This sample Rickettsia Research Paper is published for educational and informational purposes only. If you need help writing your assignment, please use our research paper writing service and buy a paper on any topic at affordable price. Also check our tips on how to write a research paper, see the lists of health research paper topics, and browse research paper examples.
Introduction
The order Rickettsiales of the a-proteobacteria contains numerous medically important bacteria, including the pathogens responsible for Rocky Mountain spotted fever and typhus. Among them are newly emergent pathogens that cause similar diseases and share many bacterial characteristics. All of the organisms are small, Gram-negative coccobacilli or bacilli, and most are transmitted to mammals by an arthropod vector. They replicate by binary fission in eukaryotic cells, either in the cytoplasm or contained within vesicles, and have a circular genome of only 1 to 1.6 Mb. Interestingly, some bacteria in this order, such as Ehrlichia and Anaplasma have lost the molecular machinery to produce lipopolysaccharide (LPS) and peptidoglycan.
Taxonomy
The classification of bacterial pathogens has traditionally relied on such characteristics as morphology, epidemiology, ecology, and clinical disease manifestations. The Rickettsiales, however, are notoriously elusive to surveillance, and their associated diseases are difficult to diagnose. The introduction of gene sequencing greatly facilitated objective classification, and the order is now partitioned based on 16S rRNA gene sequence similarities (Figure 1). The order contains two families, Rickettsiaceae and Anaplasmataceae. Rickettsiaceae contain two genera, Rickettsia and Orientia, while Anaplasmataceae comprise four genera, Ehrlichia, Anaplasma, Neorickettsia, and Wolbachia. Coxiella burnetii had traditionally been classified among the Rickettsiales, but gene sequencing now places it in the g-proteobacteria. Even though it is not phylogenetically related, the ecological, epidemiological, and clinical similarities warrant its traditional consideration along with the Rickettsiales.
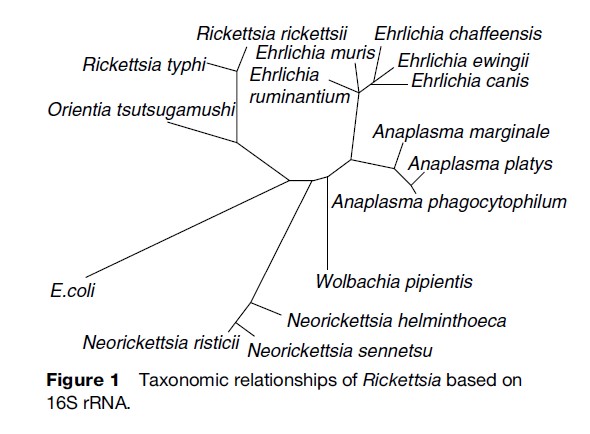
Rickettsiae And Orientia
Rickettsial diseases were described prior to the discovery of microbial causes of disease, and new rickettsial pathogens continue to be recognized. Epidemic typhus influenced the outcome of European wars from the sixteenth to the nineteenth century, and newly emergent species, such as R. africae, R. japonica, R. honei, and R. slovaca, are associated with human disease (Raoult, 1997).
Rickettsial diseases are widely distributed throughout the world, with human disease being described on every continent except Antarctica. Rickettsia are segregated into two groups, the typhus group containing R. prowazekii and R. typhi, which are the etiologic agents of epidemic typhus and murine typhus, respectively, and the spotted fever group characterized by organisms such as R. rickettsii and R. conorii, the etiologic agents of Rocky Mountain spotted fever and Mediterranean spotted fever. Numerous other spotted fever group rickettsiae have also been implicated in human disease (Table 1). Another notable rickettsial pathogen, Orientia tsutsugamushi (formerly R. tsutsugamushi), is the etiologic agent of scrub typhus. This pathogen differs from other Rickettsia, but is similar to Ehrlichia and Anaplasma, in that it lacks LPS and peptidoglycan.
Rickettsiae are also of particular importance due to their potential threat as biological weapons. Rickettsial diseases possess several important characteristics which highlight their risk for intentional dissemination. Despite the fact that natural transmission of rickettsiae occurs by arthropod exposure, infections resulting from aerosol exposure have been described with R. prowazekii, R. typhi, R. rickettsii, and R. conorii. The severe virulence and difficulty of diagnosing human rickettsioses also make them dangerous as biological weapons. In fact, R. prowazekii has an established historical significance as a biological threat. The former Soviet Union developed typhus as a biological weapon, and the Japanese government conducted human and field testing of typhus as a weapon in China during World War II.
