This sample Respiratory Syncytial Virus Research Paper is published for educational and informational purposes only. If you need help writing your assignment, please use our research paper writing service and buy a paper on any topic at affordable price. Also check our tips on how to write a research paper, see the lists of health research paper topics, and browse research paper examples.
Introduction
Respiratory syncytial virus (RSV) is the most important cause of acute lower respiratory tract infection in young children worldwide. Generally, around a quarter of childhood admissions to hospitals with acute respiratory infections are due to RSV. Severe infections are most common in the first year of life, and RSV causes a characteristic disease entity called bronchiolitis. RSV was identified first in 1956 in a group of chimpanzees and accordingly called chimpanzee coryza agent (CCA), but was later documented to be a mainly human pathogen. Because no specific treatment is available, prevention through vaccine development is a high priority.
The Organism
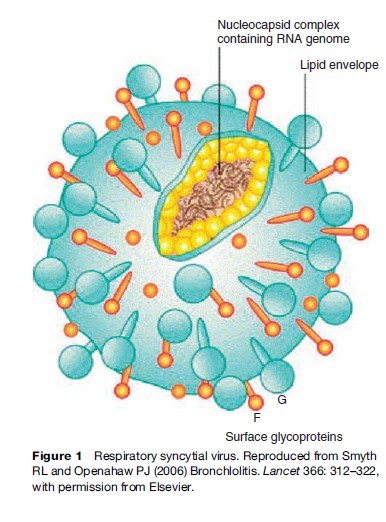
RSV is a medium-sized enveloped RNA virus (Figure 1). It is classified in the family of viruses called Paramyxoviridae, and in the genus Pneumovirus. Bovine, ovine, and caprine respiratory syncytial viruses, pneumonia virus of mice, and turkey rhinotracheitis virus also belong to this genus. The genome of RSV contains genes for ten main proteins. The two main surface proteins that are important for the generation of an immune response are the fusion protein (F protein, 70 kDa) and the attachment glycoprotein (G protein, 90 kDa). Two groups of RSV strains have been identified using monoclonal antibodies, which are called group A and group B. They differ predominantly in the G protein; the F protein is well conserved between groups. RSV is a relatively unstable virus. At 4 C, only 1% of infectivity remains after 1 week. The virus withstands freezing and thawing poorly. In culture, RSV grows best in human diploid cell lines such as Hep-2 or HeLa cells. The characteristic cytopathic effect of RSV in cell cultures is syncytia formation, from which the virus derives its name. RSV is predominantly a human virus, although RSV has also been recovered from chimpanzees, cattle, goats, and sheep.
Epidemiology
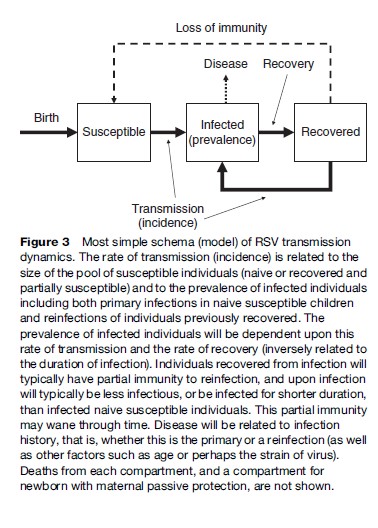
The epidemiology of RSV infection and its associated disease is determined, as for all infectious diseases, by factors that influence the rate of transmission of the virus from host to host, the development of specific immunity to reinfection within a host, and the association between infection and disease.
