This sample Puberty Research Paper is published for educational and informational purposes only. If you need help writing your assignment, please use our research paper writing service and buy a paper on any topic at affordable price. Also check our tips on how to write a research paper, see the lists of health research paper topics, and browse research paper examples.
Definition
Puberty is the phase of life characterized by physical changes that mark the transition from childhood to adulthood. It includes a process involving accelerated growth and physical maturation of the respective reproductive systems to allow fertility in boys and girls. The end result is the constellation of physical features that distinguish males and females, including secondary sexual characteristics and the major differences of size, shape, body composition, and function in many body structures and systems.
When the processes of puberty fail to follow their usual time course, fail to occur, or are affected by an altered environment or physical anomalies, there can be long-term impacts on the achievement of future adult life and fertility.
The process of physical changes is generally known as puberty. In contrast, the slower process of psychosocial, emotional, and behavioral changes that occur between childhood and adulthood, combined with the physical changes, is generally known as adolescence. There has been increasing recognition that these broader adolescent changes are associated with risk behaviors (including smoking, drug usage, early sexual activity, and eating disorders), which may become the basis for significant long-term health problems in adulthood. However, there is an opportunity to promote and develop healthy habits and behaviors during adolescence as well. Public health professionals can play an important role in this respect.
Physiological Changes
Critical changes in the interaction of endocrine functions of childhood begin as puberty approaches. These changes include the activation of the hitherto quiescent hypothalamus–pituitary–gonadal axis resulting in gonadarche, i.e., the onset of gonadal functions. The increased production of adrenal androgens giving rise to the pubic and axillary hair is known as adrenarche.
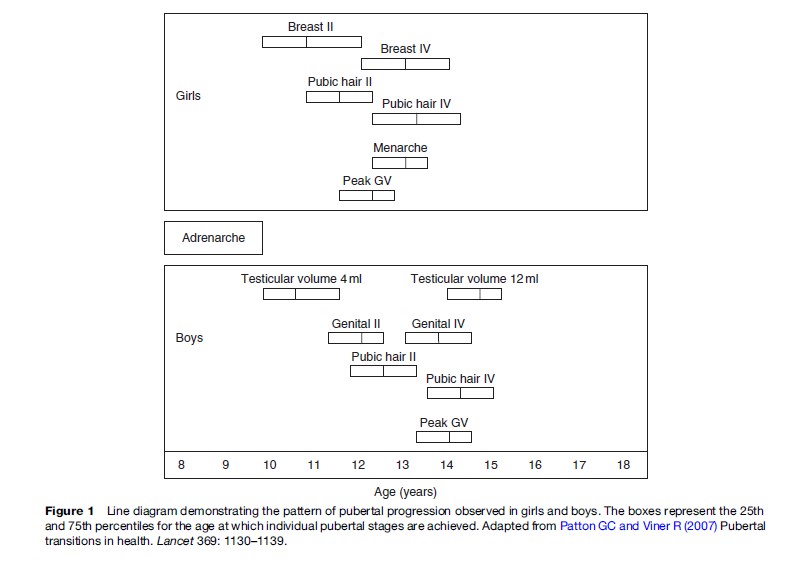
Puberty in girls generally follows the sequence of accelerated growth, breast development, adrenarche, and menarche and takes an average 4.5 years (range, 1.5–6 years), although this sequence of events is not identical for all individuals (Figure 1). For 95% of girls, secondary sexual characteristics appear between the ages of 8.5 and 13 years. For boys, the sequence is different, with genital changes, body and facial hair, adrenarche, voice changes, and accelerated growth occurring in this order (Figure 1). Pubertal onset occurs in 95% boys between the age of 9.5 and 13.5 years (Tanner, 1989).