Ecology Of Rickettsiae And Epidemiology Of Rickettsial Diseases
Rickettsial diseases occur throughout tropical, subtropical, and temperate regions. Transmission to humans is not a requirement for the maintenance of rickettsiae in nature, as all are maintained in various arthropod vectors and small animals. As a result, rickettsial diseases are classified as zoonoses. The global distribution of rickettsial diseases is dictated by the availability of a small animal host and the blood-sucking arthropod important for transmission and maintenance of rickettsiae in nature.
Rats are the primary mammalian reservoir of R. typhi, the causative agent of murine typhus, and the principal arthropod vector important for human transmission is the rat flea (Xenophsylla cheopis). R. prowazekii, the etiologic agent of epidemic typhus, was long thought to not have a zoonotic reservoir. Humans are implicated directly in the classic maintenance cycle of epidemic louse-borne typhus as lice that acquire the infection from human blood and subsequently transmit the organisms through their fecal matter die of the infection. However, investigators have also identified a zoonotic cycle involving North American flying squirrels and their ectoparasites, and typhus rickettsiae have been discovered in ticks in Mexico and Ethiopia. Therefore, alternative zoonotic cycles may also play a role in the life cycle of typhus rickettsiae.
Spotted fever group rickettsiae, such as R. rickettsii and R. conorii, are maintained in nature through transovarial transmission in infected hard ticks or mites and horizontal arthropod transmission of infection to small mammals. Similarly, O. tsutsugamushi is maintained in nature through transovarial transmission in trombiculid mites which in the larval stage feed on mammals, usually rodents, and occasionally humans, transmitting the infection.
Rickettsiae are transmitted to humans from infected arthropods, and thus the ecologic behavior of the arthropod host is an important factor in the temporal and geographic occurrence of rickettsioses (Azad, 1998). Ixodic, or hard ticks, are the vectors for spotted fever group rickettsiae, with the exception of R. akari, the etiologic agent of rickettsialpox for which the vector is a gamasid mite. As a result, most reported cases of human spotted fever rickettsioses occur in late spring and summer when human and tick activity result in exposure. The highest incidence of Rocky Mountain spotted fever occurs in children aged 5 to 9 years old (Abramson, 1999) and in older persons 40 to 64 years of age.
Lice and fleas are the vectors of R. prowazekii and R. typhi, respectively. In areas of poverty, usually associated with poor sanitation and overcrowding, epidemics of typhus can occur. Latent R. prowazekii infections may relapse resulting in recrudescent typhus (Brill-Zinsser disease) years after the primary infection. Such a case of recrudescent typhus is the index case of a subsequent epidemic.
Clinical Manifestations, Diagnosis, And Treatment Of Rickettsial Diseases
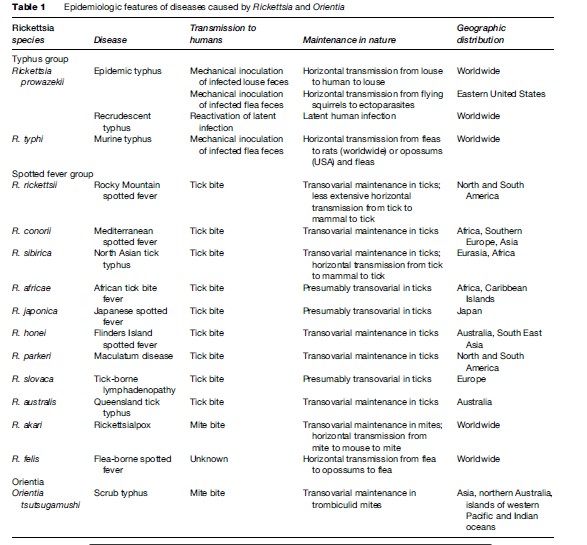
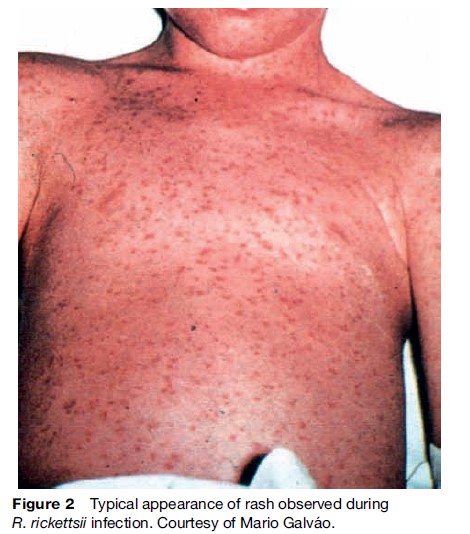
The early symptoms of rickettsial infection are similar to many other infections, particularly viral syndromes, making a clinical diagnosis difficult (Walker, 1995). Diagnosis is further complicated by the fact that tick bites in spotted fever rickettsioses are only reported about 60% of the time. Human rickettsioses are characterized by fever, severe headache, myalgias, malaise, and after several days a macular rash (Figure 2) (Thorner, 1998). Patients infected through the bite of an infected tick may or may not develop an eschar at the site of tick feeding, depending mainly on the particular rickettsial species (Figure 3). The initial diagnosis of rickettsiosis is generally based on the clinical and epidemiologic observations. To confirm the diagnosis, serologic assays are generally used, although antibodies do not appear until later in the disease course, after empiric treatment should have been initiated. Immunohistochemical detection of rickettsiae in rash biopsies is diagnostically useful after skin lesions appear.
