This sample Malaria Research Paper is published for educational and informational purposes only. If you need help writing your assignment, please use our research paper writing service and buy a paper on any topic at affordable price. Also check our tips on how to write a research paper, see the lists of health research paper topics, and browse research paper examples.
Clinical Features
The clinical features of malaria are largely dependent upon host immune status, intercurrent use of antimalarial drugs, and causative organism. Risk factors for severe disease include infection with P. falciparum and high parasite burden, age <2 in a P. falciparum-endemic region, and inadequate or ineffective chemoprophylaxis in nonimmune travelers to risk areas. Symptoms of P. falciparum infection generally manifest within 8 weeks of exposure, while those caused by other species may take several months to become apparent. In long-term residents of endemic areas, malaria infection may be completely asymptomatic.
Uncomplicated Malaria
Malaria is the most frequent cause of systemic febrile illness without localizing findings among ill travelers returning from the developing world. Fever (or a history of fever) is present in >90% of people who present to medical attention with malaria following international travel. Classic history is that of paroxysmal fevers occurring every 24–72 h, punctuated by fever-free intervals, and accompanied by chills, rigors, and sweats. These paroxysms reflect the periodicity of the malarial parasite: synchronous maturation of schizonts and lysis of parasitized erythrocytes leads to synchronous liberation of pyrogenic cytokines and inflammatory mediators, hence, the periodicity of fever. P. malariae, which causes ‘quartan’ malaria, has a 72-h life cycle, while P. vivax and ovale cause ‘tertian’ malaria, with paroxysms occurring every 48 h. P. falciparum, the species that causes the most severe disease and can lead to death, is associated with more frequent, daily paroxysms. These patterns can vary widely, and rarely demonstrate these ‘classic’ periodicities. Factors such as host immune status and intercurrent use of antimalarial drugs can greatly influence the presence or absence and periodicity of fever, as well as other presenting symptoms. Headache, nausea, vomiting, and mild delirium are frequent accompaniments of febrile paroxysms. While paroxysmal fever is more commonly reported with malaria, unremitting fever has also been described. Vague symptoms such as abdominal discomfort, myalgia, and fatigue antedate the onset of fever by 1–2 days. In semi-immune adult residents of endemic areas, low-grade fever may be the only sign to betray infection; thus, a high index of suspicion is warranted in any febrile patient with risk exposure. Rash, if present, should alert the clinician to an alternate diagnosis.
In addition to fever, anemia is a hallmark of malaria infection, whether uncomplicated or complicated. Hemoglobin level is often normal at presentation, possibly due to hemoconcentration, but falls over time. Contributors to malarial anemia are manifold, but include de facto lysis of parasitized erythrocytes, increased destruction of nonparasitized erythrocytes, and generalized bone marrow suppression in the acute stage of disease, due in part to reduced iron supply, and reduced marrow vascularity secondary to parasite sequestration. The anemia of uncomplicated disease tends to be worse in children, and in those with protracted infection.
Splenomegaly with thrombocytopenia (due to increased splenic clearance) are robust clinical predictors of those with acute malaria. Between 50–90% of patients with malaria will have nontender splenomegaly within a few days of symptom onset. Spontaneous, nontraumatic splenic rupture has been documented in ~20 cases of acute malaria, though this phenomenon may be underreported. Rupture is most likely to occur in the context of P. vivax infection and in nonimmune adults, as opposed to in residents of endemic regions where gradual splenic enlargement occurs over time, following repeated episodes of malaria. Increased intraabdominal pressure due to vomiting, rigors, or aggressive palpation on clinical exam are thought to increase the risk of splenic rupture during acute malaria. In those with chronic splenomegaly secondary to multiple attacks of malaria, frank trauma is generally implicated in splenic rupture. Abdominal pain with left upper quadrant tenderness, hypotension with orthostasis, left-sided pleural effusion, and signs of intraabdominal hemorrhage such as periumbilical ecchymoses should alert the clinician to the possibility of splenic rupture.
Acute malaria is further accompanied by defects in coagulation, characterized by enhanced intrinsic coagulation cascade activity and fibrinogen turnover, leading to increased concentration of fibrin degradation products. Hypoglycemia is a common complication of acute malaria, and arises in the context of reduced oral intake, increased catabolism due to fever, reduced hepatic gluconeogenesis and glycogenolysis, and parasite utilization of glucose as its major energy source. Cinchona alkaloids used in the management of malaria can further exacerbate this hypoglycemia by stimulating b-islet cell insulin secretion; therefore, vigilant monitoring is indicated.