The initial endocrine changes in the hypothalamus– pituitary–gonadal axis involve an increase in the pulsatile secretion of luteinizing hormone (LH) from the pituitary in response to pulsatile nocturnal gonadotropin-releasing hormone (GnRH) secretion from the hypothalamus. This nocturnal LH release precedes breast development in girls and testicular volume changes in boys by several years (Mitamura et al., 2000) and is sleep-dependent (Boyar et al., 1974). There is a progressive increase in LH pulse size as well as a gradual shift to the adult pattern of an LH pulse occurring every 90 min throughout the day and night. In contrast, follicle-stimulating hormone (FSH) does not have a diurnal pattern with nocturnal release, but there is a progressive increase in pulse amplitude until mid-puberty.
The triggers for the changes in the interaction between the hypothalamus–pituitary–gonadal axis are not well understood. Importantly, the effects of induction factors are time-dependent and many factors have only a permissive and not a contributory role. There is some evidence that leptin may play a role (Garcia-Mayor et al., 1997), providing the link between reproductive function and energy balance. A possible role for leptin is supported by the presence of leptin receptors in the hypothalamus, especially on the neuropeptide Y (NPY) neurons. Neuropeptide Y has a role in regulating brain function, including food-intake behaviors (Terasawa et al., 2001), which ties closely with theories relating to critical weight, body mass index, or body fat content as necessary prerequisites for onset of puberty (see the section titled ‘Secular Trends’). Before puberty, NPY appears to have an inhibitory role on GnRH secretion; this inhibition is thought to decline during and after puberty. Additionally, as puberty requires a functional growth hormone (GH) axis for full gonadal activation and the onset of puberty is associated with a dramatic increase in linear height growth, the role of the growth hormone–insulin-like growth factor-1 axis also appears to be critical. Recently, kisspeptin, a hypothalamic peptide, has been shown to be the key regulator of pubertal onset. Kisspeptin is also believed to provide the missing link between integration of nutritional signals (leptin and ghrelin) and pubertal onset (Seminara, 2006).
For girls, the altered LH pattern provokes a rise in estrogen production from the ovaries with a subsequent development of breast buds (Tanner stage 2), which is usually the first identified physical change of puberty. For boys, the first change is an increase in testicular volume (Mitamura et al., 2000).
Maturation of the hypothalamic–pituitary–gonadal axis results in the achievement of fertility. For girls, ovulation – the mark of attaining potential fertility – occurs usually 1–2 years after menarche (i.e., the first episode of menstrual bleeding). It appears that the later the age of menarche the longer the delay before 50% of the cycles are ovulatory, with the interval being 12 months if menarche occurs before age 12 years, 3 years when menarche occurred at age 12–12.9 years, and 4.5 years when menarche was after age 13 years. For boys, fertility is indicated by the production of sperm. While this is often difficult to detect, examination of an early morning centrifuged urine sample for the presence of spermatozoa has been increasingly shown to be effective. Researchers from developed countries have found sperm in the urine of boys at ages 11–12 years, sometimes even before pubarche (pubic hair development) (Richardson and Short, 1978).
Pubic hair development usually occurs approximately 6 months after thelarche (breast development); however, in one-third of all girls pubic hair may be the first manifestation of puberty. This is the marker of adrenarche.
Although there appears to be a temporal relationship between these two hormonal systems (the hypothalamus– pituitary–gonadal system and the hypothalamic–pituitary– adrenal system), with both contributing to the development of secondary sexual characteristics, there is considerable evidence to suggest that their control mechanisms are independent (Sklar et al., 1980; Counts et al., 1987). Premature adrenarche (precocious appearance of pubic and axillary hair before 8 years of age) is not associated with premature gonadarche. In hypergonadotropic hypogonadism (gonadal dysgenesis), where there is a failure in gonadal hormone production, adrenarche still occurs. Similarly, gonadarche is unaffected in children with adrenal insufficiency.
The adolescent growth spurt happens in girls before boys (Gordon et al., 2005). For girls, this may be the first marker of puberty, whereas in boys it invariably occurs after testicular enlargement. Estrogen has a gender nondimorphic effect on growth and is responsible for the growth spurt in both girls and boys. Local conversion of androgens to estrogens under the influence of the enzyme aromatase is crucial for these effects in boys. The growth promoting effects of estrogen are largely related to growth hormone secretion with minor effects on IGF1 production. For girls, menarche coincides with a slowing of the growth velocity to approximately 4 cm/year. Prenatal growth significantly influences pubertal development as demonstrated by normal pubertal maturation but smaller pubertal height and weight gain in babies born small for gestational age.