Due to the association of rickettsiae with an arthropod host, vector control programs and avoidance of ticks or other arthropods in areas endemic to rickettsial disease are the most prudent methods available to limit the risk of infections; however, use of protective clothing and tick repellants as well as diligent inspection for and removal of attached ticks also serve to decrease disease transmission. Currently there is no approved vaccine, although experimental vaccines have provided protection in laboratory animals.
Treatment of rickettsioses consists of appropriate antibiotic therapy. Doxycycline is the antibiotic of choice and should be administered early in the disease course as delay in antibiotic therapy has been associated with increased mortality (Dalton et al., 1995). Chloramphenicol is less effective, but may be used if doxycycline is contraindicated, as in pregnancy. Antibiotic postexposure prophylaxis following reported tick bites is not recommended as the bacteriostatic mechanism of these antibiotics may only serve to delay onset of disease.
Pathogenesis And Immunity In Rickettsial Diseases
Rickettsial diseases include some of the most severe and potentially life threatening bacterial diseases recognized today. In human infection, rickettsiae primarily infect the vascular endothelium, with the exception of R. akari, which mainly targets monocytes and macrophages. Rickettsia rickettsii also invades smooth muscle cells of the vasculature. All Rickettsia and Orientia replicate free in the cytosol of infected cells.
Human rickettsioses begin after introduction of rickettsiae through the bite of an infected tick or mite or after mechanical inoculation of infected louse or flea feces through an abrasion in the skin or the conjunctiva. Inhalational infection has also been described in laboratory workers. Following infection, rickettsiae invade the vascular endothelium, proliferate in the cell cytoplasm, and spread throughout the circulation (Figure 4a). Rickettsiae are directly cytopathic for vascular endothelium, and immune-effector responses may also play a role in vascular injury. Overall, however, the immune response is beneficial to the host. Disseminated infection leads to increased vascular permeability and accumulation of fluid in the surrounding interstitial space culminating in decreased blood volume and hypovolemic shock. Major vital organs affected are the brain and lungs, manifesting as meningoencephalitis and interstitial pneumonitis, respectively. Decreases in kidney perfusion owing to hypovolemia may also lead to acute renal failure while vascular leakage into the alveolar spaces in the lungs impairs gas exchange leading to hypoxemia.
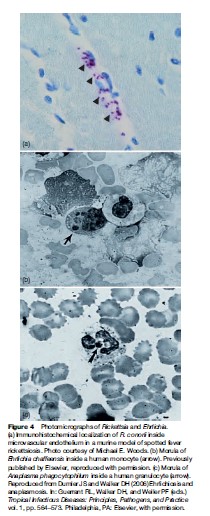
Based on the intracellular lifestyle of rickettsiae, cellular immunity is of paramount importance. Ultimately, cytotoxic T-lymphocytes are critical to clearance of rickettsiae, and the cytokines gamma interferon and tumor necrosis factor induce intracellular killing in endothelial cells through the activation of inducible nitric oxide synthase and subsequent rickettsicidal nitric oxide production. Natural killer cells also contribute to immunity against rickettsiae, primarily through the production of gamma interferon. Lastly, the humoral immune response, specifically antibody production against outer membrane proteins, may prevent reinfection.
