This sample Tetanus Research Paper is published for educational and informational purposes only. If you need help writing your assignment, please use our research paper writing service and buy a paper on any topic at affordable price. Also check our tips on how to write a research paper, see the lists of health research paper topics, and browse research paper examples.
Introduction
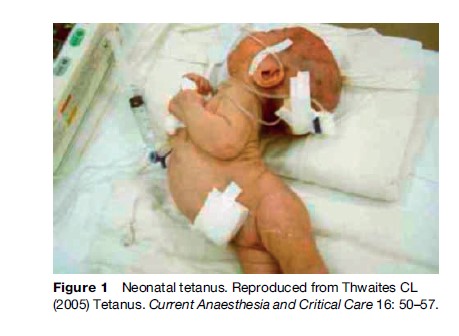
Tetanus is a disease of generalized muscle spasms and cardiovascular instability. Once established there are no specific treatments and therapy is essentially supportive, requiring modern intensive care facilities. Current lack of effective treatments means mortality remains high. The disease is completely preventable by vaccination, which was introduced in developed countries during the 1950s and 1960s. Nevertheless, in 2004 an estimated 40 million pregnant women were not immunized and 27 million children failed to complete their primary immunization course; thus, in the developing world, tetanus remains a common cause of morbidity and mortality in children and young adults but particularly in neonates, causing an estimated 257 000 neonatal deaths a year (7% of all neonatal deaths) (Figure 1).
Etiology
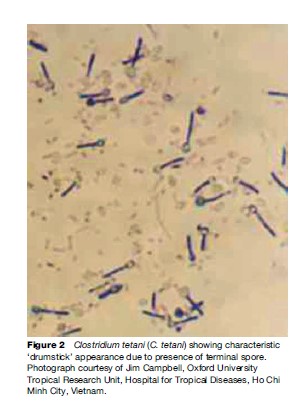
The disease is caused by the bacterium Clostridium tetani (C. tetani). This is a Gram-positive, anaerobic sporeforming bacillus (Figure 2). The spores are highly resistant and are able to exist in the environment for long periods ready to grow when suitable conditions occur. The spores are resistant to heating and dry environments and most household disinfectants. They can survive boiling for several minutes and destruction requires autoclaving at 121 C for 15–20 min. C. tetani has been isolated from human and animal feces as well as street dust, soil, and operating theaters throughout the world. Spores enter the body through minor cuts and lacerations. Often these wounds are too small to notice, or skin has healed over the top. In neonates the usual portal of entry is the umbilical stump.
Once in a suitable environment the bacteria begin to grow and multiply. Tetanus is caused by the toxin produced by C. tetani. Strains that do not produce the toxin cannot cause tetanus. The toxin is a powerful neurotoxin that is encoded on a plasmid. It is produced as a single amino acid chain that undergoes posttranslational cleavage resulting in a heavy chain and a light chain linked by a disulfide bond. The toxin is either taken up by local motor nerve endings or by distant terminals after circulation around the body. The heavy chain contains regions that bind specific regions of the motor neuron membranes and facilitate toxin entry into the cell. It also mediates retrograde transport of toxin up the axon and across the synaptic cleft where the toxin affects GABAergic inhibitory interneurons. These neurons are inhibitory neurons to the alpha motor neurons and the toxin prevents synaptic release of neurotransmitter in these neurons, removing the check on motor neuron discharge. The light chain is a zinc-dependent endopeptidase, able to cleave one of the molecules necessary for synaptic vesicle release (VAMP-2) at a single peptide bond. This is a very similar action to the closely related botulinum toxins; however, in tetanus, the action occurs within the GABA-ergic cells of the CNS, resulting in a very different clinical picture.
It is likely that the toxin causes similar disinhibition of autonomic nerve fibers resulting in the cardiovascular instability seen in severe tetanus.
Epidemiology
Tetanus is arbitrarily subdivided into tetanus affecting older individuals and neonatal tetanus (NT), which is defined by the World Health Organization (WHO) as ‘‘an illness occurring in a child who has the normal ability to suck and cry in the first 2 days of life but who loses this ability between days 3 and 28 days of life and becomes rigid and has spasms’’ (WHO, 2006). Sometimes the term maternal and neonatal tetanus (MNT) is used to describe tetanus affecting neonates and mothers in the postpartum period. Elimination of MNT has been one of the key aims of the WHO and its partners in the Expanded Program on Immunizations (EPI) since the World Health Assembly called for the ‘elimination’ of NT by 1995. (In 1999 maternal tetanus was added to the program thus the target became the elimination of MNT.) Unfortunately this target was not met and the date was postponed to 2000. Yet in 2000 there were still 57 countries in which NT was greater than the target (defined as less than 1 case per 1000 live births in every district) and the date had to be further postponed to 2005.
