This sample Prostate Cancer Research Paper is published for educational and informational purposes only. If you need help writing your assignment, please use our research paper writing service and buy a paper on any topic at affordable price. Also check our tips on how to write a research paper, see the lists of health research paper topics, and browse research paper examples.
Introduction
Prostate cancer is the most commonly diagnosed male malignancy in developed countries. With age, the total prevalence of prostate tumors reaches very high levels. Only a few percent of these become clinically significant with the potential to kill, and the challenge described by Boccon-Gibod (1996) is to distinguish tigers from pussy cats; to identify the minority of lethal cancers from the majority of nonaggressive tumors. Answers to this challenge remain elusive. We do not know what causes lethal prostate cancer; little can be done to prevent it, and, until it is possible to tell the tigers from the pussycats, this is not likely to change.
Anatomy, Physiology, And Pathology
The prostate is a walnut-sized gland located at the base of the pelvis beneath the urinary bladder, surrounding the urethra (Figure 1). Its initial development is stimulated by testosterone from the fetal testes but it remains incompletely developed until puberty, when it grows to its adult size of about 20 g around age 20 years. The prostate develops 30–50 branched ducts that are lined with glandular epithelium and open into the prostatic urethra. Its function is to contribute up to 30% of the volume of semen with slightly alkaline secretions including prostaglandins, proteolytic enzymes, acid phosphatase, zinc, and citric acid, which help maintain sperm viability.
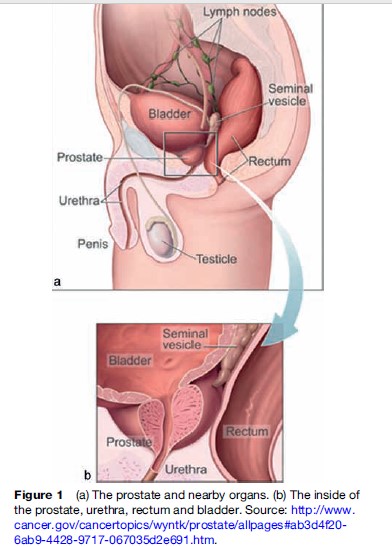
McNeal (1981) first identified the prostate’s zonal anatomy. The peripheral zone, where 60–70% of tumors occur, contains about 70% of the total volume and is accessible via digital rectal examination (DRE). The central zone, containing 25% of the volume and the ejaculatory ducts, is where inflammatory processes such as prostatitis arise. The transitional zone, where 25% of tumors occur, contains only 5% of the volume. The anterior zone is fibromuscular.
As the hormonal milieu changes with middle age, the prostate grows, a condition termed benign prostatic hyperplasia (BPH). Due to the inelasticity of the prostate’s fibrous outer capsule, increasing pressure is placed on the prostatic urethra, producing urinary symptoms. Depending on severity, these problems come to medical attention and in this context cancer is often diagnosed. BPH was once considered a risk factor for prostate cancer, but this view is no longer held; both conditions simply occur commonly in the aging prostate gland. A small proportion of cancers are found in tissue fragments from transurethral resections performed to relieve obstructive symptoms of BPH.
Virtually all prostatic tumors are adenocarcinomas arising from the glandular epithelium and most men will develop them if they live long enough. Although much research has sought tumor markers that would separate the tigers from the pussy cats, this is still beyond reach. A tumor’s potential lethality (aggressiveness) is currently based on its degree of spread beyond the capsule and microscopic inspection by a pathologist, who identifies the two largest tumor foci (primary and secondary patterns) and grades each from 1 to 5 according to the histologic features described by Gleason (Gleason, 1977) (see Figure 2).