The development of peak bone mass occurs during the teenage years, modulated by sex steroids and growth hormone. The achievement of optimum bone density also requires adequate dietary calcium, vitamin D, and exercise, particularly weight-bearing exercise. Several studies suggest that 60% of adult bone mass is acquired during the pubertal growth spurt (Gordon and Laufer, 2005). Peak bone mass is achieved by the age of 16 years in girls and 18 years in boys.
Secular Trends
The age of onset of puberty varies considerably between individuals and populations and seems to be influenced by both genetic and environmental factors. Johann Sebastian Bach recorded the age of choir boys transitioning from singing treble to alto in three churches in Leipzig between the years 1723 and 1750. It averaged around 17 years (Daw, 1970). Today, the boys of the choir in King’s College Chapel, Cambridge, United Kingdom, usually break their voices at around 13 years (Potts and Short, 1999). For girls, the onset of menarche is well documented, with records from 1850 in Scandinavia that show that the average age of menarche was about 17.5 years. Throughout the Western world, there has been a well-documented fall in age of first menstruation over the last few centuries and by as much as 6 months in a decade over much of the last century. The age appears to have reached a plateau at between 12.5 and 13 years in Europe and the United States. This secular trend has been attributed to improved nutrition and health. Whether environmental factors such as endocrine disrupters having an estrogenic effect have an influence remains to be shown. There are indications that breast development is also occurring earlier in American girls. This had led to the recommendation of lowering the age cut-off for the diagnosis of precocious puberty by the Lawson Wilkins Pediatric Endocrine Society to 6 years in black and 7 years in white girls (Kaplowitz and Oberfield, 1999). The earlier breast development has, however, not been associated with a similar trend in the age of menarche, indicating that early thelarche is associated with slower pubertal progression. The critical role of improved nutrition in the development of a secular trend is exemplified by the lack of such a trend in developing countries. While there are limited data on the pattern of pubertal development in resource-poor countries, anecdotal reports indicate later pubertal onset compared to industrialized countries. In a recent population-based prospective study, Senegalese girls were found to achieve sexual maturity on average 3 years later than their Western counterparts (Garnier et al., 2005).
Genetic factors are clearly a major influence as children with a family history of early onset of puberty will also start puberty at an earlier than average age. Also, black American girls achieve all stages of pubertal development 6 months earlier than white American girls. Other factors that appear to have an influence on the onset and rate of progression of puberty include geographic location, exposure to light, general health and nutrition, and psychological factors. Obesity and residence closer to the equator, at lower altitudes, and in an urban area are associated with earlier onset of puberty.
It has been argued that a critical body mass (47.8 kg or 105 pounds) is required to achieve menarche for girls. Rather than total weight per se, the more crucial measure is probably body composition and the percentage of body fat (thought to be approximately 16–23%). Thus, the moderately overweight (20–30% over normal weight) girls have earlier menarche, and anorexic girls and intense exercisers have delayed menarche. Nevertheless, there are inconsistencies in the data, as it has been reported in a longitudinal study that there is no change in body fat composition until after menarche. Support for the theory that body weight and puberty are linked was provided by the identification of leptin and its role (Garcia-Mayor et al., 1997). Studies have demonstrated that leptin levels increase in puberty and are low in athletes and those with anorexia, and that they delayed puberty. Additionally, rodent and monkey experiments demonstrate a role for leptin in the onset of sexual maturation.
Given the important relationship of nutrition and pubertal development, it is not surprising that a wide variety of nutritional disorders are associated with disordered pubertal development. Thus, puberty is usually delayed in individuals with malnutrition (including those with eating disorders), chronic infections, malabsorption, and anemia.