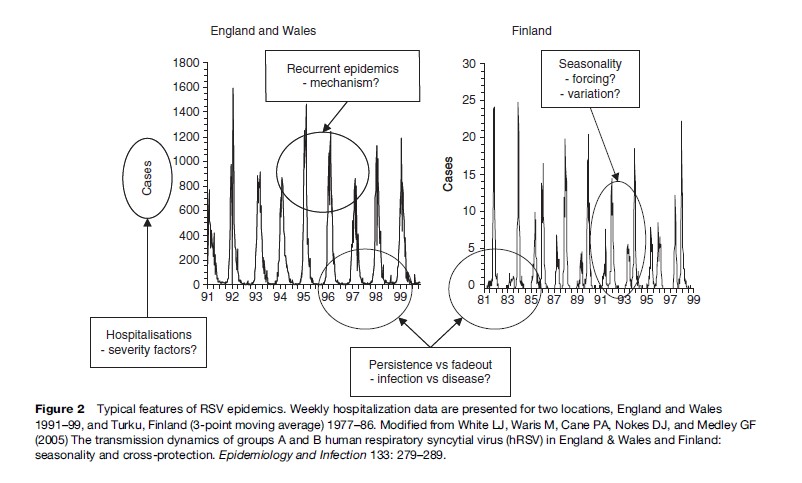
RSV is characterized by recurrent epidemics (Figure 2). This behavior of the virus in the population clearly suggests that following infection there is a refractory period (wherein new infection will not occur in a given individual), leading to a reduction in the susceptible pool, which thus constrains the continued spread of virus. Subsequently, between epidemics, there is a regeneration of the susceptible population, allowing another epidemic, and so the cycle continues (Figure 3). Replenishment of the susceptible population pool adequate to support each epidemic cycle results from at least two processes. Individuals totally susceptible to RSV (i.e., naive) accumulate through birth, following the loss of maternally derived passive protection. In addition, at least some, and probably most, of those recovered from infection again become susceptible to reinfection after a period of time. Furthermore, epidemic cycles are probably influenced by the levels of group (A and B) specific and cross-immunity which may favor transmission of one group over another according to recent dominance patterns. In the time series from Finland shown in Figure 2, the two variants show regular alternating dominance of two close epidemics of predominantly Group A, followed by two close epidemics of Group B, and so on. Data for England show a different pattern, with cycles of two epidemics of predominantly Group A, followed by only one of Group B.
Understanding what form of immunity follows infection and to what extent it wanes, is of considerable importance to understanding the transmission dynamics and persistence of RSV in human populations as well as vaccine development. The contribution made to transmission by those experiencing their first infection and those reinfected (Figure 3) depends on the relative numbers, the infectivity (duration and load of shedding), and the difference in contact patterns of each.
Most reported data for RSV is of disease occurrence, e.g., hospitalizations (Figure 2), and not of infections per se. Furthermore, these cases tend to be predominantly infants and young children, most experiencing their first infection. Evidence suggests that 60–70% of children will be infected with RSV in their first epidemic. Hence what is observed each year is largely hospitalizations of primary cases in children born since the previous epidemic (and having lost protective levels of maternal antibody) or who escaped infection in their first epidemic season. Consequently, observed cases probably represent the tip of an iceberg of total transmission in the community. Studies of individuals within the community, households, and institutions show repeated infections to be frequent in a wide cross-section of ages.
Between epidemics, cases may fade out entirely (Figure 2). It is unknown how the virus persists at these times; whether, for example, there is prolonged infection in a small proportion (e.g., immunocompromised), continuous but subclinical reinfections, or reintroduction from outside the population.
RSV has been isolated from primates (hence the original name of chimpanzee coryza virus) and ruminants. However, transmission is thought to be solely human to human and there is no evidence of reservoir hosts to account for persistence in the absence of observed human cases.
Seasonality
Epidemics of RSV show strong seasonality. This entrainment of epidemics to fixed periods of the calendar rather than irregular times indicates a periodic forcing of epidemics. While in general there is on average one epidemic per year, the pattern may differ, for example in some Scandinavian and northern European countries (Figure 2). The reasons for this variation are unclear. In temperate and Mediterranean climates, outbreaks occur mainly during the winter months, extending into spring. This temperature-dependent pattern appears to be independent of the rainfall pattern. In areas with tropical or subtropical climates and seasonal rainfall, RSV outbreaks are associated frequently with the rainy season, and not with the colder season. The peak of RSV transmission is usually 1–2 months after the onset of the rains. Outbreaks are usually sharp in onset and last between 2 and 5 months. In southern Africa, it has been shown that two populations located within 200 km and with the same climate have RSV epidemics occurring completely out of phase, and on tropical islands RSV may be present all year round. It is possible that a link with climatic patterns arises via its influence on social behavior. It has been demonstrated for measles, another member of the paramyxovirus family, that it is the timing of school opening and closing that triggers epidemics. This has not been shown for RSV. Furthermore, in The Gambia measles epidemics occurred out of phase with RSV. There is some anecdotal evidence of an association between RSV outbreaks and local festivals.
