, Noguchi confirmed the Stokes’ discovery and failed to identify L. icteroides from West African patients.
Experimental Results From The Pasteur Institute, Dakar
At the same time, a yellow fever outbreak was studied at the Pasteur Institute, Dakar, Senegal, by C. Mathis, A. W. Sellards, and Jean Laigret. They quickly confirmed the results of Stokes. Using rhesus monkeys imported from India and a strain of Ae. aegypti from Havana, Cuba, they isolated a virus from a young Syrian man, 17-yearold Francois Malayi, who had fallen ill on 20 December 1927. Like Asibi, he survived his illness, and the virus isolated from him is the ancestor of the FN (French neurotropic) strain of the yellow fever virus.
Max Theiler And The White Mouse (1930)
Now experimental study of yellow fever could begin in earnest, helped by the discovery in 1930 by Max Theiler, from the Rockefeller Foundation in New York, of the high susceptibility of the white mouse to the virus inoculated by the intracerebral route. The white mouse was a common laboratory animal and easier to handle than monkeys. Max Theiler, a future Nobel Prize of Medicine winner (1951), also finalized a neutralization test that used mice, the ‘mouse protection test,’ after observing that specific yellow fever antibodies protected the mouse from experimental disease. This relatively simple test allowed a precise determination of the geographical extent, which had been largely underestimated, of the disease in America and Africa.
The Discovery Of Jungle Yellow Fever
Until 1932, yellow fever was considered an urban disease transmitted exclusively by Ae. aegypti, a domestic and highly anthropophilic species of mosquito that bit man during the day. However, as early as 1907 an outbreak in Colombia strongly suggested that yellow fever might also be acquired in a virgin forest environment from mosquitoes other than Ae. aegypti (Monath, 1988).
The Leading Role Of Fred L. Soper (1932)
The revolutionary concept of ‘jungle’ or ‘sylvatic’ yellow fever was defined when F. L. Soper and his colleagues from the Rockefeller Foundation studied in March 1932 a small rural outbreak that occurred in the valley of Chanaan, state of Spirito Santo, Brazil. This outbreak developed during 3 months in a strictly rural area where no Ae. aegypti were found but where wild mosquitoes were prevalent, such as Aedes (O.) scapularis and Ae. (T.) flaviatilis, two species quite different from the ‘classical’ Ae. (S.) aegypti (Soper et al., 1933).
In this area, man was only contaminated when in contact with the virgin forest, for instance when woodcutters cut down trees, a mechanism by which the infected mosquitoes living together with monkeys in the canopy were thrown down to the ground with the cut trees, there able to bite and infect man. Back in their villages, the infected woodcutters could contaminate other inhabitants, thus causing the epidemic in this remote area of Brazil.
Based on this concept, F. L. Soper and his colleagues studied sporadic, fatal cases of yellow fever occurring quite silently in the rain forest of Brazil. They used a special device, the so-called ‘viscerotom,’ to remove small fragments of liver from corpses, which showed the typical lesions of yellow fever. This study led Soper and colleagues to conclude that jungle yellow fever generally occurred in ‘silent’ forest foci not discernible without the help of such field surveys (Soper et al., 1934).
Thus, in addition to the classical ‘urban’ yellow fever described by the work of Walter Reed, H. R. Carter, and W. C. Gorgas, another epidemiological form of yellow fever existed in the rainforest, and the mechanism for the conservation of the disease was located in the Amazonian canopy. Here, the virus circulated among a number of wild monkeys, such as howling monkeys (Alouatta), spider monkeys (Ateles), or douroucoulis (Aotus), and several forest mosquitoes mainly of the Haemogogus and Sabethes genera. In fact, the reported annual incidence of jungle yellow fever varied in South and Central Americas between 50 and 300 cases (Monath, 1988).
Because yellow fever was found to occur as an enzootic viral infection in wild nonhuman primates in South American rainforests, the eradication of the disease appeared impossible, and this is still true today. The only opportunity to avoid extensive epidemics in man was to carefully control Ae. aegypti, its urban vector, in large metropolises of the Americas. Control efforts against this mosquito during the 1940s associated with immunization campaigns using the 17D vaccine (see Yellow Fever Vaccines) resulted in the disappearance of urban yellow fever from South America between 1942 and the 1970s.