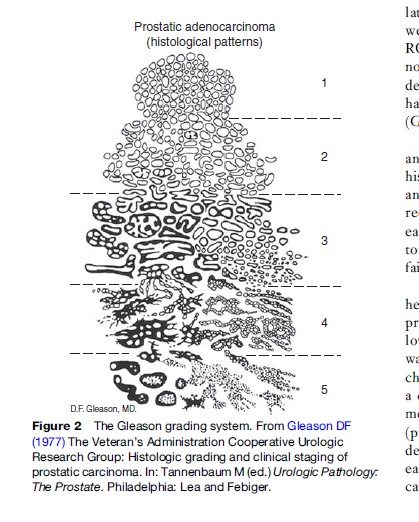
Gleason grades for the two foci, which are rarely more than one grade apart, are added to give a sum from 2 to 10. Tumors with Gleason sums below 5 are not considered aggressive. Historically, tumors with Gleason sums 5–7 were considered low risk but tumors with a Gleason score of 7 have been added to the high-grade aggressive category (formerly Gleason scores 8–10). The significance of Gleason sum 7 tumors remains controversial, especially with respect to decisions about treatment. Approximately 30% of Gleason sum 7 tumors have a primary focus of grade 4 and these are considered to be more aggressive than those having a primary focus of grade 3. Some clinicians consider that any focus of Gleason grade 4 warrants clinical suspicion.
Diagnosis, Screening, And Treatment
Prostate cancer has no specific symptoms until advanced. Its size and the presence of any lumps are assessed by DRE and ultrasound; the diagnosis of cancer is aided by measurement of the serum levels of prostate specific antigen (PSA), a protease enzyme contributed by the prostate to seminal fluid, some of which permeates into the bloodstream. Originally used clinically to monitor cancer progression after treatment, PSA is now used for early detection. High serum PSA levels almost certainly indicate the presence of malignancy, but its use also leads to overdiagnosis, that is, the diagnosis of tumors that would never have progressed to clinically significant tumors during life, the so-called pussycats.
Suspicious PSA levels are followed up with ultrasoundguided needle biopsies to establish a histopathologic diagnosis. Prostate tumors are commonly small and multifocal, and there is an element of chance in whether a tumor can be adequately identified by biopsy. The number of biopsies used to establish a diagnosis has increased over time. The PSA threshold level used to prompt clinical investigation has also fallen, especially in the United States.
PSA testing for early prostate cancer is already being performed on an ad hoc or opportunistic basis in many countries. Randomized clinical trials (RCTs) are being conducted to test the efficacy of systematic PSA screening in reducing prostate cancer mortality in the whole population (Schroder 1994). Because many controls (men who were randomly assigned not to have a PSA test) in these RCTs have in fact received PSA tests as part of their normal community care, the power of the trials to provide definitive evidence of efficacy has been reduced, and this has lengthened the duration of the trials by some years (Gohagen et al., 1994).
Screening, to be effective, requires a cheap, sensitive, and specific test, a good understanding of the natural history to identify a curable early form of the disease, and good evidence that treatment of this form of disease reduces mortality by comparison with treatment of disease diagnosed in the ordinary way at the onset of symptoms. Prostate cancer screening by PSA testing currently fails to satisfy these criteria.
Given the uncertainties that surround its biological heterogeneity and limited evidence of treatment benefits, prostate cancer management is complex. Men with low-grade, localized cancer and a low PSA may opt for watchful waiting, with regular repeat PSA tests to monitor changes, termed PSA velocity. Once PSA velocity reaches a certain point, decisions are made with respect to treatment. Radical treatments for localized disease are surgery (prostatectomy) or implantation with radioactive seeds to deliver localized radiation (brachytherapy). Advanced disease is treated by androgen deprivation/blockade, which can involve surgical or medical castration, and external beam radiation. Principal concerns are side effects, such as impotence, incontinence, and damage to the rectum and bladder neck, and the uncertainty posed by the limited evidence for substantial benefit of treatment.
Variation In Incidence And Mortality
Autopsy studies carried out by Yatani and others (1982) showed that the prevalence of microscopic, nonaggressive tumors (historically termed latent cancers) increased with age, affecting a majority of prostates by age 60 years, but varied little between populations, any variation in total incidence being due to differences in levels of invasive disease. Because of their high prevalence, the detection of nonaggressive cancers depends mainly on the intensity with which they are sought. The total incidence of prostate cancer is, therefore, highly sensitive to the medical-care setting.