Anaplasmataceae
Ehrlichia
Ecology Of Ehrlichia And Epidemiology Of Ehrlichioses
Ehrlichiae are globally distributed pathogens, of importance in both human and veterinary medicine. Ehrlichia chaffeensis, the causative agent of human monocytotropic ehrlichiosis (HME), was discovered in the midwestern United States in 1986 and subsequently was found to cause disease also in dogs. It is globally distributed throughout North and South America, Asia, and Africa. Most recognized cases are in the south central and southeastern United States, corresponding to the distribution of its main vector, Amblyomma americanum (Lone Star tick). E. chaffeensis is maintained in the major animal reservoir, Odocoileus virginianus (white-tailed deer), as a persistent infection. A second species, E. ewingii, shares the same vector, animal reservoir, and North American distribution. Distributions of the ehrlichioses outside of the United States have not been mapped; our knowledge is limited to sporadic case reports and surveillance of a few small communities (Table 2).
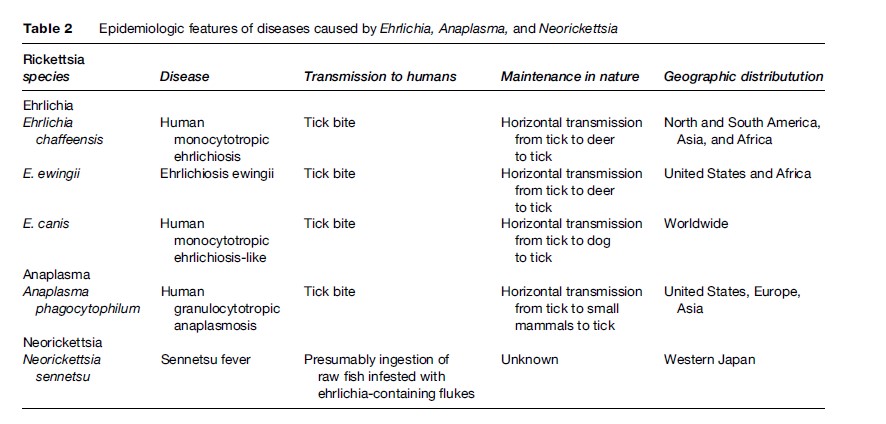
Seventy-five percent of HME cases are diagnosed in males, and most patients are older adults. Infections with E. ewingii are diagnosed mainly in the immunocompromised and cause a similar, but less severe syndrome than HME. Both HME and E. ewingii infections occur seasonally from late spring to early fall. Because they are difficult to diagnose and the symptoms are sometimes mild, the exact quantification of cases is elusive. The best efforts to enumerate infections have resulted from active, prospective surveillance for seroconversion and active infection (using PCR to detect ehrlichial DNA in blood) of at-risk populations. These studies reveal the annual incidence to be 10 to 100 cases per 100 000 population, though cases are reported at a much lower rate.
A third pathogen, E. canis, is distributed throughout the world and is emerging as a possible human pathogen. It was isolated in South America from asymptomatic individuals and from patients with suspected HME. Testing 20 patients with suspected HME for E. canis (by PCR) revealed that 30% were infected (Perez et al., 2006). The incidence of E. canis infection in humans is unclear, and it is unknown if its pathogenicity in humans is as severe as E. chaffeensis. It is transmitted by Rhipicephalus sanguineus (brown dog tick) and is maintained in dogs as chronic canine monocytic ehrlichiosis.
Clinical Manifestations, Diagnosis, And Treatment Of Ehrlichiosis
Similar to the rickettsioses, the symptoms of HME are non-specific, making clinical diagnosis difficult. The most common symptoms are fever, myalgia, malaise, and headache. Nausea, vomiting, cough, confusion, and rash occur in a minority of patients. Leukocytopenia, thrombocytopenia, anemia, and elevated liver transaminases are frequent laboratory findings. Some cases exhibit severe symptoms such as meningoencephalitis or acute respiratory distress syndrome; 2 to 3% of infected individuals die, usually with multisystem organ failure or a toxic shocklike syndrome. A history of a tick bite within 3 weeks prior to illness aids diagnosis. There is no vaccine for HME, so prevention revolves around reducing tick bites.
Diagnosis is confirmed either by a fourfold increase in antibody titer (useful only retrospectively) or by detection of E. chaffeensis DNA by PCR. It is also possible to confirm diagnosis by immunostaining of a biopsy or an autopsy sample or by isolating Ehrlichia in culture from a patient sample. However, neither technique is available outside of a specialized laboratory. Treatment should not be postponed for confirmation, but should be started as soon as HME is suspected. The most effective therapy is doxycycline; rifampin is used to treat pregnant women. E. ewingii diagnosis usually relies upon cross-reactivity with E. chaffeensis, as there is no serological test specific for E. ewingii.