Jungle Yellow Fever Also Existed In Tropical Africa
Since it was demonstrated in the New World that yellow fever was primarily a zoonosis, that is, an animal infection only occasionally transmitted to man, involving forest monkeys and mosquitoes, it was logical that an equivalent of this phenomenon would be found in Africa, especially because large rural epidemics had occurred in eastern Africa during the 1940s to 1960s. In 1941, yellow fever devastated the Nuba Mountains in Sudan, causing about 1500 deaths, in the absence of any Ae. aegypti. Moreover, in 1960–1962, a more serious rural outbreak (30 000 deaths) struck Ethiopia, propagated by Aedes simpsoni, a mosquito species quite different from Ae. aegypti.
To identify the jungle yellow fever in eastern Africa, a field study area was selected by the British virologists of the East African Virus Research Institute (EAVRI) of Entebbe, Uganda: the Bwamba forest, also located in Uganda. Here, rare sporadic cases of human yellow fever were registered in humans who lived along the edges of the forest. In this area the following epidemiological schema was established in 1948 by A. J. Haddow and colleagues (Haddow et al., 1948). In the highest canopy, when Cercopithecids monkeys settled into their nests for the night, they were bitten during their sleep by a nocturnal mosquito, Aedes africanus, and became infected with the yellow fever virus. In the day time, infected monkeys, carrying the virus in their blood, reached nearby villages to find some food in small banana plantations. Within the leaves of banana trees (Colocaria), larvae of a second mosquito species, Aedes simpsoni, were developing. The adults of Ae. simpsoni, an amphophilic and diurnal species, were then able to bite both monkeys and human beings, thus transferring the infection to humans. This process explained the occurrence of sporadic, generally mild cases of human yellow fever that were observed on the borders of the Bwamba forest. Eventually these sporadic cases might have been the spark to trigger an extensive rural outbreak.
However, this mechanism was only demonstrated for eastern Africa and, more precisely, for the Bwamba forest. Everywhere else other biological mechanisms were operating. In West and Central Africa during 1970–1980, French virologists from the Instituts Pasteur d’Outre-Mer (Dakar, Bangui, Abidjan), together with entomologists from the Office de la Recherche Scientifique et Technique Outre-Mer (ORSTOM), revealed a number of other explanations for the persistence of the disease in French territories of Africa (Monath, 1991). For instance, in the field station of Kedougou, in the eastern part of Senegal, the yellow fever virus was maintained in nature within a specific zoonotic cycle. There, forest monkeys (Erythrocebus patas, Cercopithecus aethiops) were associated with wild mosquitoes of the Aedes genus, such as Aedes opok, Ae. luteocephalus, and Ae. furcifer taylori group, ensuring the survival of the virus. From these yellow fever sanctuaries the virus might occasionally escape from its forest biotopes and infect the villages bordering the forest and surrounding savannahs.
Thus, it was possible to explain the explosion in 1965 of a devastating rural outbreak in the Djourbel area of Senegal. Originating in the southeastern forest areas, this outbreak was transmitted by Aedes aegypti, which had adapted to the rural environment and bred in clay jars or ‘canaries’ used for water storage.
Similar observations were made in the Bozo-Bouboui field station, near Bangui, in the Central African Republic. Furthermore, during these studies it was demonstrated that a number of infected female mosquitoes might transit the virus congenitally (through their ovaries) to their eggs and thus to their descendants. This process also contributed to the field conservation of the virus. Thus by several complex but efficient ways, the virus of yellow fever was maintained through zoonotic cycles in rain forests in both the New and Old Worlds, thereby making its eradication impossible.
Yellow Fever Vaccines
Fortunately, another means existed to control yellow fever, the immunization of the populations in endemic areas. For this purpose two live attenuated vaccines became available: the ‘Dakar French neurotropic vaccine’ attenuated in white mice and the ‘17D vaccine’ from the Rockefeller Foundation attenuated in tissue cultures (Barrett, 1987).
The Dakar French Neurotropic Vaccine (Dakar FNV)
This vaccine was developed by researchers of the Institut Pasteur de Dakar, Senegal, from a wild-type strain of yellow fever virus isolated during the 1927 epidemic in Dakar already discussed. This viral strain was attenuated by serial intracerebral passages in white mice and progressively lost the ability to cause systemic yellow fever in rhesus monkeys and mice. At passage 237 in mice this modified virus was considered sufficiently adapted to be used as a vaccine for human beings (Sellards and Laigret, 1932).