The main strategies involved are to increase vaccination coverage among pregnant women using current guidelines (see the section titled ‘Prevention’). In some high-risk areas, a more aggressive vaccination strategy is employed, targeting all women of childbearing age with a primary immunization course. In addition efforts have been made to educate birth attendants. Unclean delivery surfaces and traditional midwifery practices, such as covering the umbilical stump in cow dung or cutting the cord with grass, are known to be associated with increased rates of NT, thus this education is of prime importance. Undoubtedly progress has continued to be made, but WHO data show that by the end of 2005, 49 countries had still failed to eliminate MNT (WHO, 2006). Furthermore MNT elimination has to be regarded as ongoing work, since C. tetani will not be removed from the environment. Thus countries who have achieved elimination today will still need to maintain good vaccine coverage and hygiene standards to prevent a return of the disease.
Accurate data on other forms of tetanus are more difficult to obtain – part of the MNT program includes enhanced surveillance that is not necessarily extended to other tetanus cases. Incidence is estimated to be between 200 000 and 1 000 000 cases a year. Tetanus requires the full complement of modern intensive care facilities to be managed effectively and without it mortality rates range from 50 to 80%. Therefore, as most tetanus (including NT) occurs in places with poor health-care infrastructure, the mortality rates are also high. Nevertheless, improved vaccination coverage among infants and children as part of the EPI initiative has resulted in a reduction in tetanus in children (Thwaites et al., 2004). However, most immunization initiatives have focused on primary immunization in infants; long-lasting immunity requires at least five immunizations (primary series plus two boosters). Therefore there is concern as to what will happen to those given only a primary series as they get older if no further boosters are given.
In developed countries tetanus, although rare, can still be a significant problem. Those at most risk are the elderly and injecting drug users. The elderly have lower antibody levels due to natural decline with age. A recent study by McQuillan et al. (2002) in the United States showed more than two-thirds of those over 70 had subprotective antibody levels. It has long been recognized that injecting drug users are prone to tetanus infection and indeed suffer a particularly severe form of the disease. Drug users may have incomplete vaccination histories, but also certain forms of injection such as skin popping (subcutaneous injection) are associated with anaerobic foci and therefore high risk of tetanus (Beeching and Crowcroft, 2005). This was illustrated by the recent outbreak of cases in the UK (Health Protection Agency, 2004). Furthermore the drug itself can make tetanus more likely as seen in the case of heroin. This is often cut with quinine or other acidic substances, and acidic pH may enhance tissue damage and facilitate tetanus toxin entry into nerves. Other diseases, such as HIV, further complicate management of the critically ill patient.
Clinical Features
A classic triad of muscle spasms, rigidity, and autonomic disturbance characterizes tetanus. Tetanus usually develops in a predictable manner: about a week after a wound is sustained patients experience initial symptoms as muscle tone gradually increases, and eventually frank spasms occur. Initial symptoms are usually muscle stiffness, pain, or trismus. Spasms increase in frequency and duration reaching a maximum during the second and third week of illness. At about this time in severe cases, autonomic disturbance becomes apparent. More severe tetanus tends to progress more rapidly, although in the elderly progression is more variable. The terms incubation period (time from injury to first symptom) and period of onset (time from first symptom to first spasm) are commonly used to indicate the rate of disease progression. In severe cases, incubation periods of 7 days or less are common, with period of onset at 24–48 h. Using these and other clinical features on presentation, researchers have attempted to predict the eventual severity of tetanus. Several scores have been created, but the best validated and most useful are the Tetanus Severity and Dakar scores (Thwaites et al., 2006b).
Muscle spasm involving either the laryngeal muscles (resulting in airway obstruction) or respiratory muscles are the most common causes of death in settings without access to mechanical ventilation. In these settings mortality from tetanus is extremely high in all age groups. If ventilators are available then spasms can be controlled using muscle paralysis agents and mortality rates are reduced (Thwaites et al., 2004). Cardiovascular instability due to autonomic disturbance then becomes the most frequent cause of mortality. Other problems common to critically ill patients such as nosocomial infection and thromboembolus are also common. Thus, even in settings with good intensive care facilities mortality is still significant, at between 10 and 20% (CDC, 2004b; Thwaites et al., 2004).
Diagnosis
Microbiological diagnosis is difficult and unreliable. One-quarter of patients have no wound from which to take samples and in many others healing has occurred before clinical tetanus develops. Thus, the diagnosis is entirely clinical, based on the presence of the directly observed features described above; microbiological culture of C. tetani is purely supportive.