Variation Within The Normal Range
As there is considerable individual variation in onset and duration of puberty, there can be concern that the pubertal development of a young person may be abnormal, either delayed or precocious. Often this is not the case, and the boy or girl simply represents either end of the normal spectrum.
Delayed puberty can result in a young person being physically immature and short compared to peers. Since adolescence is a time when the young person is becoming more independent, and the establishment of a peer group is an important step in this process, delayed puberty can have a negative impact on overall well-being. This response varies considerably among individuals but boys in particular may be more concerned by their short stature than by any delay in the development of their secondary sexual characteristics. Early pubertal development may also have a negative psychosocial impact. These children appear more physically mature than their level of intellectual development and are prone to sexual abuse.
Abnormal Pubertal Development
Pubertal development can be precocious or delayed. Precocious puberty is defined as pubertal onset (stage II breast development in girls and testicular volume more than 4 ml in boys) before the age of 8 years in girls and 9.5 years in boys. No underlying cause is identified in most girls with precocious puberty, in contradistinction to boys who usually have an identifiable etiology. Precocious puberty may be caused by increased gonadotropin production (gonadotropin-dependent) or autonomous gonadal hormone production (gonadotropin-independent). Gonadotropin-dependent precocious puberty may be related to a wide variety of intracranial pathologies including brain tumors, congenital malformation, and neurological insults emphasizing the need for thorough neurological evaluation (including neuroimaging). Gonadotropinindependent precocious puberty is rare in girls and most commonly related to estrogen-producing ovarian cysts. This form of puberty is much more common in boys and caused by increased adrenal or testicular androgen production. Besides the significant psychosocial impact, the most important consequence of precocious puberty is short stature. While these children are tall compared to their peers at presentation, their bone age is disproportionately advanced, resulting in compromised final height. The most effective treatment for gonadotropin-dependent precocious puberty is gonadotropin-releasing hormone (GnRH) analogs. Prolonged stimulation by these analogs results in the desensitization of the pituitary and reversal of pubertal changes. These agents are expensive, however, and should be used only in individuals with progressive puberty and compromised growth potential. Medroxyprogesterone acetate and cyproterone acetate are economical and effective in preventing menarche but have no effect on final height. They may be used in girls with intellectual disability and early puberty if height is not a major issue of concern. Gonadotropinindependent precocious puberty is treated by agents that decrease sex hormone production (aromatase inhibitors) or action (antiandrogens in boys and selective estrogen receptor modulators in girls).
Delayed puberty is defined by the absence of pubertal development by the age of 13 years in girls and 14 years in boys. In addition, inappropriate progress of puberty with no menarche within 5 years of thelarche indicates delayed puberty. Delayed puberty may be caused by disorders of the hypothalamic–pituitary axis (hypogonadotropic hypogonadism) or of the gonadal functions (primary gonadal failure or hypergonadotropic hypogonadism). Hypogonadotropic hypogonadism is most commonly reversible and related to systemic diseases, nutritional disorders, and increased physical activity. On rare occasions, it is caused by intracranial tumors, congenital malformations, and neurological insult such as radiotherapy and surgery. Hypergonadotropic hypogonadism is most commonly associated with chromosomal disorders (with Turner’s syndrome in girls being the most common). With increasing numbers of young people surviving childhood malignancies, the potentially negative effects of chemotherapy and radiotherapy on the gonads, including possible gonadal failure, need to be remembered. Importantly, delayed puberty may represent a normal variation of pubertal development (constitutional delay of puberty and growth). The condition is particularly common in boys and associated with delayed skeletal maturation and paternal history of delayed puberty. Treatment of delayed puberty should be started at an appropriate age (13 years in girls and 14 years in boys). Low-dose estrogens (10% of adult doses) should be started in girls and gradually increased over 2 years to adult doses. Progesterone should be added after the onset of withdrawal bleeding. In boys, parenteral testosterone treatment should be started at low doses (100 mg monthly) and increased to adult doses (300 mg every 3 weeks) over 2 years. Boys with constitutional delay of puberty and growth respond well to three to six doses of testosterone.