Prior to PSA testing, a 30-fold international variation in prostate cancer incidence was observed, with high rates in Western countries, particularly the United States, and low rates in Asia. Although age-standardized incidence rates differed between countries, trends were stable for decades prior to PSA testing (see Figure 3). After PSA testing became widespread in the late 1980s, incidence more than doubled in some populations. This happened because nonaggressive tumors were in effect transferred from the pool of previously undiagnosed disease to tumors requiring clinical attention. The dramatic increase occurred earlier and rose higher in the United States than elsewhere. After peaking in 1992, incidence fell, but it has remained at a higher level than before. Countries where PSA testing is not common do not show this huge increase.
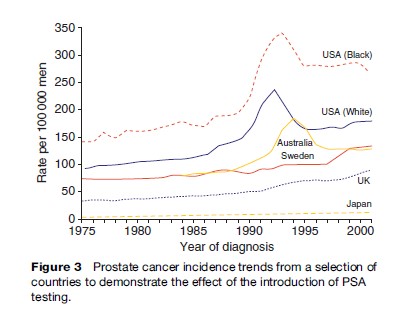
Figure 4 shows age-standardized prostate cancer incidence and mortality rates for a selection of countries centered on 1985 and 1995, before and after PSA testing. The remarkable features are the huge differences in incidence and minor differences in mortality. PSA testing was slow to emerge in the UK and is rare in Denmark, and this is reflected in the rates.
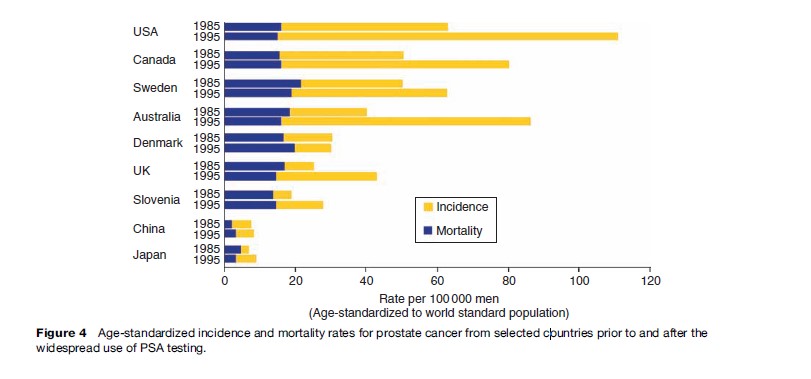
Against a long-term trend of slowly increasing prostate cancer mortality, some countries have experienced a small decrease that has sparked debate about the contribution of PSA testing. It is impossible, however, to distinguish possible early detection effects from simultaneous improvements in treating advanced cancers. Mortality has also fallen in countries without PSA testing and this argues against the impact of PSA testing on mortality. Such opportunistic testing is unlikely to have reduced mortality so quickly unless the lead time (advancement of diagnosis by the test) were extremely short, which is improbable. We will have to wait for randomized controlled trials (RCTs) to resolve this question.
Total prostate cancer survival has improved since the introduction of PSA testing, due mainly to the diagnosis of an increased proportion of nonaggressive tumors. Relative survival estimates at 5 years are virtually 100% for localized cancer and are around 33% for cancer with distant metastases.
Prostate cancer shows strong ethnic variation, with African-Americans, especially those of Jamaican descent, having the highest reported rates of incidence and mortality in the United States. It had long been considered that native Africans were at low prostate cancer risk but recent surveys suggest that the incidence for thoroughly screened African populations may be as high as for African-Americans.
Japanese men have historically experienced a low incidence of prostate cancer, but this changed among Japanese migrants to the United States. Incidence rates for first-generation Hawaiian Japanese rose above those for Japan and the rates for the second generation rose even further. It is impossible to ascribe this to specific changes in environment because little is known about the environmental causes of prostate cancer. The fact that mortality rates also increased in the migrants argues that at least some of the change might be due to environmental factors.