Geographical Spread
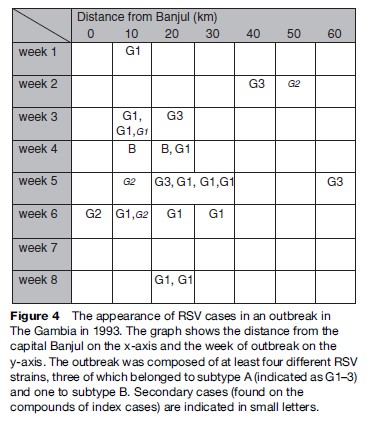
Molecular epidemiological studies and phylogenetic analyses can assist in understanding global patterns. RSV appears ubiquitous in human communities. Globally, RSV epidemics occur as seasonal clusters with starting months differing from region to region and appearing to spread out from coastal foci (low altitude, and perhaps an association with population size as major cities are located proximate to the coast). It appears, however, that contiguous spread from a point source is not the case, as genetically very similar strains arise simultaneously in geographically widely separated locations. Instead, temporal patterns in epidemic clusters are interpreted as sequential independent outbreaks arising as conditions for spread become favorable. The spread of an epidemic composed of viruses of different strains in The Gambia is shown in Figure 4. The ability of the virus to spread rapidly globally within the space of 2–4 years has been well documented, as in the case for a variant of RSV Group B with a signature 60-nucleotide duplication in the attachment G gene.
Age Distribution
The peak incidence of hospitalized cases of RSV is in the age group 2–5 months, with some variation between epidemic years. Serological studies suggest that about half of all children are infected during the first year of exposure, and that almost all have been infected after the second outbreak that they encountered. Studies show that primary infection tends to be most severe, with the vast majority of infection resulting in clinical signs and in around 20–40% of cases involving the lower respiratory tract. Around 1% of all infants are hospitalized with RSV – irrespective of whether they reside in industrialized or low-income countries, which is a tremendous global public health burden. Subsequent reinfections are progressively less severe. The relationship between age and severity is probably the result of development of acquired immunity to disease but also a function of size of a child’s airways, which may be more compromised by the inflammation of infection when smaller (see description of the section titled ‘Immunity and pathogenesis’ below). The relative importance of the two factors is difficult to tease apart.
The best estimates of the incidence of RSV lower respiratory tract infections are around 150–240 cases per 1000 child years in infancy, determined from community cohort-based studies in developed and developing countries. Estimates from hospital out-patient or admission data are in general markedly lower at 10–30 cases per 1000 years in infants, other than for North American native peoples (who have a higher incidence described). Hospital data clearly suffer from the inherent bias of underreporting due to inequality of access to health services.
RSV Infection In Elderly People
Recent work has highlighted an important contribution of RSV to flu-like disease in adults and the elderly. Outbreaks have been described in nursing homes, affecting a high percentage of residents, with complications occurring in up to 15% of the infected persons. Epidemiological studies correlating RSV outbreaks with excess deaths from respiratory infections indicate that RSV might be as important a cause of increased mortality in elderly people as influenza.
Sex Distribution
Boys are more commonly affected by severe disease, on average two-thirds of hospitalized children are male. This male preponderance corresponds to the generally higher incidence of acute respiratory infections of any etiology in boys. However, in mild disease the distribution between sexes is equal.
Transmission
RSV is shed in respiratory secretions and transmitted to other individuals via large droplets (not via aerosol) or by contamination of materials or surfaces (fomites). The virus has short-term viability in the environment. Given this requirement for relatively close contact (compared with measles or rubella where virus is transmitted in aerosol form), it is perhaps surprising that RSV spreads so rapidly through a population, such that the vast majority of children are infected by the end of their second year of life. Transmission is clearly dependent upon specific human behavior, and in particular the contact patterns within schools and within the home will predispose to transmission. School-age siblings are a major risk factor for household introduction, and the infection rate is increased by daycare attendance.
The duration of shedding and the shedding load will both contribute to the capacity of the infection to spread. Data suggest that primary infected children tend to spread for longer period at higher levels of shedding than older individuals (presumably undergoing reinfections), hence per capita their contribution to transmission will be greater.
Transmission occurs mainly from older children, who infect young infants. The incubation period of illness from RSV has been reported as being between 2 and 8 days, most commonly between 4 and 6 days.
Risk Factors And Risk Groups
Risk Factors
Studies in industrialized countries have reported that risk factors for hospitalization with acute lower respiratory tract infections caused by RSV are lack of breastfeeding, crowding, a low level of maternal education, the presence of atopy or asthma in the parents, and parental smoking. From developing countries, one published study from The Gambia confirmed the role of crowding as a main risk factor, but most of the other factors found were minor in their importance and did not appear to lend itself to public health interventions.