Abnormal adrenal function can result in early adrenarche (independent of gonadarche) with resultant pubic and axillary hair, acne, and an increase in growth rate with advance in skeletal height. The condition is usually nonprogressive and does not require extensive evaluation. Features of virilization (change in voice, clitoromegaly, and increased muscularity) should prompt evaluation for hyperandrogenic conditions such as congenital adrenal hyperplasia and virilizing adrenal or ovarian tumors.
The psychosocial repercussions of achieving physical maturation well ahead of the cognitive and psychosocial development, and thus being out of synchrony with peers, can have major consequences in increasing health risk behaviors in these young people.
Disorders Of Sexual Development
Although some of the conditions associated with disorders of sexual development are diagnosed at birth or in early childhood (for example, girls with ambiguous genitalia at birth due to congenital adrenal hyperplasia, girls with descended testes due to the complete androgen insensitivity syndrome, children with ambiguous genitalia at birth due to mixed gonadal dysgenesis, partial androgen insensitivity, or true hermaphroditism), some of these conditions are not diagnosed until pubertal development is noted to be abnormal. It is estimated that genital anomalies occur in 1 in 4500 births. Likewise, structural anomalies of the urogenital tract may have required initial corrective surgery in the neonatal period, but further interventions relating to the altered anatomy nevertheless may be required when the young person passes through puberty. This is particularly the case for young women with obstructive genital tract anomalies that may not have been appreciated until menstruation begins. Delays in making these diagnoses can have a negative impact on future fertility.
Virilization at puberty, delayed puberty, or primary amenorrhea may all be presentations of disorders of sexual development. Correct identification of the underlying cause is important to ensure an optimal long-term outcome.
The impact of having a disorder of sexual development also needs careful psychological attention – because issues of fertility and infertility, being normal or abnormal, that is being different from one’s peers – are important issues for adolescents in terms of their psychosocial development (Liao, 2004). Thus, even for those young people in whom the diagnosis and management of a disorder of sexual development was commenced in infancy, issues regarding genital appearance, sexual function, and general well-being are ideally addressed by a multidisciplinary team (Warne et al., 2005). A shift in health-care provision is required in adolescence to ensure that the young person – rather than the parents on behalf of the child – is the focus of care.
The issues for teenage boys and girls with disorders of sexual development are similar. Body image and self-identity, satisfaction with genital appearance and function, and prospects of being fertile or infertile are important. Although there are cultural differences in response to these issues, increasing evidence across cultures reveals that disclosure is important for all individuals.
Conclusions
The timing of puberty has changed over time, with a range of factors influencing its onset and course. Although the knowledge regarding these processes has improved, there are still many triggers and influences that are poorly understood. The end result of a physically mature, fertile individual is the outcome of a developmental process that is coupled closely to the cognitive and psychosocial maturation process. When the pubertal processes fail to progress normally, there are often clear consequences in terms of failure to achieve full sexual and reproductive health as well as mental health. Conversely, failure to achieve psychosocial maturity also has implications for achieving the physical endpoint of a healthy and successful sexual and reproductive life.
Bibliography:
- Boyar RM, Rosenfeld RS, Kapen S, et al. (1974) Human puberty: Simultaneous augmented secretion of luteinising hormone and testosterone during sleep. Journal of Clinical Investigation 54: 609–618.
- Counts DR, Pescovitz OH, Barnes KM, et al. (1987) Dissociation of adrenarche and gonadarche in precocious puberty and in isolated hypogonadotrophic hypogonadism. Journal of Clinical Endocrinology and Metabolism 64: 1174–1178.
- Daw SF (1970) Age of puberty in Leipzig,1727–49, as indicated by voice breaking in JSBach’s choir members. Human Biology 42: 87–89.
- Garcia-Mayor RV, Andrade MA, Rios M, et al. (1997) Serum leptin levels in normal children: Relationship to age, gender, body mass index, pituitary-gonadal hormones, and pubertal stage. Journal of Clinical Endocrinology and Metabolism 82: 2849–2855.