Severe And Cerebral Malaria
Key events in the pathogenesis of malaria include (1) invasion of host erythrocytes by the malarial parasite, (2) adherence of parasitized erythrocytes to cell surface molecules expressed in the vasculature of vital organs (sequestration), and (3) induction of proinflammatory mediators in response to infection. Severe complications arise due to each of these mechanisms and the host’s immune response to them. The similarity of early-stage malaria to many systemic viral illnesses can lead to diagnostic and therapeutic delay, which enables the parasite burden to increase and the pathogenesis of severe malaria to ensue. Severe and cerebral malaria are almost universally caused by P. falciparum, and carry mortality rates of 10–30%, even in the setting of appropriate parenteral antimalarial drug therapy. While deaths due to the other three species infecting humans have been reported, these cases are extremely rare. However, infection with P. vivax has been reported to cause severe manifestations such as acute renal failure (ARF), severe thrombocytopenia, shock, acute respiratory distress syndrome (ARDS), and encephalopathy, though these sequelae are much less likely to occur with P. vivax than with P. falciparum infection. The clinical features of severe malaria differ between adults and children, with end-organ failure occurring more commonly in adults, and severe malarial anemia with cerebral involvement occurring more commonly in children. Criteria for the diagnosis of severe or complicated malaria are listed in Table 1.
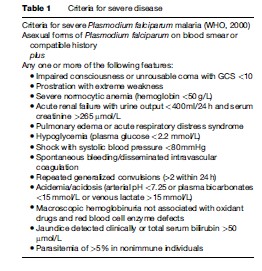
Consistent predictors of intensive care unit mortality in patients with severe malaria include unrousable coma, pulmonary edema, shock, and metabolic acidosis, with those patients who die having the highest day-1 parasitemias, lowest Glasgow Coma Scale (GCS) scores, greatest need for mechanical ventilation, lowest arterial pH, and highest serum lactate levels. Metabolic acidosis is the single best independent predictor of adverse outcome in children and adults with severe malaria.
Nonimmune Adults
Adults traveling to P. falciparum-endemic areas, particularly sub-Saharan Africa, and those residing in regions where transmission is sporadic (Southeast Asia) are at risk of severe malaria. In addition to fever, common presenting signs include jaundice (2/3 patients), hyperparasitemia (1/2 patients), prostration (1/2 patients), ARF (1/3 patients), and bleeding diatheses (1/6 patients). Less than 10% of adult patients with severe malaria will present with severe acidosis, coma, seizures, ARDS, or shock, however, those in whom diagnosis and appropriate management are delayed are at increased risk of developing these complications.
Jaundice in severe malaria arises due to dysfunction at prehepatic, hepatic, and posthepatic levels. Hemolysis, hepatocyte dysfunction (as evidenced by reduced clotting factor synthesis and gluconeogenesis), and an acalculous cholestatic phenomenon all contribute to hyperbilirubinemia, the latter of which can persist for weeks. Acute tubular necrosis secondary to glomerular filtration of hemoglobin liberated from lysed red cells is the underlying pathogenesis of the oliguric ARF seen in severe malaria. ‘Blackwater fever’ refers to the voiding of cola-colored urine in the setting of significant intravascular hemolysis due to antimalarial drug hapten formation, and while this condition in and of itself creates a massive hemoglobin load for the kidney to process, it is rarely accompanied by ARF. Thrombocytopenia coupled with prolonged prothrombin (PT) and partial thromboplastin (PTT) at times leads to stigmata of bleeding, including gastrointestinal hemorrhage. Disseminated intravascular coagulation is the most severe on the spectrum of malaria-related coagulopathies.
Lactic acidosis, while less common than jaundice, ARF, and severe thrombocytopenia at presentation, is a major predictor of poor outcome in severe malaria. Hypovolemia, anemia, microvascular obstruction due to sequestration or parasitized erythrocytes, and cytotoxicity of circulating inflammatory mediators all contribute to metabolic acidosis. Serum levels of lactate are occasionally normal in the setting of metabolic acidosis, supporting that other acid metabolites play a role in the pathogenesis of this severe manifestation of malaria.
Pulmonary edema is a feature of severe malaria that occurs in the absence of reduced left ventricular ejection fraction, and reflects increased pulmonary vascular permeability. Along with pulmonary edema, metabolic acidosis with compensatory respiratory alkalosis, and malarial anemia both contribute to the development of ARDS, itself a harbinger of mortality. ARDS is one of the later complications to occur in the setting of severe, acute malaria, usually arising on day 4 or 5 after admission. Anticipation of this complication is prudent.
Cerebral malaria is typified by unrousable coma (GCS <11 with no localizing pain response) in a patient with asexual P. falciparum parasitemia. The cerebral malaria that occurs in adults differs from that occurring in children. Typical clinical manifestations of cerebral malaria in adults include: coma (which occurs several days following the onset of benign symptomatology), seizures (20–50%), upper motor neuron lesions (characterized by increased tone and hyperreflexia), decorticate or decerebrate posturing, dysconjugate gaze, and retinal hemorrhages (15%). Pupillary, oculocephalic, and oculovestibular reflexes are generally intact. Coma likely results from a combination of vasoocclusion due to sequestion, metabolic acidosis, toxic cytokine milieu, and compromise of the blood–brain barrier leading to increased cerebral vascular permeability. Long-term neurological sequelae occur in only ~5% of adults who survive cerebral malaria.