High-Risk Groups
Children most likely to develop a severe course of illness are those with underlying medical conditions: Premature infants, those with congenital heart disease, chronic lung disease and prematurity, other chronic lung disease, and those with immunosuppression.
Malnutrition
In developing countries, most of the children hospitalized with RSV are not visibly malnourished, and several studies indicate that malnutrition is less of a risk factor for the development of severe RSV infection than for respiratory infections of other etiologies.
A summary of transmission and protective characteristics is made in Table 1.
Immunity And Pathogenesis
Following RSV infection, the human immune system produces both serum and mucosal IgM, IgA, and IgG antibodies. Primary RSV infection induces IgM response in 5–10 days and IgM antibodies usually persist for 1–3 months. The maximal response of IgG antibody occurs within 20–30 days after the onset of symptoms. By 1 year, RSV-specific IgG levels have declined to low levels. The serum IgA response occurs several days later than IgM and IgG responses. During RSV infection, free RSV-specific IgE and cell-bound IgE are found in nasopharyngeal aspirates. RSV structural proteins are important determinants of antibody responses. Studies showed that responses to the F protein of RSV were often cross-reactive with different RSV strains, whereas antibody responses to the G protein were subgroup-specific. Primary immune responses against RSV is relatively ineffective, but after reinfection a significant booster effect is noted.
Besides antibody production, immune response to RSV infection leads to specific cell-mediated changes such as lymphocyte transformation, cytotoxic T cell responses, and antibody-dependent cellular cytotoxicity. Cell-mediated immune responses include both CD4þ and CD8þ T cells and T helper-1 (Th1) and T helper-2 (Th2) type cell responses. Th-1 cell responses are characterized by high levels of interferon-gamma production, the typical response seen in viral infections. In contrast, asthma and atopy are typically characterized by Th-2 cells producing interleukin 4 and interleukin 5. In some studies, analysis of nasal lavage and peripheral blood samples from RSV-infected children showed reduced interferon gamma: Interleukin 4 ratios in infants with acute wheezing compared to children with signs of upper respiratory tract infection alone. These data are consistent with excessive Th-2 and deficient Th-1 immune responses in RSV bronchiolitis. During the course of RSV infection, IgE antibodies interact with mast cells with subsequent release of inflammatory mediators. The interaction between RSV and the respiratory epithelium also results in the release of a variety of cytokines and chemokines, thereby mobilizing other cells to the site of disease.
Like many viruses, RSV is able to control and manipulate the host in order to escape its immune response. Major RSV structural proteins share structural homology with human proteins. RSV glycoprotein G binds the human CX3CR1 chemokine receptor, potentially facilitating chemotaxis of inflammatory cells. The RSV fusion protein binds Toll-like receptor 4 (TLR4), upregulates surface expression of TLR4 on bronchial epithelial cells, and sensitizes airway epithelial cells to bacterial endotoxin and other TLR4 ligands. Once RSV has infected a cell, it rapidly synthesizes nonstructural proteins that cause species-specific resistance to certain interferons.
Pathological changes in the lungs of children who have died of RSV bronchiolitis include a peribronchiolar mononuclear infiltration, necrosis of the epithelium of the small airways, plugging of the lumina of the small airways, and hyperinflation and atelectasis.
The relation between RSV infection and subsequent episodes of wheezing has been consistently shown in clinical studies. By contrast, uncomplicated common colds and other common viral infections in early childhood seem to protect against wheeze. The increased bronchial reactivity seen in RSV infection may be due to anatomic restrictions of the neonatal bronchial tree or tissue damage produced by the infection itself or ongoing inflammation after the symptoms of acute disease have resolved. There is no clear explanation for the association between RSV bronchiolitis and recurrent wheeze in later life. Lower than normal lung function prior to RSV infection is a risk factor for the development of bronchiolitis. RSV bronchiolitis could act as a marker for a predisposition to airway disease or the association of RSV infection and allergic sensitization or atopic illness could be causal, with RSV infection leading to long-term changes in the lungs.
Other possible immune mechanisms such as imprinting or programing of the immune system at a vulnerable stage of postnatal development or persistent infection are subjects of current debate.