Pathogenesis And Immunity In Ehrlichioses
Ehrlichia species are transmitted to the mammalian host during tick feeding, being inoculated along with the tick saliva. Once introduced into the dermis, E. chaffeensis and E. canis have selective tropisms for monocytes and macrophages, while E. ewingii targets neutrophils and occasional eosinophils. On blood smears, the bacteria can be visualized as morulae, which are microcolonies of Ehrlichia inside host cell vesicles (see Figure 1). Unlike the Rickettsiales, Ehrlichia do not replicate in the cytoplasm. Following uptake of the bacteria into vesicles, phagocytes disseminate hematogenously to the major target organs: liver, lung, spleen, and, for E. chaffeensis, the central nervous system. Ehrlichiae directly compromise the killing ability of these cells by preventing superoxide radical production and phagolysosomal fusion.
The immune system is both the target of the bacteria and the natural cure for the disease; thus, knowledge of its role in mediating tissue damage and in controlling bacterial replication is essential to understanding the pathogenesis. Animal models have demonstrated that a gamma interferon producing CD4+ T lymphocyte response is necessary for control of ehrlichial infection. Antibodies and CD8+ cytotoxic T lymphocytes contribute to protective immunity. Lymph node studies from HME patients suggest that infected macrophages are highly activated by E. chaffeensis but do not appropriately induce cytotoxic T cells (Dierberg and Dumler, 2006). These T cells may contribute more to pathogenesis than to resolution, possibly by producing a cytokine storm that induces a toxic shocklike syndrome. Overproduction of TNF-alpha and IL-10 and weak CD4+ T lymphocyte responses contribute to illness severity (Ismail et al., 2004).
Anaplasma
Ecology Of Anaplasma And Epidemiology Of Anaplasmosis
Members of the genus Anaplasma infect mainly ruminants (cud-chewing animals such as cattle, sheep, and goats), but one species in particular is a significant human pathogen. Anaplasma phagocytophilum is the causative agent of human granulocytic anaplasmosis (HGA). HGA has been identified in Europe and Asia but is most commonly reported in the United States. A. phagocytophilum is maintained in deer mice and other animals and is transmitted by Ixodes scapularis (blacklegged tick) in the eastern United States, by Ixodes pacificus (western blacklegged tick) in the western United States, and by Ixodes ricinus in Europe. Following the distribution of its vectors, HGA is distributed throughout the upper Midwest and northeastern states as well as parts of California. Its incidence peaks in the late spring to early fall, and seroprevalence has been reported to be 15 to 36% in endemic areas.
Clinical Manifestations, Diagnosis, And Treatment Of Anaplasmosis
The clinical presentation of HGA is similar to HME except for rarity of central nervous system involvement, rash, and severe complications. Even with high seroprevalence, few patients require hospitalization, suggesting frequent asymptomatic or undiagnosed infections. Mortality is less than 1% and often due to an opportunistic super-infection. Diagnosis is confirmed serologically as in HME, but the tests for HME and HGA are distinct. Morulae are more frequently detected in peripheral blood smears in HGA, but are found in neutrophils rather than in monocytes. HGA responds to the same antibiotics as the ehrlichioses, doxycycline or rifampin, which should be begun early to shorten illness and prevent complications.
Pathology And Immunity In Anaplasmosis
The most prominent pathology observed in HGA is liver injury. The lungs may also be involved, and all cellular components of blood may decrease in number. Because organisms are not prominent at sites of tissue damage, an immune-mediated mechanism has been suggested. A. phagocytophilum parasitizes granulocytes, especially neutrophils, in the mammalian host. Bacteremia sufficient to infect feeding ticks, and thus maintain the pathogen in nature, is achieved by prolonging the neutrophil life span past the normal 6 to 12 h. Anaplasmae halt apoptosis, phagocytosis, and nitric oxide production in the neutrophil, compromising the innate immune system. T cell numbers and antibody production may also be suppressed, enhancing susceptibility to opportunistic fungal, bacterial, and viral infections. Mouse models have demonstrated that inflammation, specifically the nonspecific innate immune cascade, contributes to tissue damage, but does not control bacterial replication (Scorpio et al., 2006).
Neorickettsia
Ecology
Neorickettsiae infect digenetic trematode worms (flukes) and are maintained transovarially. The fluke vector has a complex parasitic life cycle that requires development within one or two intermediate hosts (mollusks and sometimes insects or fish) followed by sexual reproduction within a mammalian host. Because of transovarial transmission, neorickettsiae only require replication within trematode worms for maintenance in nature. However, neorickettsiae are capable of replicating in mammalian tissues following the ingestion of a trematode-infested host. It is the replication within mammalian cells that causes the neorickettsial diseases of human and veterinary importance.