Management
Initial management aims to eliminate C. tetani, minimizing further toxin production and removing any unbound toxin. A careful search should therefore be made for any possible source of infection and the wound thoroughly cleaned and debrided as necessary. Antibiotics (preferably metronidazole, but penicillin may also be used) should be given as well as antitoxin. Antitoxin is available in two forms. Ideally human tetanus immune globulin (HIG) should be given but if this is not available – a common occurrence in much of the world due to its cost – then equine antitoxin may be substituted.
Beyond these specific therapies, management is essentially supportive. Spasms are minimized using intravenous benzodiazepines. Diazepam is used most frequently due to its cost and widespread availability, but shorter-acting drugs, more suitable for prolonged intravenous infusion, are preferable. High doses are commonly required. If spasms are not controlled adequately, and ventilators are available, muscle relaxants are used and ventilatory support is given.
Cardiovascular complications are treated with increased sedation (including morphine and high-dose benzodiazepines) and specific cardiovascular drugs such as calcium antagonists or inotropes as indicated. Beta-adrenoceptor blockers are not favored as their use has been linked to periods of profound hypotension and cardiac arrest. An exception may be esmolol, which has a very short-acting half-life, and is unlikely to result in such long-lasting hypotension. The alpha-2 agonist clonidine has been used with success in tetanus but no large-scale studies have been reported.
Recent interest has surrounded the use of magnesium sulfate to control muscle spasms and cardiovascular instability. Initial uncontrolled studies by Lipman et al. (1987) and Attygalle and Rodrigo (2002) have reported favorable results – even that mechanical ventilation may be avoided. However these have not been borne out by a large, randomized controlled trial, although magnesium sulfate does appear to have beneficial effects on cardiovascular control and reduce the need for sedatives and muscle relaxant drugs (Thwaites et al., 2006a).
Finally it should be remembered that tetanus does not result in future immunity. Thus all patients should receive a full immunization course to prevent recurrence.
Prevention
Tetanus can be prevented by adequate immunization and/or effective wound care. The former is most likely to be successful as tetanus can arise from extremely minor wounds or abrasions. Most cases of tetanus occur in superficial wounds not deemed worthy of medical attention. Nevertheless, tetanus has been reported in those with complete vaccination history thus thorough wound cleaning and if necessary debridement should be routine.
Immunization ideally consists of a primary course of three vaccinations in infancy followed by two or three boosters at strategic intervals. Tetanus vaccines are made from tetanus toxoid, which is not particularly immunogenic, so is further absorbed onto aluminum to increase immunogenicity. They are available as single-dose tetanus toxoid (TT), or combined with diphtheria toxoid of which there are two preparations: high-dose diphtheria toxoid (DT) for use in children under 7 years, or low-dose diphtheria toxoid (dT) for use in older individuals. Combinations of DT or dT are available with whole-cell or acelluar pertussis (DTwP, DtaP, dTwP, or dTaP). Even more comprehensive preparations are now available containing hemophilus influenza B, hepatitis B, or polio.
Tetanus toxoid (including the standard combination vaccines) is extremely safe. Adverse effects are unusual and usually very mild. Tetanus toxoid can safely be used during pregnancy (indeed a vital element of MNT prevention) as well as in immunodeficient individuals, including those with HIV. Care should be taken in HIV patients as immune responses may be diminished. Malaria also reduces transplacental antibody passage and may decrease the effectiveness of NT prevention.
The WHO recommendations are for a primary course in infancy followed by boosters at ages 4–7 years and 12–15 years and one more booster in adult life, for example, during the first pregnancy or for military service (WHO, 2006). This differs slightly from recommendations in the UK, where this sixth booster is not recommended routinely. However, if a person sustains a tetanus-prone wound (Table 1) or is traveling to a country in which booster vaccination may be difficult to obtain if a wound is sustained, then a booster should be given (Salisbury and Begg, 1996). U.S. guidelines are similar to the WHO’s except an additional dose is given at 14–16 months (CDC, 2004a).