- Garnier D, Simondon KB, and Be´ne´fice E (2005) Longitudinal estimates of puberty timing in Senegalese adolescent girls. American Journal of Human Biology 17: 718–730.
- Gordon CM and Laufer MR (2005) The physiology of puberty. In: Emans SJ, Laufer MR, and Goldstein DP (eds.) Pediatric and Adolescent Gynecology, pp. 120–155. Philadelphia, PA: Lippincott, Williams, and Wilkins.
- Kaplowitz PB and Oberfield SE (1999) Reexamination of the age limit for defining when puberty is precocious in girls in the United States: Implications for evaluation and treatment. Drug and Therapeutics and Executive Committees of the Lawson Wilkins Pediatric Endocrine Society. Pediatrics 104: 936–941.
- Liao LM (2004) Development of sexuality: Psychological perspectives. In: Balen AH, Creighton SM, Davies MC, MacDougall S, and Stanhope R (eds.) Paediatric and Adolescent Gynaecology – A Multidisciplinary Approach, pp. 77–93. Cambridge, UK: Cambridge University Press.
- Mitamura R, Yano K, Suzuki N, et al. (2000) Diurnal rhythms of luteinizing hormone, follicle-stimulating hormone, testosterone, and estradiol secretion before the onset of female puberty in short children. Journal of Clinical Endocrinology and Metabolism 85: 1074–1080.
- Patton GC and Viner R (2007) Pubertal transitions in health. Lancet 369: 1130–1139.
- Potts M and Short RV (1999) Growing up. In: Potts M and Short RV (eds.) Ever Since Adam and Eve, pp. 161–163. Cambridge, UK: Cambridge University Press.
- Richardson DW and Short RV (1978) The time of onset of sperm production in boys. Journal of Biosocial Science 5: 15–25.
- Seminara SB (2006) Mechanisms of disease: The first kiss – A crucial role for kisspeptin-1 and its receptor G-protein-coupled receptor 54, in puberty and reproduction. Nature Clinical Practice. Endocrinology and Metabolism 2: 328–334.
- Sklar CA, Kaplan SL, and Grumbach MM (1980) Evidence for dissociation between adrenarche and gonadarche: Studies in patients with idiopathic precocious puberty, gonadal dysgenesis, isolated gonadotroph deficiency, and constitutionally delayed growth and adolescence. Journal of Clinical Endocrinology and Metabolism 51: 548–556.
- Tanner JM (1989) Fetus into Man: Physical Growth from Conception to Maturity. Cambridge MA: Harvard University Press.
- Terasawa E and Fernandez DL (2001) Neurobiological mechanisms of the onset of puberty in primates. Endocrine Reviews 22: 111–151.
- Warne G, Grover S, Hutson J, et al. (2005) A long-term outcome study of intersex conditions. Journal of Pediatric Endocrinology and Metabolism 18: 555–567.
- Balen AH, Creighton SM, Davies MC, MacDougall S, and Stanhope R (2004) Paediatric and Adolescent Gynaecology – A Multidisciplinary Approach. Cambridge, UK: Cambridge University Press.
- National Center for Health Statistics (2007) 2000 CDC Growth Charts. United States Department of Health and Human Services. http://www.cdc.gov/growthcharts (accessed February 2008).
- Potts M and Short RV (1999) Ever Since Adam and Eve. Cambridge, UK: Cambridge University Press.
- Rogol AD, Clark PA, and Roemmich JN (2000) Growth and pubertal development in children and adolescents: Effects of diet and physical activity. American Journal of Clinical Nutrition 72: S521–S528.
- Sperling MA (2002) Pediatric Endocrinology. 2nd edn. Philadelphia, PA: Saunders.
- Speroff LGR and Kase N (1999) Clinical Gynecologic Endocrinology and Infertility, 6th edn. Philadelphia, PA: Lippincott Williams and Wilkins.
See also:
Free research papers are not written to satisfy your specific instructions. You can use our professional writing services to buy a custom research paper on any topic and get your high quality paper at affordable price.