Epidemiology
Three species are recognized as rare, emerging pathogens, and more species are continuing to emerge. Neorickettsia helminthoeca infects dogs that ingest uncooked salmon or trout harboring infected flukes. Following a 14-day incubation period, the infected dog will develop salmon poisoning disease (SPD), a syndrome marked by fever, vomiting, diarrhea, dehydration, weakness, swollen lymph nodes, and thrombocytopenia. Without appropriate treatment (tetracycline or doxycycline), most dogs succumb to infection. Diagnosis is made by identification of organisms in a lymph node biopsy. SPD has only been reported along the Pacific coast of the United States and Canada with a few reports along the Atlantic coast of Brazil. Another bacterium, Neorickettsia risticii, infects horses following ingestion of insects that harbor infected flukes. It causes a syndrome similar to SPD called Potomac horse fever (PHF) or equine monocytic ehrlichiosis. The fluke vector Acanthatrium oregonense is believed to utilize bats as its primary mammalian host, and N. risticii have been isolated from bat tissues.
A third organism, Neorickettsia sennetsu, causes sennetsu fever and is the only known neorickettsia capable of infecting humans. Unlike SPD and PHF, no fatalities have been reported from sennetsu fever. The clinical course is self-limited and similar to infectious mononucleosis, including fever, body aches, headache, and swollen lymph nodes. The disease has only been reported in western Japan during late summer and fall and is probably related to eating uncooked fluke-infested fish. Routine diagnostics have not been developed, though a few specialized laboratories are capable of detecting N. sennetsu DNA in tissue biopsies by PCR. Doxycycline is an effective treatment.
Pathology And Immunity
Very little is known about the pathological consequences of and immunity to N. sennetsu. Most of the current knowledge regarding neorickettsiae is derived from studies of the veterinary pathogens. The mechanisms of exit from the fluke and entry into the mammal are unknown, but once introduced into the mammal, neorickettsiae infect macrophages in lymphoid tissues throughout the body. Lymph node biopsies from SPD cases demonstrate infiltration of lymphocytes and infected macrophages. Similar lesions are observed in the spleen and intestinal lymphoid tissues at necropsy. Animal studies with N. risticii suggest that development of neutralizing antibodies may be important in generating a protective immune response.
Wolbachia
Wolbachiae are endosymbionts of arthropods and filarial nematodes. In contrast with all other Anaplasmataceae, these bacteria have not been reported to infect mammals. Arthropods and filariae, therefore, serve as hosts but not vectors. With few exceptions, wolbachiae are parasitic to arthropods, altering their sexual reproductive capacity. Only as mutualistic symbionts in filariae does their existence relate to human disease. Many filarial species, including some that infect humans, depend on wolbachiae for survival, and the eradication of wolbachiae from these nematodes leads to worm death at embryonic, larval, and adult stages. Interestingly, filarial wolbachiae have lost the genes necessary for cellular invasion and are passed to successive generations strictly transovarially, like mitochondria.
Wolbachia have been estimated to infect 20 to 76% of insects and many nematodes in a worldwide distribution. Most nematodes known to cause filariasis in humans carry wolbachiae, including Wuchereria bancrofti, Brugia malayi, Brugia timori, and Mansonella ozzardi, but not Mansonella perstans. Wolbachia are also found in Onchocerca volvulus, the causative agent of onchocerciasis (river blindness), and Dirofilaria immitis, the canine heartworm. Loa loa, in contrast, live independently of bacterial endosymbionts. During filarial invasion of a mammalian host, wolbachiae contribute to the immunopathology by enhancing neutrophil activation. Promising animal studies suggest that doxycycline directed at the Wolbachia may be an efficient, novel treatment for filariasis and onchocerciasis.
Coxiella
Coxiella Burnetii
Ecology
C. burnetii, the etiologic agent of the zoonosis Q fever, is a pathogen primarily of sheep, goats, and cattle, but may also infect other livestock, pets, wild mammals, birds, reptiles, and humans. C. burnetii has two morphologic forms, the small cell variant and the large cell variant, which are distinguishable by electron microscopy. The large cell variant replicates inside the acidified phagolysosome and is resistant to the low pH and lysosomal enzymes. The small cell variant can persist in the soil free of a eukaryotic host. This form is resistant to many chemical disinfectants, heat, and cold. C. burnetii is passed among its primary reservoirs (cattle, sheep, and goats) by bacteria-laden aerosols. It replicates to high titers in the placenta and the lactating mammary gland; parturition and slaughter generate infectious aerosols that may be inhaled or contaminate soil. Consumption of unpasteurized milk can also cause infection. Because of its high infectivity (the minimum infective dose is one organism) and its environmental stability, the Centers for Disease Control and Prevention has classified C. burnetii as a category B biological threat agent.