Semi-Immune And Nonimmune Children
Ninety percent of deaths due to falciparum malaria occur in African children under the age of 5 years. Unlike in adults, severe malaria in children is more likely to be characterized by severe anemia, hypoglycemia, and cerebral manifestations including repeated generalized seizures, and less likely to be accompanied by pulmonary edema, jaundice, and coagulopathy.
Severe malarial anemia refers to a hemoglobin level <50 g/L (<5 g/dl) coupled with a parasitemia >10 000 parasites per cubic ml, and arises due to parasite-dependent lysis of red blood cells, autoimmune hemolysis, and bone marrow toxicity. Up to 20% of African children under 5 years of age with malaria will fulfill criteria for severe malarial anemia, and an additional 10% will fulfill criteria for cerebral malaria, the latter of which carries a mortality rate of 10–30%. Compared to adults with cerebral malaria, coma is rapid in onset and resolution in children. Generalized seizures are both more common and frequent in children with cerebral malaria versus adults. Children are also more likely than adults to exhibit focal cranial nerve and brainstem reflex abnormalities in the context of cerebral malaria. Furthermore, in children with cerebral malaria, tone tends to be more flaccid than rigid, which is more reminiscent of lower motor neuron disease. Retinal hemorrhages are found in up to one-third of children with cerebral malaria. Children who have survived cerebral malaria carry double the risk of long-term neurologic sequelae of adults. Persistent neurologic abnormalities include seizure disorder, developmental delay, behavioral disturbance, hemiparesis, ataxia, aphasia, blindness, and tone deficits, most likely spasticity.
Malaria In Pregnancy
Malaria in pregnancy can have catastrophic consequences for both the mother and fetus, and is more likely to take a severe or complicated course, including pulmonary edema and severe hypoglycemia, than in nonpregnant women. Severe and cerebral malaria in pregnancy also carry higher mortality rates, with up to 50% of pregnant women with cerebral malaria dying of the disease. Reduced systemic and placental cell-mediated immunity can lead to hyperparasitemia with concomitant severe anemia. Placental sequestration and cytokine activation can further lead to placental insufficiency, which translates into fetal intrauterine growth restriction, and ultimate low birth weight. In holo and hyper endemic areas of malaria transmission, this risk of low birth weight is confined to the first pregnancy, whereas in areas of low or unstable transmission, this risk applies to multigravid women as well.
Malaria And HIV Co-Infection
Current evidence supports that HIV and malaria each negatively affect the outcome and course of the other. It has been suggested that HIV-related immune suppression compromises innate host malaria clearance mechanisms. In regions where malaria transmission is low or unstable, HIV-positive adults with malaria are two to fivefold more likely to have a complicated or severe course. HIV has also been shown to impair the response to antimalarial therapy, with higher rates of treatment failure among those who are co-infected. Malaria has been shown to upregulate HIV transcription, with those who are co-infected demonstrating a two to sevenfold increase in HIV viral load during acute malaria infection. Whether this acute rise in viremia is clinically significant is debatable. However, at least one study in Uganda has shown a significant decline in CD4 counts in HIV-positive patients following episodes of malaria.
Management
There are three cardinal questions that must be addressed for optimal management of malaria:
- Is the infection caused by P. falciparum?
- Is the malaria complicated or severe?
- Is the parasite likely to be drug-resistant?
The answers to these questions will greatly influence the decisions made in managing these patients.
Is The Infection Caused By P. Falciparum?
Species identification of the infecting organism is critical to appropriate management. P. falciparum can cause life-threatening disease in nonimmune adults and children, and should be considered a medical emergency. The clinical descent down the slope of severe manifestations can be rapid; therefore, prompt initiation of therapy is essential. While the management objectives for P. vivax, P. malariae, and P. ovale are to cure disease and, in the case of P. vivax and P. ovale, to prevent relapse, because P. falciparum can cause fatal disease, its treatment objectives are to cure disease and to prevent death. Thick and thin Giemsa-stained blood smears allow for species identification and quantification of parasitemia. Point-of-care speciation is also available through use of sensitive rapid dipstick assays, which detect P. falciparum antigens such as HRP-2. While molecular assays also permit speciation, as yet, their turnaround times are prohibitive for point-ofcare diagnostics.
Is The Malaria Complicated Or Severe?