Risk Factors For Prostate Cancer
Apart from age, family history, and race, few risk factors have been established for prostate cancer, especially for its lethal forms. It is thus not currently possible to prevent prostate cancer. Given the amount of research conducted to identify modifiable risk factors, this is disappointing. One problem has been the biological heterogeneity of prostate tumors. Another has been the dominance of reports in the literature from small case-control studies that, due to their retrospective design, inherent biases and poor statistical power have produced inconsistent findings concerning a large number of possible risk factors.
In the late twentieth century, the first reports began to appear from large cohort studies. Unfortunately, the prostate cancers in these studies have been diagnosed during the PSA era, and the majority of tumors are nonaggressive. Without rigorous efforts to identify risk factors for aggressive cancer and distinguish tigers from pussycats, progress in our understanding of prostate cancer will continue to be slow.
Family History And Genetic Factors
Having a first-degree relative with prostate cancer incurs a twofold to threefold risk and this increases with the number of relatives affected and earlier age at diagnosis. These observations are consistent with an inherited genetic predisposition. Analyses of families with multiple cases of prostate cancer have inferred dominant patterns of inheritance, as well as recessive and X-linked patterns. It is therefore considered that mutations in more than one gene might increase susceptibility to prostate cancer. Several loci have been identified, for example, on chromosomes 1, 17, 20, and X, but reports have been difficult to replicate. Putative prostate cancer susceptibility genes have also been reported, for example, ELAC2, RNASEL, and MSR1, but none has been generally accepted. The BRCA2 gene associated with increased risk of breast cancer for women is a possible exception, as male BRCA2 mutation carriers have increased risk of early-onset prostate cancer.
A region on chromosome 8q found in Icelandic families has been replicated in two other studies and may contribute to increased risk for African-Americans (Amundadottir et al., 2006). The risk associated with this region appears regardless of severity, so the putative gene is likely to be associated with early events in carcinogenesis.
The search for prostate cancer genes may have been thwarted by tumor heterogeneity, since different genes may be involved in susceptibility to aggressive and nonaggressive tumors. There is evidence that men with a family history are more likely to have PSA testing and this will lead to increased diagnosis of nonaggressive tumors in families and make the search for susceptibility genes more difficult.
Association studies have been carried out for common polymorphisms in candidate genes known to be in pathways important to prostate development and function. Candidates have been selected from pathways such as steroid hormone metabolism, DNA repair, insulin, and insulin-like growth factors (IGFs) and response to infection and inflammation. For example, much has been published concerning genetic variants in the androgen receptor, 5 alpha reductase type 2, the vitamin D receptor, IGF-1, interleukins, toll-like receptors, and a number of cytochrome P450 enzymes. This approach has had a similar lack of success to the search for susceptibility genes. Seldom have initial reports generated from small, poorly designed studies been replicated by studies with greater statistical power. It is increasingly recognized that genetic association studies need to be substantially larger than previously thought. Some very large studies are under way, but an equivalent emphasis on tumor aggressiveness is also required if knowledge is to be advanced.
Hormones
Androgens are required for normal growth and function of the prostate. Testosterone and its active form dihydrotestosterone (DHT) bind to the androgen receptor and result in increased transcription and cell division. Some argue that androgens are permissive factors for prostate cancer, in that their presence is essential for other carcinogenic factors to play their part. Androgen deprivation in almost all forms leads to involution of the prostate, a fall in PSA levels, and apoptosis of prostate cancer and epithelial cells. Prostate cancer is termed hormone-dependent because when advanced it is initially responsive to androgens and can be controlled by surgical or chemical castration. Men with congenital 5-alpha reductase deficiency cannot convert testosterone to DHT and do not develop a normal prostate. It is often said that eunuchs do not develop prostate cancer but the evidence for this observation is slight.
It has long been reasoned that the hormone dependency of advanced disease might also influence incidence, the androgen hypothesis. Many case-control studies tested this hypothesis, with contradictory results. Early cohort study analyses supported the androgen hypothesis, because circulating levels of testosterone were associated with prostate cancer risk, while those of sex-hormone binding globulin (SHBG) were protective.