Clinical Features
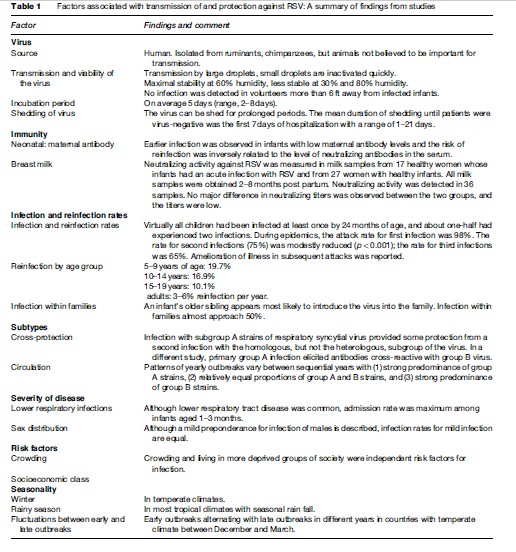
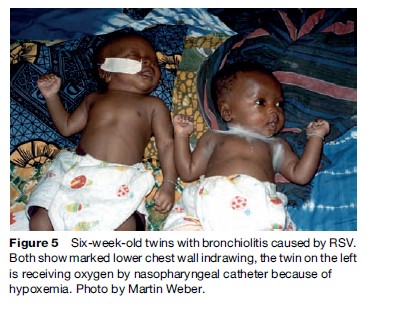
The first infection of an infant with RSV is almost always apparent, but clinical features vary between a runny nose and severe pneumonia. Children who develop a lower respiratory illness may do so again in the following years, but these episodes are generally less severe. The most common manifestation of RSV acute lower respiratory tract infection is pneumonia, the ratio between cases of pneumonia and bronchiolitis ranges between 7:1 and 1:1. The clinical signs of bronchiolitis are expiratory wheeze (Figure 5), hyperinflation of the lung (Figure 6), and fine crepitations on auscultation. Often the signs of both entities overlap, and pneumonia appears to be a continuum of bronchiolitis.
The most common signs of RSV infection are cough (97–100%), rhinitis (56–82%), dyspnea (50–78%), rhonchi (59–78%), wheeze (45–76%), and crepitations (27–72%). Fever is less common in younger children than in older ones. Clinical assessment of children is directed mainly at the detection of hypoxemia.
Useful signs indicating possible hypoxemia are inability to feed, severe respiratory distress with signs such as head nodding, and cyanosis. Younger children become hypoxemic more frequently than older ones. However, all these signs have limited sensitivity and specificity, so, where available, a pulse oximeter (which measures blood oxygen levels) should be used in the assessment of children with RSV acute respiratory infection. Hypoxemia in RSV infection is probably due to a low ventilation-perfusion ratio rather than to shunting through unventilated lung. Most children admitted to hospital improve sufficiently within 4–7 days to be discharged, but inflammation in the lung may persist longer, with abnormalities in gas exchange and wheezing.
Bacterial Coinfections
Of major clinical importance is the frequency of bacterial coinfections in children with RSV, as this determines the need for antibiotic therapy. Most published studies looking at this issue were small and found bacteria only occasionally. In The Gambia, bacteria were found in 3.5% of 255 children. All these children had a high temperature on admission. The only large study with a high bacterial isolation rate was one conducted in Pakistan, in which bacteria were found in 31% of all cases of RSV infection. In most studies, Streptococcus pneumoniae was the most frequently isolated organism, followed by Haemophilus influenzae.
Diagnosis
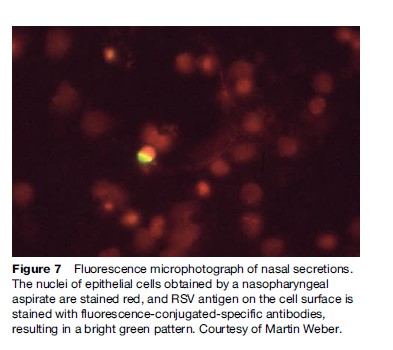
The suspicion of RSV infection is high if children present with bronchiolitis during the RSV season. The diagnosis can be confirmed by viral culture, the detection of viral RNA by polymerase chain reaction, or by the detection of RSV antigen in nasal secretion by immunofluorescence or antigen detection ELISA (Figure 7). The latter are available commercially, and can be done by personnel with limited training. However, as the consequences of a positive test result are limited, these tests will not be done routinely in countries with limited resources. In industrialized countries, testing will mostly be done for cohorting of hospitalized patients.