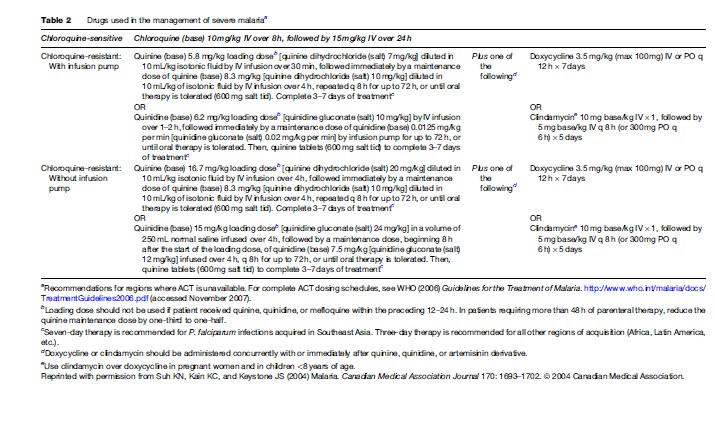
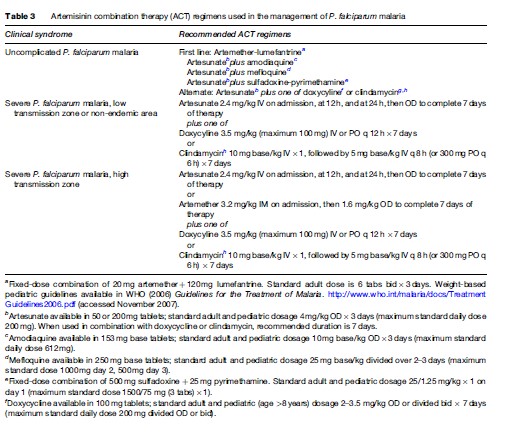
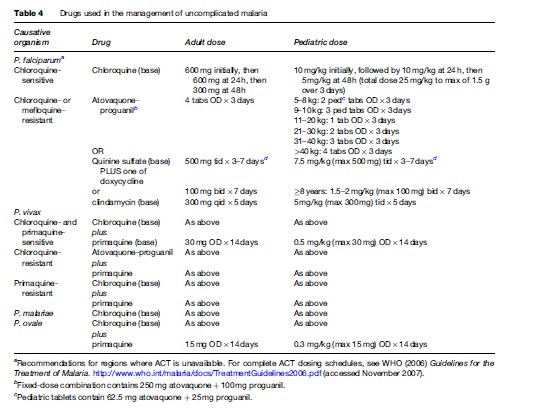
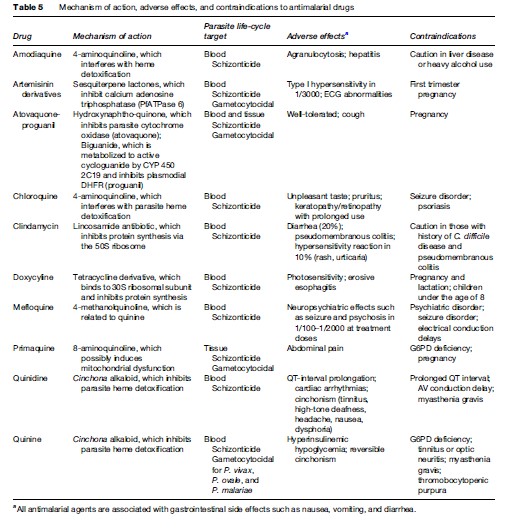
Severe or cerebral malaria necessitates immediate administration of combination parenteral antimalarials (Table 2) and ongoing supportive care. Artemisinin derivatives are the most potent antimalarials known to man, and are derived from the leaves of the Artemisia annua plant. Artemisinin combination therapy (ACT), which pairs an artemisinin derivative with another antimalarial drug, is recommended by the WHO for management of all P. falciparum malaria, whether severe or not, in areas where these drugs are available. However, the lack of availability of ACT in many Western countries limits its use predominantly to endemic areas in Africa and Southeast Asia. The choice of therapy will also therefore depend on location and local formularies. Table 2 summarizes the current recommendations for therapy of severe P. falciparum malaria in areas where ACT is unavailable. Table 3 lists the currently recommended ACT regimens for P. falciparum malaria. A comprehensive discussion of ACT dosing schedules and practicalities of administration can be found in the 2006 WHO Guidelines for the Treatment of Malaria. Oral therapy alone, while indicated for uncomplicated malaria (Table 4), is inadequate in the setting of severe disease, or in patients who are vomiting. Adverse effects and contraindications to currently used antimalarials are listed in Table 5. Vigilant patient monitoring in an ICU setting is ideal, with particular attention paid to vital signs, glycemia (accuchecks q 2–4 h), neurologic status, arterial blood gas, urine output, and parasitemia.