Early analyses failed to take into account tumor heterogeneity, combining all cancers as if they were the same, the majority of those being nonaggressive. Later studies that have stratified the analysis on Gleason score or tumor grade have reported that circulating levels of androgens are associated with increased risk of nonaggressive tumors but a reduced risk of aggressive tumors. This is consistent with the known function of androgens in maintaining prostate cellular differentiation.
Clearly, the relationship between androgens and prostate carcinogenesis is complex. Putting their role in the genesis of nonaggressive tumors to one side, the question remains of how and at what stage androgens might lose their protective influence on cellular differentiation and promote aberrant proliferation. A pertinent observation is that prostate cancer increases dramatically with age from the fifth decade, a time linked with the androcline and changes in hormonal milieu toward falling androgen and rising estrogen levels.
There is evidence that IGF-1 and its binding protein, IGFBP-3, are associated with risk, but findings from cohort studies are contradictory. A review of early studies reported a positive association with IGF-1 and a negative association with IGFBP-3 levels. Larger studies later found little evidence of association with IGF-1 and some have found positive associations with IGFBP-3, in direct contrast with the earlier observations. The latter, if real, may help explain a link with obesity. IGFBP-3 levels are reported to decrease with increasing levels of physical activity and are potentially modifiable.
Sexual Activity
The androgen hypothesis has also influenced research about sexual behaviors and markers of androgen influence, for example, age at starting shaving, sexual activity and marriage, the number of sexual partners including prostitutes, and the frequency of sexually transmissible infections (STIs). With the exception of STIs, these reports have lacked consistency. Some studies also report risk associated with diminished opportunity for sexual activity, for example, increased prostate cancer mortality for Roman Catholic priests, somewhat analogous to breast cancer in nuns. The case-control literature on these topics has its usual flaws but additionally lacks a standard approach to measuring sexual activity.
A large case-control study and a prospective cohort study using a standard questionnaire on ejaculatory frequency by decade of life reported similar findings, that men having high ejaculatory frequency, especially in early adulthood, reduced their risk by about one-third. The significance of the observation is not clear; hypotheses range from considerations of prostatic duct hygiene to more subtle hormonal feedback relationships between the prostate, testes, and the pituitary gland. The contrasting age-related relationships between androgen levels, ejaculatory frequency, and prostate cancer incidence may offer some insight.
Infection And Inflammation
There is general support for the hypothesis that infection might be causally related to prostate cancer. Self-reports of STIs are unreliable, however, and few studies have attempted to measure evidence of infection directly. Further, many common STIs in men are asymptomatic or insufficiently bothersome to come to medical attention and it is not possible to identify culpable organisms for a substantial proportion of those that do.
The findings concerning human papilloma virus infection are essentially null, an earlier positive association failing to be replicated by later studies. Studies have also failed to demonstrate any consistent association with herpes simplex viruses. There is limited serologic evidence that syphilis infection is associated with risk. On the other hand, a protective effect of serum positivity to Chlamydia trachomatis infection has been reported, consistent across different serotypes and demonstrating dose–response relationships. This raises the possibility that some STIs might be able to increase the prostate’s immune response.
The idea that one consequence of infection, chronic inflammation, might contribute to prostate carcinogenesis is gaining ground. Inflammation in prostate specimens is common and foci of proliferative inflammatory atrophy (PIA) are commonly observed in the peripheral zone. Some consider PIA to be a precancerous lesion or a marker of an intraprostatic environment that is favorable to cancer development, because it is often found adjacent to areas of both intraepithelial neoplasia and cancer. Although not fully consistent, a number of studies have shown a protective effect of aspirin and nonsteroidal antiinflammatory drugs on prostate cancer risk, particularly for advanced disease. Further evidence for the possible causal significance of inflammation arises from reports that variants in genes involved in the inflammatory response to infection, for example, the interleukin and toll-like receptor signaling pathways, are associated with risk. Intriguingly, some putative susceptibility genes, for example, MSR1 and RNASEL, have roles in this pathway.