Treatment And Follow-Up
Treatment of RSV infection is largely supportive, aimed at mechanically clearing secretions obstructing the airways and maintaining nutritional and fluid status and oxygenation. Children who are hypoxemic receive supplemental oxygen. As the main abnormality in the affected lungs is a mismatch of ventilation and perfusion, relatively low concentrations of inspired oxygen are usually sufficient. This can be achieved with nasal prongs or nasal cannulae and low oxygen flow rates, typically 1 l/min (Figure 8). One important factor is nutritional support. As children with severe RSV infection have an increased work of breathing, and might be unable to feed, they need to be monitored closely. If there is concern about their ability to suck, the mother should express breast milk and feed by cup, or by nasogastric tube. If a nasogastric tube is passed, care has to be taken to clear nasal secretions to minimize airway obstruction.
Bronchodilators play a limited role in the treatment of RSV bronchiolitis. They appear to be least useful in younger children. Some authorities recommend a trial of bronchodilator therapy and to continue with this treatment if there is clinical improvement after the application. Steroids do not appear to be useful.
In developing countries, the main concern is the risk of concomitant bacterial infection, which is still unresolved, as indicated above (see section ‘Bacterial Coinfections’). Therefore, in primary care settings, for the classification of severity, the WHO algorithm for pneumonia is used. This classifies the disease based on simple signs such as fast breathing and lower chest wall indrawing. No distinction is made between the treatment of RSV pneumonia and bronchiolitis. Children with fast breathing who have no danger signs and are able to feed receive an oral antibiotic. Children with lower chest wall indrawing, or with danger signs are admitted for inpatient treatment with an injectable antibiotic. Children under 2 months of age who are irritable, unable to feed, or dyspneic, would fulfil the criteria for neonatal sepsis and accordingly be treated as inpatients with a combination of the locally adequate sepsis regimen. Undoubtedly, this approach will result in considerable overtreatment of children with a purely viral illness, but so far no safe approach has been established to enable less-skilled workers to distinguish between a purely viral infection and an infection with a bacterial component.
Figure 7 Fluorescence microphotograph of nasal secretions. The nuclei of epithelial cells obtained by a nasopharyngeal aspirate are stained red, and RSV antigen on the cell surface is stained with fluorescence-conjugated-specific antibodies, resulting in a bright green pattern. Courtesy of Martin Weber.
Figure 8 A child receiving oxygen with nasal prongs. Reproduced from Pocket Book of Hospital Care for Children, p. 282, with permission from the World Health Organization.
Follow-up is done according to the severity of disease. Children not admitted should be seen after 2 days, and the mother should be told to seek care earlier if the child becomes sicker or is unable to feed.
Prevention
There is no effective treatment modality, thus making prevention a higher priority. The antiviral agent ribavirin has been promoted for severe cases for years, but a metaanalysis questions its efficacy for the prevention of death and respiratory deterioration. Intravenous immune globulin or aerosol-administered immune globulin and RSV hyperimmune globulin (RSV-IGIV) are not efficacious in treatment, despite the clear benefit of the latter when a humanized monoclonal RSV antibody (Palivizumab) is used in preventing RSV hospitalization in high-risk groups. The cost of passive prophylaxis and its requirement for monthly injections prohibits its use in the general population. Albeit, simple hand washing and cleaning of environmental surfaces have been shown to prevent both development of ALRI in infants and children and to prevent nosocomial spread of RSV. In many hospitals, children with RSV are cohorted or barrier-nursed.
Background For Vaccine Development
The development of a RSV vaccine, then, would appear to be a worthwhile objective. Attempts at vaccine development have been hampered by several major obstacles. The severest cases of RSV disease are in young infants less than 6 weeks old. Thus, there is a limited window of opportunity to administer such a vaccine. The immunologic factors that are responsible for protection are not completely understood. RSV infections themselves do not prevent subsequent recurrences, although there is generally a reduction in severity with subsequent infection. Vaccine development has been slowed by the catastrophic consequences seen with an older RSV vaccine. Studies of a formalin-inactivated vaccine observed more severe lower respiratory illness after exposure to natural infection during RSV epidemics among RSV-naive vaccinees compared with controls not receiving the vaccine. This enhancement of disease on exposure to natural infection is likely related to an imbalance in the immune response favoring a Th2 type response to RSV protein when the formalin-inactivated vaccine was administered to RSVnaive infants. These infants mounted a humoral response, but neutralizing antibodies were not produced. It has been hypothesized that on exposure to wild virus, RSV replicated in the lungs of these infants, eliciting a Th2 response that was responsible for the enhanced pathology seen in the lungs of children who died. In animal models, a similar Th2 predominance has been observed, with subunit vaccines preventing the development of such vaccines in the infant population where they are most needed.
