This sample Tuberculosis Prevention Research Paper is published for educational and informational purposes only. If you need help writing your assignment, please use our research paper writing service and buy a paper on any topic at affordable price. Also check our tips on how to write a research paper, see the lists of health research paper topics, and browse research paper examples.
Introduction
Tuberculosis (TB) remains one of the deadliest diseases in the world. It is a social disease with medical implications. It has always occurred disproportionately among disadvantaged populations such as the homeless, malnourished, and overcrowded. Within the past two decades the spread of human immunodeficiency virus (HIV) infection and migration of persons from areas of high TB incidence have resulted in an increased number of tuberculosis cases in certain countries. The World Health Organization (WHO) estimates that there were 8.8 million new cases of TB in 2003, of which 3.9 million were smear-positive and 674 000 were co-infected with HIV. An estimated 1.7 million people died from TB in 2003, including those coinfected with HIV.
For most people tuberculosis prevention means the diagnosis and treatment of individuals with latent tuberculosis infection (LTBI). But tuberculosis can also be prevented in other ways – among communities and even countries. Thus actions can be taken to prevent spread of the tubercle bacilli to other persons from an infectious host, or to prevent development and the spread of drug-resistant strains of tuberculosis. In this new age of globalization, where movement of population is on a massive scale, prevention of TB in high-income countries may require a more global approach to tuberculosis control worldwide.
In this research paper, we focus on preventing TB on an individual level by detecting and treating latent infection, thus reducing the risk of development of active disease. This, in turn, should impact on population prevention by reducing transmission from contagious cases. On the community prevention level, we briefly mention contact tracing, outbreak management, and public health intervention. We also discuss vaccination and the prevention of drug resistance, since these have an impact on disease prevention.
Prevention At The Individual Level: Diagnosis And Treatment Of Latent Tuberculosis
Pathogenesis Of Latent Tuberculosis
TB is dependent upon human hosts for its survival. Primary infection is usually self-limited and followed by latent infection, which can last from three months to a lifetime. In some of those infected, latent infection may reactivate to cause post-primary disease.
At the time of the initial infection, the distribution of inhaled droplet nuclei is determined by the pattern of lung ventilation and thus tends to favor the middle and lower lung zones. In immunocompetent hosts, alveolar macrophages ingest the M. tuberculosis organisms. When innate macrophages’ microcidal activity is inadequate to destroy the initial few tubercle bacilli, they start replicating in the macrophage. Although at the site of implantation the lesion is usually insignificant, TB bacilli will spread to regional lymphatic nodes and then disseminate through the bloodstream to all parts of the body. The lymph node spread and bacillemia occur within four to six weeks of primary infection even in perfectly normal healthy hosts. Fortunately cell-mediated immunity and delayed-type hypersensitivity develop at this time, limiting further bacterial multiplication and creating granulomata in all the sites at which bacillary seeding has occurred. At this time the infection enters the latent phase, but this hematogenous seeding sets the stage for later reactivation of tuberculosis and explains why disease can develop in pulmonary or nonpulmonary organs.
Diagnosis Of Latent Tuberculosis Infection
Indication For Testing
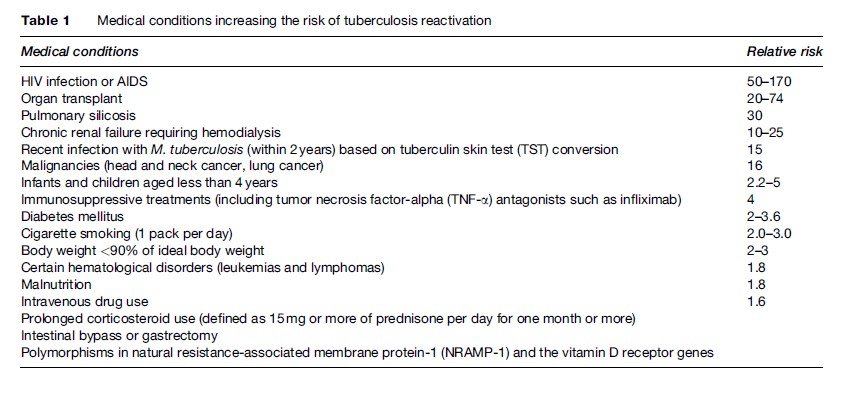
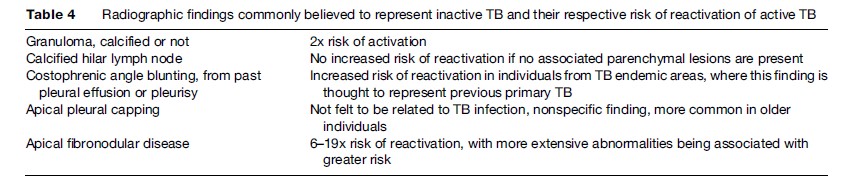
As cases of TB decrease in high-income, low-prevalence countries, there has been renewed interest and focus on the screening and treatment of latent TB as an important TB-control strategy. In general, testing for latent TB infection is indicated when the risk of development of disease, if infected, is increased. There are three general situations of increased risk: (1) recent infection, since 3 to 10% of recently infected persons will develop active TB disease within 1 to 2 years; (2) infection associated with conditions which decrease immunity (Table 1); and (3) radiological evidence on a chest X-ray of old inactive tuberculosis (see Table 4) but no prior adequate treatment. Recent infection is most commonly seen in people who have been in contact with patients with active, smearpositive pulmonary tuberculosis. Recent infection is also seen in health-care workers serving high-risk patients or having unprotected exposure to patients with TB disease before the diagnosis is made and adequate airborne precautions are instituted. A fourth category, which differs slightly from the previous ones because it does not necessarily carry a greater risk of disease but rather is associated with an increased prevalence of infection, comprises the following:
- Residents and employees of high-risk settings such as correctional facilities, long-term-care facilities, and homeless shelters
- Certain populations who are medically underserved and who have low income, as defined locally
- Populations at high risk who are defined locally as having an increased incidence of TB disease
- Infants, children, and adolescents exposed to adults in high-risk categories.