Dietary And Nutritional Factors
A large number of inconsistent dietary associations have been reported from ecological correlation studies and case control studies. The measurement error associated with dietary assessment coupled with the biases inherent in case-control studies provides a strong rationale for focusing attention on reports from well-conducted cohort studies. Cohort studies (mostly North American) have reported positive associations with saturated fat and/or meat, dairy products (calcium), and zinc and negative associations with tomato-based foods (lycopene), vitamin D, vitamin E, and selenium, fish (omega-3 fatty acids), soy, isoflavones, and polyphenols. Cohort study findings are also not fully consistent, possibly because the majority of cancers in these studies are PSA-detected and advanced tumors are rare. Although there is a trend toward analysis by tumor grade or stage, statistical power is usually a limiting factor.
It is clear from cohort studies that fruit and vegetables are not associated with risk (Key et al., 2004). Although a protective role for phytoestrogens has been promoted, there is little evidence that men who consume foods containing significant amounts are at lower risk. The evidence regarding cruciferous vegetable consumption is also poor. No effect of cruciferous vegetables was observed in either the European Prospective Investigation of Cancer (EPIC) or the Health Professionals Follow-Up Study (HPFS). EPIC had no information on tumor stage but in the HPFS there was evidence of a protective effect for disease confined to the prostate in men aged less than 65 years (Giovannucci et al., 2003).
Although the data from different studies are not entirely consistent, tomato-based foods (especially tomato sauce), and their principal carotenoid, lycopene, may be protective and, more so, against advanced disease. Lycopene is concentrated in the prostate and has antioxidant ability twice that of beta-carotene and ten times that of vitamin E. Associations with other carotenoids are weaker than for lycopene but are similar in direction.
Micronutrients of interest for chemoprevention have been identified incidentally from RCTs having other principal outcomes. The Alpha Tocopherol Beta Carotene (ATBC) trial in male smokers showed an unexpected deficit in prostate cancer in the alpha tocopherol (vitamin E) arm but no effect of beta carotene (Albanes et al., 1996). There is little evidence that vitamin E within the normal dietary range is protective. Similarly, a trial of selenium for skin cancer prevention also showed a deficit of prostate cancer. These agents, both of which have antioxidant properties, are now subject to RCTs. A concern for the RCTs is the outcome, which is unlikely to be aggressive cancer but a screen-detected endpoint such as elevated PSA or microscopic disease. The ATBC trial’s other unexpected outcome was the increased risk of lung cancer in the beta carotene arm, a salutary example to those who would promote the supplementary consumption of micronutrients in high doses. It is matched by the finding that men who take high doses of zinc supplements put themselves at increased risk of prostate cancer.
Meat, particularly red meat, remains under suspicion, but it has been difficult to separate an effect of meat from that of fat, for which meat-based foods provide a major source. Some hold the view that the association with red meat might be related to carcinogenic by-products of cooking methods, particularly char grilling. There is little available evidence, and similar associations have not been observed with other grilled meats such as chicken or fish. Fish consumption appears to be protective against advanced prostate cancer, possibly as a rich source of marine fatty acids with known anti-inflammatory properties that might be relevant to prostate cancer prevention.
Cohort studies have yielded mixed results concerning fat, but those that do report associations have indicated them more consistently with advanced prostate cancer. Analyses by type of fat and for specific fatty acids have produced similarly inconsistent results, the strongest association being reported with alpha-linolenic acid. Although the evidence is inconclusive, several hypotheses have been advanced to explain an association with fat, including an effect on serum androgen levels and the possibility that certain types of fatty acids or their metabolites may initiate or promote carcinogenesis.
Dairy foods have been suspected for their fat and calcium content. It has been hypothesized that high calcium intake, possibly by lowering 1,25(OH)2 vitamin D levels, is associated with poorer differentiation in prostate cancer and thereby with fatal prostate cancer. Rigorous reviews and meta analyses have concluded that the risk associated with dairy foods, if any, is very small.
Vitamin D is also manufactured by the skin when exposed to sunlight. Vitamin D is known to promote differentiation and impede proliferation of prostate cells and its deficiency is, therefore, hypothesized to decrease risk, but epidemiological studies are inconclusive.