Fluid therapy in the context of severe malaria remains controversial, and must be assessed on an individual basis. A balance must be struck between the risk of fluid overload, to which adults with severe disease are particularly vulnerable, and dehydration, which will necessarily worsen concomitant renal failure. Children with severe malaria are more likely than adults to be volume depleted, and may respond favourably to a fluid bolus. The hyperventilation and respiratory distress seen in children with severe malaria is attributable to metabolic acidosis and severe anemia. Blood transfusion is therefore indicated in children with hemoglobin <50 g/L (<5 g/dl) who are from high-transmission zones. A more conservative threshold for transfusion of hemoglobin, <70 g/L (<7 g/dl), can be applied to those with severe anemia who reside in low-transmission zones or are residents of the developed world who have returned from travel to an endemic area.
Acute pulmonary edema should be managed as per standard of care with supplemental oxygen, positive pressure ventilation if available and the patient is hypoxic, and therapeutic maneuvers that reduce preload on the heart, including diuresis, venodilation, hemofiltration, and dialysis. In patients with significant coagulopathy, vitamin K and fresh frozen plasma can be given. Correction of hypovolemia and anemia are key to ameliorating metabolic acidosis. To date, no other intervention has proven valuable.
Seizures are a common complication of cerebral malaria, especially in children. Administration of intravenous benzodiazepines is the mainstay of treatment of seizures due to cerebral malaria. The WHO currently recommends against use of phenobarbital in children with malarial seizures, due to its association with increased mortality (WHO, 2006). Preemptive administration of antiseizure medication is not recommended.
Hyperparasitemia is a major risk factor for death from severe malaria. Parasitemia should therefore be followed every 6–12 h over the first 24–48 h of therapy. In addition to parenteral antimalarials, exchange transfusion or aphoresis has been touted as an effective way to reduce parasite burden, though evidence to support this maneuver is scant and it thus remains controversial. Its use should probably be limited to situations in which prognosis is grave, parasitemia is >30%, or parasitemia is >10% with accompanying evidence of end-organ dysfunction including cerebral, pulmonary, or renal manifestations.
The continued high rate of mortality in severe and cerebral malaria has led to the investigation of a number of adjuvant therapies for P. falciparum malaria including corticosteroids, monoclonal antibodies to TNFa, TNFa inhibitors, heparin, prostacyclin, and desferroxamine. None of these adjuvant therapies has proven efficacious in clinical trials, and therefore none is recommended.
In cases of severe malaria, where presentation is that of a systemic febrile illness with evidence of multisystem involvement, the concurrent use of parenteral broadspectrum antibiotics should be liberal. Intercurrent bacterial infection is common, particularly in children, and may be overshadowed by the diagnosis of malaria. Thus, this entity must be anticipated, especially in the setting of unexplained deterioration despite effective antimalarial and supportive therapy.
Because P. falciparum infection in pregnancy is associated with low birth weight, adverse fetal outcome, severe maternal anemia, and increased risk of severe sequelae, particularly pulmonary edema and hypoglycemia, administration of antimalarial therapy must not be delayed. Prompt initiation of parenteral therapy is indicated in pregnant women with severe malaria. In later stages of pregnancy, artesunate may be preferred over quinine due to the latter’s association with recurrent hypoglycemia.
Pharmacotherapy of uncomplicated malaria is summarized in Table 4. Unless a patient cannot tolerate oral medication due to vomiting or is believed to have poor gastrointestinal absorption, therapy of uncomplicated malaria should be via the oral route. Outside of Africa, P. vivax is the predominant species causing disease. P. vivax and P. ovale have a persistent liver phase that is responsible for clinical relapses. In order to reduce the risk of relapse following the treatment of symptomatic P. vivax or P. ovale infection, primaquine may be indicated to provide ‘radical cure.’ Primaquine use is contraindicated in pregnancy; therefore, P. vivax and P. ovale infections occurring during pregnancy should be treated with standard doses of chloroquine. Relapses can then be prevented by weekly chemosuppression with chloroquine until after delivery. Because of primaquine’s oxidant hemolytic potential, patients should undergo testing for glucose-6phosphate dehydrogenase deficiency prior to initiation of primaquine therapy.
Is The Parasite Likely To Be Drug-Resistant?
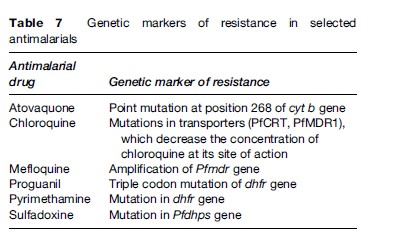
In most areas of the world where malaria is transmitted, it is caused by drug-resistant parasites (Table 6). Antimalarial resistance has been described in all species of Plasmodium infecting humans, except P. ovale. P. falciparum resistance to most antimalarials, with the exception of artemisinin derivatives, has been documented. However, in only a few of these drugs can genetic markers of resistance be identified (Table 7). Chloroquine resistance in P. falciparum is widespread, with efficacy confined to P. falciparum-endemic areas north of the Panama Canal in Central America and the Caribbean. Mefloquine and multidrug resistance in P. falciparum is, for the most part, confined to the Thai–Burmese and Thai–Cambodian borders, along with parts of Vietnam and eastern Burma. P. falciparum resistance to quinine monotherapy has been reported in Southeast Asia, but is of little consequence when quinine is used as a component of combination therapy.