This vaccine was extensively used in the French colonies and territories of Africa between 1939 and 1952. In fact, 38 667 543 vaccinations were performed on a total population of about 17.5 million. In the form of a ‘dry’ (lyophilizated) vaccine, it was found adequately stable and highly effective when administered by scarification of the skin. It was also possible to use the dry vaccine in association with the Jenerian vaccine (against smallpox) in large campaigns of double immunization of entire populations. In French West Africa this vaccination program resulted in the disappearance of the disease from this area from 1939 to 1965.
On the contrary, in the British West African colonies, where large-scale immunization campaigns were not conducted, several outbreaks were registered during the 1950s, mainly in Gold Coast (Ghana) and Nigeria (Monath, 1988).
However, it was also proved that a number of cases of encephalitis were directly related to the administration of the Dakar vaccine. This appeared quite evident during the epidemic affecting the region of Djourbel, Senegal, in 1965, a direct consequence of the abandonment of immunization programs by the Senegalese authorities after accession to independence. As a result of vaccinations performed in an emergency situation, a great number of cases of encephalitis occurred, mainly in children under 14 years old. This sanitary disaster led the World Health Organization (WHO) to prohibit the use of Dakar vaccine in young populations and eventually to its progressive abandonment during the 1970s, preference being given to the safer 17D vaccine.
The Rockefeller 17D Vaccine
The 17D vaccine was developed by Max Theiler and his coworkers in 1936 (Theiler et al., 1937), using the Asibi strain of yellow fever virus isolated in 1927 from a sick man of that name in the Gold Coast, as previously discussed. This wild strain was passaged first in mouse and chick embryo tissue cultures, and then in chick embryo tissue cultures from which the nervous tissues had been removed in order to eliminate any neurotropic content. Finally the 17D vaccine was prepared by inoculating fertile hen eggs (Theiller and Smith, 1937) and administrated by subcutaneous injection. The initial vaccine trials were conducted in Brazil in 1937–1938, during which 59 000 people were vaccinated without any adverse reaction.
Later on some problems appeared at the beginning of its large-scale utilization, mainly during the Second World War, when cases of jaundice were observed after vaccination of U.S. soldiers, caused by accidental contamination of the vaccine batches by infected human sera. Once these products were removed, the 17D vaccine was safe again and was officially approved by the WHO.
The only serious difficulty encountered with this vaccine was its poor thermostability in the field, thus excluding its large-scale use in tropical areas where high ambient temperatures might inactivate the vaccine. Research to stabilize it was then undertaken in a number of pharmaceutical laboratories, which led to new 17D thermostable yellow fever vaccine preparations. These were finally available for intensive use in tropical areas in Africa and South America, including the hottest ones.
Today, the 17D vaccine is largely used either individually (for travelers going to endemic areas) or collectively for populations at risk. For instance, in tropical developing countries, the thermostable 17D vaccine is frequently associated with six other vaccine components in the Expanded Program on Immunization (EPI) for the greater advantage to young children.
American Or African Origin Of Yellow Fever
For a long time many American and European authors considered yellow fever to be primarily an American disease that first emerged in the Yucatan, Mexico, in 1648. However, in 1931, the great American epidemiologist H. R. Carter claimed that the true origin of the disease was in Africa (Carter, 1931).
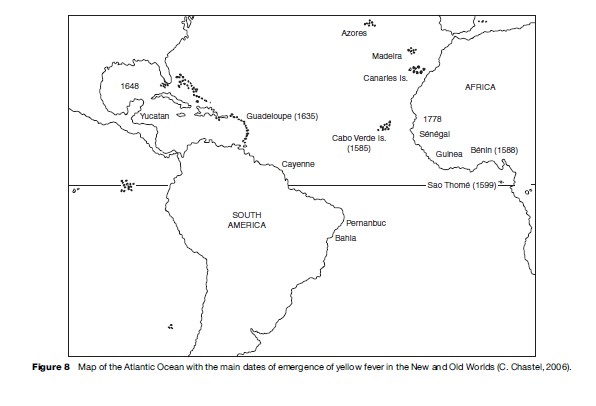
In fact, there are now many convincing arguments for the African origin of yellow fever. If one considers the history of epidemics occurring in the early European colonies established in the New World (Guadeloupe, 1635; Yucatan and West Indies, 1648), or aboard the fleets putting into ports or trading posts of the West Africa coasts (Cabo Verde Islands, 1585; Benin, 1588; Sao Tome, 1599), it appears that yellow fever was active in West Africa before 1778, the ‘official date’ of the first occurrence of the disease in Senegal (see Figure 8).