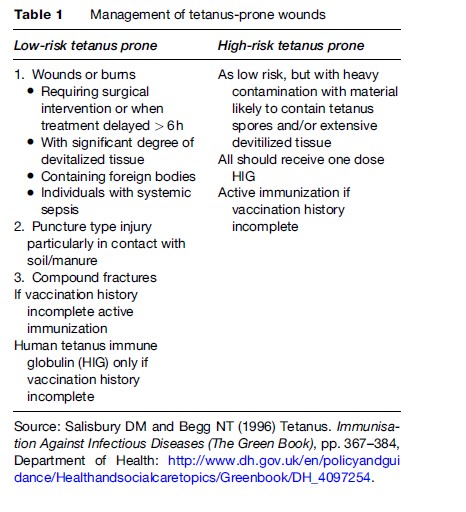
In nonimmunized adolescents and adults a three-dose primary course is advised with the first two doses given at least 4 weeks apart and the third at least 6 months after the second. If two further boosters are given, the total of five doses is expected to confer lifelong protection. In pregnant women, the same schedule is recommended if there is incomplete or unknown vaccination history: at least two doses (usually dT) should be given at least 4 weeks apart during the pregnancy, with a third 6 months later and two boosters at yearly intervals. If the person has had a primary course in infancy then just two further doses (at least 4 weeks apart) are recommended. This can be reduced to one dose if they also had a childhood booster. A final (sixth) booster at least 1 year later should give lifelong immunity.
Tetanus immunization should also be given after certain tetanus-prone wounds. In very high-risk situations additional passive immunization with HIG should be given to neutralize any existing tetanus toxin.
Bibliography:
- Attygalle D and Rodrigo N (2002) Magnesium as first line therapy in the management of tetanus: A prospective study of 40 patients. Anaesthesia 57(8): 811–817.
- Beeching NJ and Crowcroft NS (2005) Tetanus in injecting drug users. British Medical Journal 330(7485): 208–209.
- Centers for Disease Control Prevention (CDC) (2004a) Recommended childhood and adolescent immunization schedule – United States, January–June 2004. Morbidity and Mortality Weekly Report (MMWR) 53(1): Q1–Q4.
- Centers for Disease Control Prevention (CDC) (2004b) Summary of notifiable diseases – United States, 2002. Morbidity and Mortality Weekly Report (MMWR) 51(53): 17.
- Health Protection Agency (2004) Ongoing national outbreak of tetanus in injecting drug users. Communicable Disease Report CDR Weekly 14(9): 26.
- Lipman J, James MF, et al. (1987) Autonomic dysfunction in severe tetanus: Magnesium sulfate as an adjunct to deep sedation. Critical Care Medicine 15(10): 987–988.
- McQuillan GM, Kruszon-Moran D, et al. (2002) Serologic immunity to diphtheria and tetanus in the United States. Annals of Internal Medicine 136(9): 660–666.
- Salisbury DM and Begg NT (1996) Tetanus. Immunisation Against Infectious Diseases (The Green Book), pp. 367–384. Department of Health: http://www.dh.gov.uk/en/policyandguidance/ Healthandsocialcaretopics/Greenbook/DH_4097254.
- Thwaites CL, Yen LM, et al. (2004) Impact of improved vaccination programme and intensive care facilities on incidence and outcome of tetanus in southern Vietnam, 1993–2002. Transactions of the Royal Society of Tropical Medicine and Hygiene 98(11): 671–677.
- Thwaites CL, Yen LM, et al. (2006a) Magnesium sulphate for the treatment of severe tetanus: A randomised controlled trial. The Lancet 368(9545): 1436–1443.
- Thwaites CL, Yen LM, et al. (2006b) Predicting the clinical outcome of tetanus: The tetanus severity score. Tropical Medicine and International Health 11(3): 279–287.
- World Health Organization (WHO) (2006) Immunization surveillance, assessment and monitoring: Tetanus. http://www.who.int/ immunization_monitoring/diseases/tetanus/en/index.html (accessed October 2007).
- Centers for Disease Control Prevention (CDC) (1991) Update on adult immunization. Recommendations of the Immunization Practice Advisory Committee (AICP). Morbidity and Mortality Weekly Report 40: 47–52, 67–71, 73, 77–81, 86–94.
- Thwaites CL (2005) Tetanus. Current Anaesthesia and Critical Care 16: 50–57.
- Udwadia FE (1994) Tetanus. New York: Oxford University Press.
- World Health Organization (WHO) (2002) Department of Vaccines and Biologicals Vaccine Assessment and Monitoring Team Vaccines and Biologicals Global 2002 Summary.
- Geneva, Switzerland: WHO. World Health Organization (WHO) (2006) Tetanus vaccine. Weekly Epidemiological Record 81(20): 198–208.
- http://www.cdc.gov/vaccines/pubs/pinkbook/downloads/tetanus.pdf – CDC Vaccines and Immunizations: Publications.
- http://www.immunisation.nhs.uk/article.php?id=97 – National Health Service: Information on Immunization.
- http://www.who.int/topics/tetanus/en – World Health Organization: Tetanus.
See also:
Free research papers are not written to satisfy your specific instructions. You can use our professional writing services to buy a custom research paper on any topic and get your high quality paper at affordable price.