Subunit RSV Vaccines
Several subunit vaccines containing various combinations of RSV proteins have been developed, but while most have remained in preclinical trials, a few have been tested in children and none in infants. An alternative strategy, given the young age of infants when they become ill, is maternal immunization. Mothers could receive a subunit vaccine and protective antibodies will be transferred transplacentally to their babies, thus providing the neonates with protection during the most vulnerable period. Unfortunately, development of this vaccine was curtailed due to the low immunogenicity and difficulties in obtaining large quantities of immunogen. An optimal strategy may still be live attenuated vaccines that have been modulated to be immunogenic but not produce too many significant clinical symptoms or lead to viral transmission.
Live Attenuated RSV Vaccines
Current research to develop a pediatric RSV vaccine is focused on live attenuated strains for intranasal administration. These induce both local and systemic immunity and can be used in infancy. The major problem in this attenuation process appears to be obtaining the right balance between immunogenicity (the ability to elicit a protective immune response) and reactogenicity (side effects) in order to deliver to young infants. Some strains have been too attenuated to be protective, others caused considerable illness in young infants.
Passive Immunoprophylaxis – Immunoglobulins
During the 1980s, passive immunoprophylaxis was studied as an alternative to the live vaccines, which had failed to provide acceptable protection against RSV infection. Early studies in rats, as well as clinical observations of infants infected with RSV, demonstrated that titers of RSV antibodies needed to be between 1:200 and 1:400 to prevent lower respiratory tract infection. Standard immunoglobulin preparations did not adequately protect the lower respiratory tract against RSV infection, seemingly due to the low titers that could be achieved.
Introduction Of Respiratory Syncytial Virus Immune Globulin
Respiratory syncytial virus immune globulin (RSV-IGIV (RespiGamTM) contains a sixfold higher concentration of RSV neutralizing antibodies than does standard immunoglobulin preparations. It was developed to provide passive immunity against RSV in infants who were born preterm, before the third trimester when maternal IgG antibodies are typically passed from the mother to the fetus.
In studies, RSV-IGIV was found to be effective, safe, and well tolerated, though it also has various limitations. For example, it is not effective in children with congenital heart disease or cyanotic heart disease because of blood hyperviscosity, which can be worsened by the immunoglobulin. RSV-IVIG, in clinical studies, was responsible for more hypercyanotic events than albumin. In addition, administration of RSV-IGIV is time-consuming and inconvenient, involving 3to 4-h-long monthly intravenous infusions of large fluid volumes and protein loads. This can lead to fluid overload in some children and is of special concern in children, particularly infants, with chronic cardiopulmonary conditions.
Monoclonal Antibodies
Monoclonal antibodies were investigated in an effort to avoid the difficulties associated with RSV-IGIV. The first monoclonal preparations could be administered intranasally, thereby protecting the portal of entry and precluding the difficulties associated with parenteral therapy.
In a study using rhesus monkeys, a mouse monoclonal IgA antibody against RSV F glycoprotein was administered as nose drops. The monkeys developed high titers of RSV neutralizing antibodies, but this result was not repeated in human phase III clinical trials, and efficacy could not be proved. Regardless, because the half-life of IgA is short, dosing schedules would require repeated applications, reducing the likelihood of compliance.
Likewise, a clinical trial with the intramuscular IgG humanized monoclonal antibody SB 209763 also failed to produce favorable results.