Overweight and obesity are products of imbalance between energy intake and expenditure by way of physical activity. The evidence that body size and composition are associated with the risk of prostate cancer is inconsistent. Some inconsistencies are due to variations in design, power, and physical measurements, many studies relying on self-report rather than direct measurement. A comprehensive meta-analysis of the associations reported from cohort studies concluded that body mass index was weakly associated with prostate cancer risk (particularly for advanced tumors). Few studies have examined central obesity and prostate cancer risk, though there is growing recognition of its possible importance. One cohort study that made direct body measurements has shown waist circumference to be positively associated with the risk of aggressive disease.
Several prospective cohort studies have found that obese men are more likely to die from prostate cancer. Also, among men with prostate cancer, obese men are more likely to experience biochemical progression (rising PSA levels). It has been postulated that obesity might differentially influence the development of disease, reducing the risk of nonaggressive disease, while increasing the risk of aggressive disease.
Given the positive associations observed between measures of obesity and prostate cancer, it would be expected that physical activity would be protective against prostate cancer. Reviews have concluded that physical activity may be inversely associated with prostate cancer risk but the evidence is inconsistent, the magnitude of the risk reduction observed is small, and these could be attributed to the studies’ methodological limitations.
Other Factors
Alcohol
Reviews generally conclude that there is no association between low to moderate alcohol consumption and prostate cancer, but cannot exclude the possibility of an association with heavy drinking. There is a single case-control study report of a protective effect of red wine consumption, which, if real, may be related to the antioxidant properties of red wine pigments such as resveratrol.
Tobacco
Overall, epidemiological studies do not support an association between smoking and the risk of developing prostate cancer. There is evidence, though, that smoking is associated with increased prostate cancer mortality.
Radiation
The prostate is one of the few organs in which cancer is not associated with ionizing radiation, even at high doses. There is weak evidence of a negative association with ultraviolet light exposure, possibly via vitamin D synthesis.
Occupation
Although a large literature exists on occupational associations with prostate cancer, very little has been firmly established. The risks that have been observed may be the result of uncontrolled confounding with social class. In the PSA era, the degree of detection bias in occupational studies has increased further, because men who believe that they have been exposed to a possible carcinogen tend to seek PSA tests more frequently and are more likely to have a diagnosis made than colleagues.
Early studies focused on occupational exposures to cadmium. As the prostate concentrates zinc, it was considered that cadmium could replace zinc and be procarcinogenic, but follow-up studies of cadmium smelter and battery production workers failed to confirm early suggestions of increased risk.
Dioxin, a contaminant of a herbicide used in Vietnam, is similar to many components of herbicides used in farming. A review of the linkage between dioxin and prostate cancer risk by the National Academy of Sciences reported that the available data on the association between dioxin exposure and prostate cancer was inconclusive. A number of studies have looked at the risks associated with farming, particularly related pesticide and herbicide exposures, but most have been ecologic and have failed to measure exposures at the individual level or to control for confounders.
Vasectomy
Studies have been inconsistent with respect to possible risks of prostate cancer following vasectomy. Reviews have concluded that virtually all studies had been deficient in their handling of bias that could account for the modest risks observed in the positive studies.
Future Prospects
Prostate cancer has changed irrevocably since the widespread use of the PSA test for early detection. In many countries, incidence continues to increase, while in others early detection has yet to begin. Although many men now diagnosed with prostate cancer would have died with the disease rather than as a result of it, their diagnosis is just as meaningful in terms of public health services as would have been a diagnosis based on symptoms. The manmade epidemic of prostate cancer will continue to impact hugely on the use of health services not only with respect to initial diagnosis and treatment, but also in relation to the management of the long-term side effects of treatment, with their enormous potential to diminish quality of life.
There is little prospect of change until advances are made in the molecular taxonomy of these tumors, so that the tigers can be discriminated from the pussycats. Without such progress, the epidemic of prostate cancer will continue unabated.
Bibliography:
- Albanes D, Heinonen OP, Taylor PR, et al. (1996) Alpha-to-copherol and beta-carotene supplements and lung cancer incidence in the alpha-to-copherol, beta-carotene cancer prevention study: Effects of base-line characteristics and study compliance. Journal of the National Cancer Institute 88(21): 1560–1570.