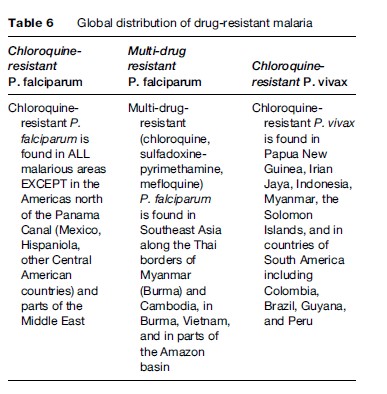
Chloroquine resistance in P. vivax is mainly found in Papua New Guinea and Papua (Irian Jaya), along with other countries of Oceania such as Indonesia, East Timor, and the Solomon Islands. There have also been isolated reports of chloroquine-resistant P. vivax from the Amazonian regions of Peru, Brazil, and Guyana. Chloroquine remains an effective antimalarial for most P. vivax acquired in Southeast Asia (other than in Thailand, Korea, and Myanmar), subcontinental India, the Middle East, and Latin America. Only recently has chloroquine resistance in P. malariae from Indonesia been described (Maguire et al., 2002). To date, P. ovale remains fully sensitive to the existing antimalarial pharmacologic armamentarium.
Treatment is considered to have failed if fever and parasitemia fail to resolve or recur within 14–28 days of treatment initiation. These failures may arise due to drug-resistant organisms, malabsorption of the drug (vomiting or diarrhea), or poor adherence. A full course of retreatment with an alternate regimen is indicated in these cases. If fever and parasitemia recur >2 weeks post-treatment, this likely reflects recrudescence, or reinfection in an endemic zone.
Prevention
Prevention In Travelers
Malaria is a preventable disease in travelers. A general approach to the prevention of malaria in travelers involves an assessment of individual risk, a discussion of mosquito bite avoidance measures, and the prescription of chemoprophylactic agents where appropriate.
Assessment Of Individual Risk
Many factors contribute to an individual’s risk of acquiring malaria when traveling and include, but are not limited to:
- Geographic destination;
- Type of travel (urban vs. rural);
- Type of accommodations (tents vs. screened rooms);
- Itinerary during travel (trekking, altitude, river or jungle exposure);
- Season of travel (wet vs. dry);
- Duration of travel (short-term vs. long-term);
- Likelihood of compliance with preventive measures.
All travelers to malarious areas should receive specific pretravel counseling from a qualified professional who can provide an educated assessment of the individual traveler’s risk of acquiring malaria abroad, and who can address the following two pillars of malaria prevention.
Mosquito Bite Avoidance
Use of personal protective measures and behaviors to reduce the likelihood of being bitten by a female anopheline mosquito is key to the prevention of malaria. These measures and behaviors include, but are not limited to:
- Avoidance of outdoor activity after dusk (Anopheles mosquitos bite from dusk from dawn);
- Use of long-sleeved shirts and long pants when exposure is likely;
- Use of N,N-diethyl-3-methylbenzamide (DEET)based mosquite repellants;
- Use of permethrin or other insecticide-impregnated bed net.
Aerosol, pump, and gel-based formulations containing 20–50% DEET can be used safely and efficaciously in adults and children over 2 months of age. Picardin is another commonly used repellant that is safe and effective in both adults and children, and tends to cause less skin irritation than DEET. Insecticide-impregnated bed nets can be used safely by both pregnant women and children. Permethrin is also available in liquid or spray formulations for impregnation of clothing.
Chemoprophylactic Agents
Following a detailed assessment of individual risk of malaria and counseling around personal protective measures for bite avoidance, the pretravel encounter can be directed toward a needs assessment for chemoprophylaxis. In order to prescribe an appropriate agent, the travel medicine practitioner will want to determine:
- If the traveler will be exposed to malaria;
- Which species of Plasmodium predominates in his or her region of travel;
- If there is likely to be drug-resistant P. falciparum at his or her destination;
- Whether or not the traveler is likely to adhere to the prescribed regimen;
- If there are any contraindications to the prescribed regimen such as pregnancy or likelihood of pregnancy;
- Whether or not the traveler will have ready access to medical attention during travel.
A detailed past medical history is important to gather in order to determine if there are contraindications to a particular class of antimalarials.