Indigenous peoples of the Americas appeared highly susceptible to yellow fever, as were the Europeans coming recently from Spain, Portugal, or France. However, the black slaves brought from Africa to the Americas were found to be relatively resistant, as though they had been already infected and immunized in their home countries.
Aedes aegypti, the vector of urban yellow fever, has long been the only American mosquito belonging to the Stegomya subgenus. It was probably not a true member of the original fauna of the Americas but was transported from Africa by the slave ships, which initiated their shameful traffic as early as 1501–1511 to both Santo Domingo and Cuba.
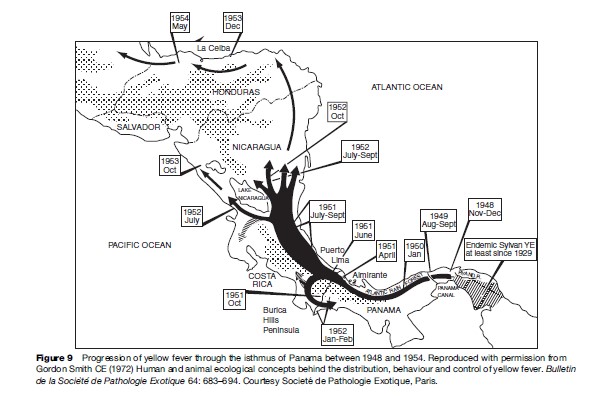
In America, yellow fever is a fatal disease of wild monkeys, and jungle yellow fever develops in successive waves of high mortality in these primates, spreading through the forests, as it did between 1948 and 1954 in Panama and Central America (Figure 9). This pattern clearly indicates that the yellow fever virus has been only recently introduced in the Americas and has not adapted yet there to its vertebrate hosts. On the contrary, jungle yellow fever in Africa is a silent disease with no death among monkeys and inducing generally only a mild disease among human beings contaminated in rainforests. This indicates an ancient and efficient adaptation of the virus to its environment (Chastel, 1998).
Molecular biological studies of yellow fever virus strains have resulted in the definition of at least five geographical genotypes: West Africa I, West Africa II, Central and Eastern Africa, South America I, and South America II. The most recent molecular studies have favored an African origin of this disease (Tolou et al., 1998).
Yellow Fever Today
Global Epidemiological Situation (December 2006)
Today, the disease occurs only in sub-Saharan Africa and tropical South America, where it is endemic and intermittently epidemic. A total of 18 735 cases and 4522 deaths were reported from 1987 to 1991 (Robertson et al., 1996), and the global epidemiological situation remained quite nasty in 2006.
In Africa, where most cases are reported, the fatality rate is greater than 20%, and infants and children are exposed to a greater risk of infection. A survey in rural West Africa during interepidemic or ‘silent’ periods indicates that such periods may last for years and even decades. The incidence of yellow fever was estimated to be 1.1–2.4 cases per 1000 persons. In 1991, an urban epidemic of yellow fever reappeared in Abidjan, Ivory Coast; among the 280 cases there were 6 deaths.
In South America, cases occur most frequently in young men who have occupational exposure to forest dwelling mosquito vectors in forested or transitional areas of Bolivia, Brazil, Colombia, Ecuador, Venezuela, Guyana, French Guiana, and Peru. In these countries the incidence of yellow fever is lower than in Africa because the mosquitoes which transmit the virus between monkeys in the forest canopy do not often come in contact with humans and because immunity in indigenous populations is high. Urban epidemic transmission has not occurred in South America for many years, but the risk of introduction of the virus in towns and cities is ever present.