- Amundadottir LT, Sulem P, Gudmundsson J, et al. (2006) A common variant associated with prostate cancer in European and African populations. Nature Genetics 38(6): 652–658.
- Boccon-Gibod L (1996) Significant versus insignificant prostate cancer – Can we identify the tigers from the pussy cats? Journal of Urology 156(3): 1069–1070.
- Giovannucci E, Rimm EB, Liu Y, Stampfer MJ, and Willett WC (2003) A prospective study of cruciferous vegetables and prostate cancer. Cancer Epidemiology Biomarkers and Prevention 12(12): 1403–1409.
- Gleason DF (1977) The Veteran’s Administration Cooperative Urologic Research Group: Histologic grading and clinical staging of prostatic carcinoma. In: Tannenbaum M (ed.) Urologic Pathology: The Prostate. Philadelphia, PA: Lea and Febiger.
- Gohagan JK, Prorok PC, Kramer BS, and Cornett JE (1994) Prostate cancer screening in the prostate, lung, colorectal and ovarian cancer screening trial of the National Cancer Institute. Journal of Urology 152(5): 1905–1909.
- Key TJ, Allen N, Appleby P, et al. (2004) Fruits and vegetables and prostate cancer: No association among 1104 cases in a prospective study of 130, 544 men in the European Prospective Investigation into Cancer and Nutrition (EPIC). International Journal of Cancer 109(1): 119–124.
- McNeal JE (1981) The zonal anatomy of the prostate. The Prostate 2: 35–49.
- Schroder FH (1994) The European Screening Study for Prostate Cancer. Canadian Journal of Oncology 4(supplement 1): 102–105.
- Yatani R, Chigusa I, Akazaki K, et al. (1982) Geographic pathology of latent prostatic carcinoma. International Journal of Cancer 29(6): 611–616.
- Bostwick DG, Burke HB, Djakiew D, et al. (2004) Human prostate cancer risk factors. Cancer 101(supplement 10): 2371–2490.
- Boyle P, Severi G, and Giles GG (2003) The epidemiology of prostate cancer. Urologic Clinics of North America M30(2): 209–217.
- Donn AS and Muir CS (1985) Prostatic cancer: Some epidemiological features. Bulletin Cancer 72(5): 381–390.
- Friedenreich CM and Thune I (2001) A review of physical activity and prostate cancer risk. Cancer Causes and Control 12(5): 461–475.
- Friedrich MJ (1999) Issues in prostate cancer screening. Journal of the American Medical Association 281(17): 1573–1575.
- Godley PA (1999) Prostate cancer screening: Promise and peril – A review. Cancer Detection and Prevention 23(4): 316–324.
- Platz EA (2002) Energy imbalance and prostate cancer. Journal of Nutrition 132(supplement 11): 3471S–3481S.
- Platz EA and Giovannucci E (2004) The epidemiology of sex steroid hormones and their signaling and metabolic pathways in the etiology of prostate cancer. Journal of Steroid Biochemistry and Molecular Biology 92(4): 237–253.
- Platz EA, De Marzo AM, and Giovannucci E (2004) Prostate cancer association studies: Pitfalls and solutions to cancer misclassification in the PSA era. Journal of Cell Biochemistry 91(3): 553–571.
- Ross RK, Pike MC, and Coetzee GA (1998) Androgen metabolism and prostate cancer: Establishing a model of genetic susceptibility. Cancer Research 58(20): 4497–4504.
- Schaid DJ (2004) The complex genetic epidemiology of prostate cancer. Human Molecular Genetics 13(1): R103–R121.
- Shimizu H, Ross RK, Bernstein L, et al. (1991) Cancers of the prostate and breast among Japanese and white immigrants in Los Angeles County. British Journal of Cancer 63(6): 963–966.
- Whittemore AS (1994) Prostate cancer. Cancer Surveys 19–20: 309–322.
See also:
Free research papers are not written to satisfy your specific instructions. You can use our professional writing services to buy a custom research paper on any topic and get your high quality paper at affordable price.