Epidemiology, Clinical Manifestations, And Diagnosis
Humans with close occupational contact with livestock and other infected animals, such as workers in the dairy and meat-processing industries, veterinarians, hunters, and farmers, are at greatest risk for exposure to C. burnetii. Human infections usually result from the inhalation of aerosols generated from birth products, feces, urine, or milk from infected animals; 60% of infections are asymptomatic. When symptoms do arise, they are usually flulike and present after an incubation period of 2 to 3 weeks as high fever, myalgia, malaise, and nonproductive cough. Thirty to fifty percent of symptomatic patients develop atypical pneumonia, heralded by cough and pleuritic chest pain. Chest radiographs show nonspecific changes. Q fever may also manifest as hepatitis. Chronic infections with Coxiella burnetii also occur.
Q fever infection is diagnosed serologically using commercially available enzyme linked immunosorbent assay (ELISA) or indirect immunofluorescence assay (IFA) kits. Diagnosis of acute disease requires IgM titers of greater than 1:50 and IgG titers greater than 1:200 to phase II variant C. burnetii; diagnosis of chronic infection requires an IgG titer equal to or greater than 1:1600 to phase I variant. C. burnetii may be isolated in shell-vial culture, but this technique is rarely used due to a lower sensitivity and a higher risk to laboratory personnel.
Acute Q fever infections are treated with doxycycline alone; chronic infections require the addition of hydroxychloroquine. Hydroxychloroquine raises the pH inside the phagolysosome, increasing the effectiveness of doxycycline. In treated patients, the mortality rate is less than 1%. Some patients, however, do not clear C. burnetii during treatment, and viable organisms have been isolated even after 12 months of antibiotic therapy. No vaccine for Q fever is commercially available in the United States, but in Australia a vaccine successfully protects people at risk due to occupational exposure. Prior to vaccination, individuals must be tested for seropositivity to C. burnetii as severe adverse reactions occur at the injection site and systemically in seropositive individuals.
Pathogenesis And Immunity
Acute Q fever generally occurs after inhalation of viable C. burnetii, which first infect alveolar macrophages. The small cell variant of C. burnetii enters the phagosome of monocytes and macrophages passively and delays phagolysosomal fusion, apparently to allow the change to the large cell variant. C. burnetii then replicates inside the phagolysosome. Dissemination throughout the body occurs with C. burnetii infecting monocytes and macrophages with the formation of granulomas in infected organs.
Chronic Q fever occurs when a substantial bacterial burden persists apparently owing to host factors. During chronic infection, C. burnetii undergoes gene reduction typically involving LPS synthesis genes. Chronic Q fever infections are normally manifested as infective endocarditis. Over 90% of patients with C. burnetii infective endocarditis have underlying cardiac valvular disease (rheumatic, degenerative, or congenital) or prosthetic heart valves. In Europe 3 to 5% of endocarditis cases are caused by C. burnetii. However, in the United States, only seven cases of endocarditis due to Q fever have been described. These data suggest that there may be geographic differences in the pathogenesis of C. burnetii.
Sterilizing immunity to C. burnetii is rare, as organisms can persist despite immunologic control. Control is dependent on effective T-lymphocyte responses and granuloma formation. Granuloma formation is aided by signaling through Toll-like receptor (TLR) 4, a host cell receptor known to bind LPS and trigger an inflammatory cascade. Animals that lack TLR4 have significantly decreased numbers of granulomas (Honstettre et al., 2004).
Bibliography:
- Abramson JS and Givner LB (1999) Rocky Mountain spotted fever. The Pediatric Infectious Disease Journal 18: 539–540.
- Azad AF and Beard CB (1998) Rickettsial pathogens and their arthropod vectors. Emerging Infectious Diseases 4: 179–186.
- Dalton MJ, Clarke MJ, Holman RC, et al. (1995) National surveillance for Rocky Mountain spotted fever, 1981–1992: Epidemiologic summary and evaluation of risk factors for fatal outcome. American Journal of Tropical Medicine and Hygiene 52: 405–413.