Risk For Travelers
Only a small proportion of yellow fever cases are officially reported because the disease occurs in remote areas. So travelers in endemic areas in both African and South American areas may take the risk of contracting the disease if they are not vaccinated. Four out of five cases of yellow fever among travelers from the United States and Europe in 1996–2002 were exposed to the virus in South America. All five cases were fatal and occurred among unvaccinated travelers. The risk of illness in travelers from yellow fever has been estimated to be 0.4–4.3 cases per million travelers in yellow fever-endemic areas (Center for Disease Control and Prevention, Atlanta, GA, 12 December 2006; see Relevant Websites).
Bibliography:
- Barett ADT (1987) Yellow fever vaccines. Bulletin de l’Institut Pasteur 85: 103–124.
- Carter HR (1931) Yellow Fever. An Epidemiological and Historical Study of Its Place of Origin. Baltimore, MD: Williams and Wilkins.
- Chastel C (1998) Origin of yellow fever. Me´decine Tropicale, Marseilles 58: 59S–66S.
- Chastel C (2003) Centenary of the discovery of yellow fever virus and its transmission by a mosquito. Bulletin de la Socie´te´ de Pathologie Exotique 96: 250–256.
- Chaves-Carballo E (2005) Carlos Finlay and yellow fever: Triumph over adversity. Military Medicine 170: 881–885.
- Flexner S (1929) Hideyo Noguchi: A biographical sketch. Science 69: 653–660.
- Gorgas WC (1912) Sanitation at Panama. The Journal of the American Medical Association 58: 907–909.
- Gordon Smith CE (1972) Human and animal ecological concepts behind the distribution, behaviour and control of yellow fever. Bulletin de la Socie`te´ de Pathologie Exotique 64: 683–694.
- Haddow AJ, Smithburn KC, Dick GWA, Kitchen SF, and Lumsden WHR (1948) Implication of the mosquito Aedes (stegomyia) africanus (Theo) in the forest cycle of yellow fever in Uganda. Annals of Tropical Medicine and Parasitology 42: 218–223.
- Hudson NP (1966) Adrian Stokes and yellow fever research: A tribute. Transaction of the Royal Society of Tropical Medicine and Hygiene 60: 170–174.
- Monath TP (1988) Yellow fever. In: Monath TP (ed.) The Arboviruses: Ecology and Epidemiology, pp. 142–231. Boca Raton, FL: CRC Press.
- Monath TP (1991) Yellow fever: Victor, Victoria? Conqueror, conquest? Epidemics and research in the last forty years and prospects for the future. American Journal of Tropical Medicine and Hygiene 45: 1–43.
- Porterfield JS (1989) Yellow fever in West Africa: A retrospective glance. British Medical Journal 299: 1555–1557.
- Robertson SE, Hull BP, Tomori O, Bele O, Le Duc JW, and Esteves K (1996) Yellow fever: A decade of emergence. Journal of American Association 276: 1157–1162.
- Sellards W and Laigret J (1932) Vaccination de l’homme contre la fie` vre jaune. Comptes Rendus de l’Academie des Sciences Paris 194: 1609–1611.
- Soper FL, Penna H, Cardoso E, et al. (1933) Yellow fever without Aedes aegypti: Study of rural epidemic in the Valle do Chanaan, Espirito Santo, Brazil, 1932. American Journal of Hygiene 18: 555–587.
- Soper FL, Richard ER, and Crawford PJ (1934) The routine post-mortem removal of liver tissue from rapidly fatal yellow fever foci. American Journal of Hygiene 19: 549–566.
- Theiler M and Smith HH (1937) The use of yellow fever virus modified by in vitro cultivation for human immunization. Journal of Experimental Medicine 65: 767–786.
- Tolou H, Pisano MR, and Durand JP (1998) Molecular epidemiology of yellow fever. Me´decine Tropicale, Marseilles 58: 37–41.
- http://www.pasteur.fr/recherche/banque/CRORA – CRORA / Centre Collaborateur OMS de reference et de recherche´ pour les arbovirus et les virus des fie` vres he´ morragiques. IRD / Institut Pasteur de Dakar, Se´ ne´ gal.
- http://www.cdc.gov/health/diseases.htm – Center for Disease Control and Prevention (CDC), Atlanta, Georgia, United States.
- http://www.pasteur.fr/socpatex/pages – Socie´ te´ de Pathologie Exotique, Paris, France.
See also:
Free research papers are not written to satisfy your specific instructions. You can use our professional writing services to buy a custom research paper on any topic and get your high quality paper at affordable price.