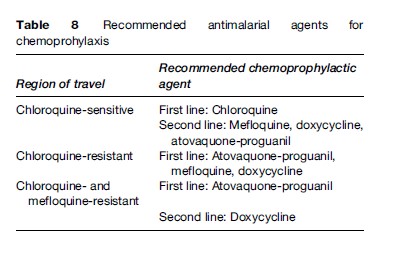
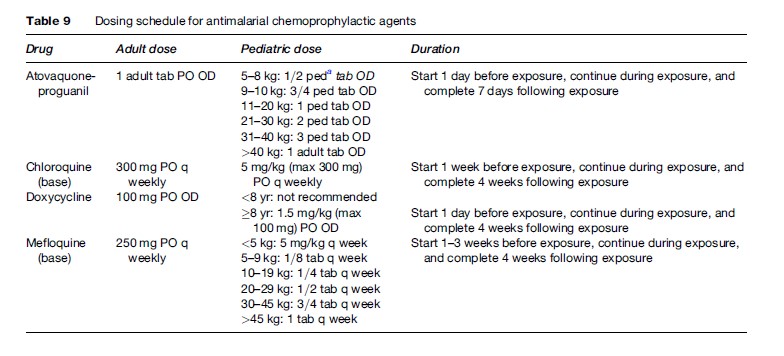
Tables 8 and 9 summarize the antimalarials that are currently recommended for chemoprophylaxis in travelers to malarious regions. It is important to emphasize to the traveler that all chemoprophylactic regimens must be started prior to arrival in the risk area, taken throughout travel in the risk area, and continued for one to four weeks post-travel, depending on the regimen. Drugs such as atovaquone-proguanil that work by preventing the preerythrocytic development of the parasite (causal prophylaxis) need only be taken one week post-travel. Conversely, drugs such as mefloquine, chloroquine, and doxycycline that work by suppressing blood stage infection need to be taken for a full four weeks post-travel.
In travelers who will not have timely access to medical care, standby therapy (self-treatment) can be life-saving. Self-treatment may also be an appropriate option for those in whom the chemoprophylactic regimen is suboptimal due to underlying medical condition. Options for standby therapy include atovaquone-proguanil, chloroquine (in a chloroquine-sensitive region), or quinine plus doxycycline. Dosing is as outlined in Table 4. It should be emphasized to travelers that self-treatment in no way obviates the need for timely medical attention, nor should it be perceived as an alternative to prophylaxis. In addition, travelers should be discouraged from purchasing self-treatment regimens overseas or from switching their prophylactic regimen while abroad.
Prevention of malaria in the pregnant traveler presents a challenge due to widespread chloroquine resistance in P. falciparum, and the lack of chemoprophylactic agents that can be used safely in all trimesters. Chloroquine can be safely used during all trimesters of pregnancy, and is appropriate for prophylaxis against chloroquine-sensitive P. falciparum. The decision to travel to an area with high transmission of chloroquine-resistant P. falciparum while pregnant should not be taken lightly. P. falciparum malaria can have grave consequences for mother, fetus, and neonate. If transmission is thought to be high and likelihood of exposure will be great, and travel cannot be deferred, then mefloquine prophylaxis is a reasonable option. While there is mounting evidence that atovaquoneproguanil is likely to be safe in pregnancy, insufficient data are available to recommend its use as a prophylactic agent in pregnancy. Doxycycline and primaquine are contraindicated in pregnancy.
Prevention In Residents Of Endemic Areas
Due to the prohibitive costs and infrastructural requirements of mass prophylaxis campaigns, insecticide-treated bed nets and targeted chemoprophylaxis remain the mainstays of malaria prevention in endemic areas. Children less than 5 years of age and pregnant women are candidates for intermittent preventive therapy (IPT) or continuous chemoprophylaxis. IPT consists of twice or thrice pre-emptive therapy during the course of pregnancy (or infancy) with an agent such as chloroquine or chloroquine-proguanil. This strategy in pregnancy reduces the risk of severe malarial anemia, low birth weight, and severe disease in pregnant women, though these benefits are largely seen in primigravidae. Insecticidetreated bed nets have been shown to significantly reduce the burden of childhood mortality secondary to malaria, and to reduce the incidence of anemia and malaria in pregnancy.
Malaria Vaccine
Malaria vaccine initiatives have been ongoing for over 30 years now; however, only recently have candidate malaria vaccines been tested in humans in clinical trials. To date, investigational vaccines have been designed to target specific stages of the parasite (notably P. falciparum) life cycle, including the preerythrocytic/liver stages, asexual erythrocytic stages, sexual blood stages, and mosquito stages. The complexity of the parasite life cycle has hampered the development of successful candidate vaccines, as immunity to one life cycle stage (e.g., liver stage) confers no protection to other stages (e.g., blood stages). Natural immunity to P. falciparum is highly strain-specific and ephemeral, and requires multiple episodes of infection (boosting) to maintain both humoral and cell-mediated immunity. That P. falciparum expresses approximately 5300 antigens further hinders vaccine development, as it is currently unknown which of these antigens are key players in the genesis of immunity.