The development of a humanized monoclonal antibody produced by recombinant DNA technology – palivizumab – was a major advance in protection against RSV. Palivizumab (Synagis) is a humanized monoclonal antibody (IgG1) directed to an epitope on the A domain of the F glycoprotein on the surface of the respiratory syncytial virus. Its mechanism of action is to neutralize and inhibit the fusion activity of both types A and B clinical RSV isolates on respiratory epithelial cells. Unlike RSV-IGIV, palivizumab is not derived from human blood and does not require intravenous administration. Its greater safety and convenience of use are clear advantages to previous methods of passive immunoprophylaxis. It is administered seasonally to high-risk individuals by monthly intramuscular (IM) injections. Studies showed that overall hospitalization rates for RSV infection were reduced in children that received palivizumab compared with rates in children that received placebo. Hospitalization rates were reduced in children with bronchopulmonary disease. In premature infants who had received palivizumab, there was a reduction in subsequent wheezing and asthma 2–4 years later. Palivizumab was safe in children with hemodynamically significant congenital heart disease.
So far, the search for a safe and effective vaccine against RSV has not succeeded, and clinical outcomes in studies of children treated symptomatically for RSV with bronchodilators, steroids, and antiviral agents (ribavirin) have not been improved. Until such a vaccine is discovered and proven, palivizumab remains the only safe, effective, and convenient treatment to prevent RSV disease in young children at risk.
Prognosis
Mortality
The mortality of children admitted to hospital with RSV-ALRI is low in developed countries, and even in developing countries with a generally higher hospital mortality, only approximately 1–3% of hospital admissions die, mostly those with an underlying illness, such as congenital heart disease or bronchopulmonary dysplasia. However, where oxygen is not routinely available, or where inpatients are not routinely monitored to detect complications or the inability to feed, mortality may be considerably higher. The impact of RSV on mortality in the community is unknown.
Further Wheezing
There is debate about whether RSV triggers further episodes of wheezing. Data from The Gambia indicate that children with severe RSV infection are at higher risk to be admitted again with respiratory problems over the next few years, but appear to have no higher risk of asthma later. A recent study from Europe and Canada shows that by preventing RSV lower respiratory tract infection (LRTI) in infancy (using palivizumab) there was a 50% reduction of subsequent physician-diagnosed recurrent wheezing in the next 3–4 years. Long-term follow-up of these future subjects is under way.
Bibliography:
- Smyth RL and Openshaw PJ (2006) Bronchiolitis. Lancet 368: 312–322.
- White LJ, Waris M, Cane PA, Nokes DJ, and Medley GF (2005) The transmission dynamics of groups A and B human respiratory syncytial virus (hRSV) in England & Wales and Finland: seasonality and cross-protection. Epidemiology and Infection 133: 279–289.
- Bordley WC, Viswanathan M, King VJ, et al. (2004) Diagnosis and testing in bronchiolitis: a systematic review. Archives of Pediatric and Adolescent Medicine 158: 119–126.
- Hall CB (2001) Respiratory syncytial virus and parainfluenza virus. New England Journal of Medicine 344: 1917–1928.
- Nokes DJ, Okiro EA, Ngama M, et al. (2004) Respiratory syncytial virus epidemiology in a birth cohort from Kilifi district Kenya: Infection during the first year of life. Journal of Infectious Diseases 190: 1828–1832.
- Ogra PL (2004) Respiratory syncytial virus: The virus, the disease and the immune response. Paediatric Respiratory Review 5(supplement A): S119–S126.
- Simoes EA (2002) Immunoprophylaxis of respiratory syncytial virus: Global experience. Respiratory Research 3(supplement 1): S26–S33.
- Simoes EA (2003) Environmental and demographic risk factors for respiratory syncytial virus lower respiratory tract disease. Journal of Pediatrics 143: S118–S126.
- Stensballe LG, Devasundaram JK, and Simoes EA (2003) Respiratory syncytial virus epidemics: The ups and downs of a seasonal virus. Pediatric Infectious Diseases Journal 22: S21–S32.
- Subcommittee on Diagnosis and Management of Bronchiolitis and American Academy of Pediatrics (2006) Diagnosis and management of bronchiolitis. Pediatrics 118: 1774–1793.
- Weber MW, Mulholland EK, and Greenwood BM (1998) Respiratory syncytial virus infection in tropical and developing countries. Tropical Medicine and International Health 3: 268–280.
- http://www.cds.gov/nciod/durd/revb/respiratory/rsufeat.htm – Centers for Disease Control and Prevention (COC) article on respiratory syncytial virus.
- http://www.nlm.nih.gov/medlineplus/respiratorysyncy tialvirus infections.htm – medline plus on respiratory syncytial virus infections.
See also:
Free research papers are not written to satisfy your specific instructions. You can use our professional writing services to buy a custom research paper on any topic and get your high quality paper at affordable price.