- Dierberg KL and Dumler JS (2006) Lymph node hemophagocytosis in rickettsial diseases: A pathogenetic role for CD8 T lymphocytes in human monocytic ehrlichiosis (HME)? BMC Infectious Diseases 6: 121.
- Dumler JS and Walker DH (2006) Ehrlichiosis and anaplasmosis. In: Guerrant RL, Walker DH, and Weller PF (eds.) Tropical Infectious Diseases: Principles, Pathogens, and Practice vol. 1, pp. 564–573. Philadelphia, PA: Elsevier.
- Honstettre A, Ghigo E, Moynault A, et al. (2004) Lipopolysaccharide from Coxiella burnetii is involved in bacterial phagocytosis, filamentous actin reorganization, and inflammatory responses through Toll-like receptor 4. Journal of Immunology 172: 3695–3703.
- Ismail N, Soong L, McBride JW, et al. (2004) Overproduction of TNF-alpha by CD8+ type 1 cells and down-regulation of IFN-gamma production by CD4+ Th1 cells contribute to toxic shock-like syndrome in an animal model of fatal monocytotropic ehrlichiosis. Journal of Immunology 172: 1786–1800.
- Perez M, Bodor M, Zhang C, Xiong Q, and Rikihisa Y (2006) Human infection with Ehrlichia canis accompanied by clinical signs in Venezuela. Annals of the New York Academy of Sciences 1078: 110–117.
- Raoult D and Roux V (1997) Rickettsioses as paradigms of new or emerging infectious diseases. Clinical Microbiology Review 10: 694–719.
- Scorpio DG, von Loewenich FD, Gobel H, Bogdan C, and Dumler JS (2006) Innate immune response to Anaplasma phagocytophilum contributes to hepatic injury. Clinical and Vaccine Immunology 13: 806–809.
- Thorner AR, Walker DH, and Petri WA (1998) Rocky Mountain spotted fever. Clinical Infectious Disease 27: 1353–1360.
- Walker DH (1995) Rocky Mountain spotted fever: A seasonal alert. Clinical Infectious Diseases 20: 1111–1117.
- Bakken JS and Dumler JS (2006) Clinical diagnosis and treatment of human granulocytic anaplasmosis. Annals of the New York Academy of Sciences 1078: 236–247.
- Chapman AS (2006) Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever, ehrlichioses, and anaplasmosis – United States: A practical guide for physicians and other health-care and public health professionals. MMWR Morbidity and Mortality Weekly Report 55(No. RR-4).
- Fishbein DB, Dawson JE, and Robinson LE (1994) Human ehrlichiosis in the United States, 1985 to 1990. Annals of Internal Medicine 120: 736–743.
- Marrie TJ (2006) Q fever. In: Guerrant RL, Walker DH, and Weller PF (eds.) Tropical Infectious Diseases: Principles, Pathogens, and Practice vol. 1, pp. 574–577. Philadelphia, PA: Elsevier.
- Raoult D and Walker DH (2006) Typhus group rickettsioses. In: Guerrant RL, Walker DH, and Weller PF (eds.) Tropical Infectious Diseases: Principles, Pathogens, and Practice vol. 1, pp. 548–556. Philadelphia, PA: Elsevier.
- Sexton DJ and Walker DH (2006) Spotted fever group rickettsioses. In: Guerrant RL, Walker DH, and Weller PF (eds.) Tropical Infectious Diseases: Principles, Pathogens, and Practice vol. 1, pp. 539–547. Philadelphia, PA: Elsevier.
- Walker DH, Raoult D, Dumler JS, and Marrie T (2005) Rickettsial diseases. In: Kasper DL, Braunwald E Fauci AS, et al. (eds.) Harrison’s Principles of Internal Medicine, 16th edn., pp. 999–1008. Chicago, IL: McGraw-Hill.
- Watt G and Walker DH (2006) Scrub typhus. In: Guerrant RL, Walker DH, and Weller PF (eds.) Tropical Infectious Diseases: Principles, Pathogens, and Practice vol. 1, pp. 557–563. Philadelphia, PA: Elsevier.
- http://wwwn.cdc.gov/travel/contentDiseases.aspx – Centers for Disease Control and Prevention: Diseases Related to Travel.
- http://www.cdc.gov/ncidod/dvrd/rmsf/index.htm – Centers for Disease Control and Prevention: Rocky Mountain Spotted Fever.
See also:
Free research papers are not written to satisfy your specific instructions. You can use our professional writing services to buy a custom research paper on any topic and get your high quality paper at affordable price.