One preerythrocytic stage vaccine, RTS,S/AS02, has shown promise in early clinical trials. This vaccine was designed to target the malarial circumsporozoite protein, and the vaccine antigen is comprised of a fusion protein (RTS) expressed in yeast, which binds to hepatitis B surface antigen (S) to form RTS,S. When mixed with an adjuvant, AS02, and given intramuscularly to volunteer vaccinees, RTS,S induces a high-titer antibody response to both circumsporozoite protein and hepatitis B surface antigen. In a randomized controlled trial in the Gambia, adults given three doses of RTS,S/AS02 were protected from developing natural P. falciparum malaria (Bojang et al., 2001). Vaccine efficacy was 71% in the first nine weeks of the surveillance period, and 34% during the entire 15-week surveillance period (Bojang et al., 2001). No protection was afforded by the vaccine in the final six weeks of surveillance, reiterating that immunity is very short-lived. In a follow-up study, it was shown that the protection conferred by RTS,S/AS02 was not strainspecific (Alloueche et al., 2003). Additional studies are ongoing in Mozambique. While there are many other candidate vaccines entering the early stages of clinical evaluation in humans, RTS,S/AS02 is the first to demonstrate efficacy in natural P. falciparum infection. These results are very promising, and a commercially available vaccine is on the horizon.
Bibliography:
- Alloueche A, Milligan P, Conway DJ, et al. (2003) Protective efficacy of the RTS,S/AS02 Plasmodium falciparum malaria vaccine is not strain specific. American Journal of Tropical Medicine and Hygiene 68: 97–101.
- Bojang KA, Milligan P, Pinder M, et al. (2001) Efficacy of RTS,S/AS02 malaria vaccine against Plasmodium falciparum infection in semi-immune adult men in The Gambia: A randomized trial. Lancet 358: 1927–1934.
- Maguire JD, Sumawinata IW, Masbar S, et al. (2002) Chloroquineresistant Plasmodium malariae in south Sumatra, Indonesia. Lancet 360: 58–60.
- World Health Organization (2000) Severe falciparum malaria. Transactions of the Royal Society of Tropical Medicine and Hygiene 94(supplement 1): 1–90.
- World Health Organization (2006) Guidelines for the Treatment of Malaria. Geneva, Switzerland: World Health Organization.
- Baird JK (2005) Effectiveness of antimalarial drugs. New England Journal of Medicine 352: 1565–1577.
- Franco-Paredes C and Santos-Preciado JI (2006) Problem pathogens: Prevention of malaria in travelers. Lancet Infectious Diseases 6: 139–149.
- Greenwood BM, Bojang K, Whitty CJM, and Targett GA (2005) Malaria. Lancet 365: 1487–1498.
- Hewitt K, Stekete R, Mwapas V, et al. (2006) Interactions between HIV and malaria in non-pregnant adults: Evidence and implications. AIDS 20: 1993–2004.
- Leder K, Black J, O’Brien D, et al. (2004) Malaria in travelers: A review of the GeoSentinel surveillance network. Clinical Infectious Diseases 39: 1104–1112.
- Mackintosh CL, Beeson JG, and Marsh K (2004) Clinical features and pathogenesis of severe malaria. Trends in Parasitology 20: 597–603.
- Moorthy VS, Good MF, and Hill AVS (2004) Malaria vaccine developments. Lancet 363: 150–156.
- Schlagenhauf P and Kain KC (2007) Malaria chemoprophylaxis. In: Keystone J, Kozarsky P, Nothdurft H, Freedman D, and Conner B (eds.) Travel Medicine, 2nd edn. ch.12. Philadelphia, PA: Elsevier Science.
- Suh KN, Kain KC, and Keystone JS (2004) Malaria. Canadian Medical Association Journal 170: 1693–1702.
- Targett GA (2005) Malaria vaccines 1985–2005: A full circle? Trends in Parasitology 21: 499–503.
- Warrell DA (1997) Cerebral malaria: Clinical features, pathophysiology, and treatment. Annals of Tropical Medicine and Parasitology 91: 875–884.
- White NJ (2004) Malaria. In: Cook GC and Zumla AI (eds.) Manson’s Tropical Diseases, 21st edn, pp. 1205–1295. London: WB Saunders.
- http://www.who.int/ith – World Health Organization, International Travel and Health, 2007.
- http://www.cdc.gov/malaria – Centers for Disease Control and Prevention, Malaria.
- http://www.cdc.gov/malaria/diagnosis_treatment/tx_clinicians.htm – Centers for Disease Control and Prevention, Treatment of Malaria (Guidelines for Clinicians).
- http://www.cdc.gov/travel/regionalmalaria/index.htm – Centers for Disease Control and Prevention, Travelers’ Health.
- http://wwwn.cdc.gov/travel/contentYellowBook.aspx – Centers for Disease Control and Prevention, CDC Health Information for International Travel, 2008.
See also:
Free research papers are not written to satisfy your specific instructions. You can use our professional writing services to buy a custom research paper on any topic and get your high quality paper at affordable price.