Tuberculin Skin Testing
A century-old test, the tuberculin skin test (TST) has evolved since the time of its introduction to clinical practice, but with the ever-changing epidemiology, clinical features, and practice, the interpretation of the TST remains controversial. When interpreting the TST, it must be considered whether the patient belongs to a high-risk population such as HIV-infected intravenous drug users, aging population, immunocompromised hosts, or patients taking immunomodulation therapy for inflammatory bowel diseases or rheumatologic afflictions.
The material, manufacturer, and technique of administration are all factors that can affect the result of a TST. Currently, the only accepted material for use is purified protein derivative (commonly called PPD) (Landi and Gupta, 1975). Manufacturers must test each batch of PPD against the original batch lot of PPD-S to ensure standardization. Although different strengths of the testing material are available, the WHO strongly recommends that testing be done using 5 tuberculin units (5TU) of PPD-S or its equivalent (Guld, 1954).
The administration methods vary. The most common ones are the Mantoux method of intradermal injection, or the multi-puncture techniques such as the Heaf or Tine tests.
The Heaf test was performed mainly in the United Kingdom but has recently been removed from the market. The Tine test uses a small device that has four to six short needles (tines) coated with tuberculin antigens. The tines are pressed into the skin, usually on the inner side of the forearm, forcing the antigens into skin. The test is read by measuring the size of the largest papule. A negative result is the presence of no papules. Because it is not possible to precisely control the amount of tuberculin used in the tine test, a positive test should be verified by using the Mantoux test in order to interpret its significance. In addition, sensitivity is about 15% lower than with the Mantoux method. For this reason, the Tine test is considered to be less reliable than the Mantoux test and is therefore not recommended.
The Mantoux method was found to have better reproducibility (Furcolow et al., 1967) and a lower rate of false negatives when compared to the two previously mentioned multipuncture devices. Based on currently available evidence, it can be strongly recommended that for all tuberculin skin testing a dose bioequivalent to 5TU of PPD-S (or 2TU of RT-23, an equivalent tuberculin testing material more widely used in Europe (Comstock et al., 1964)) be administered via the Mantoux technique of intradermal injection with a 26-gauge needle. The inner aspect of the forearm is commonly used as it is the most convenient site, but the site of injection is not important, as long as it is free of hair and scar.
Since all the data regarding risk of TB according to clinical situation and size of tuberculin reaction are based on TST measured 48–72 h after testing, readings should take place 48–72 h following administration. Induration can be defined by palpation or by the ballpoint method. We recommend the ballpoint method, which consists of running the ball of a pen at a 45 angle toward the site of injection; the tip should stop at the edge of the induration. The induration, not redness, should be measured and recorded in millimeters (Table 2).
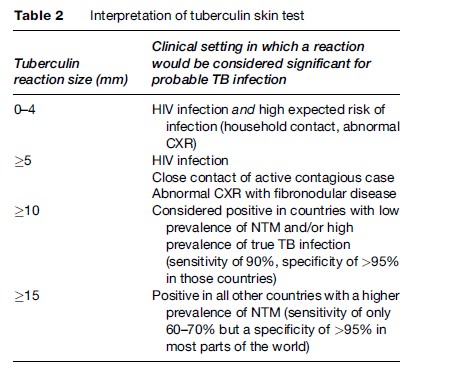
The interpretation of the TST does not consist only of measuring the degree of the reaction but takes into account the predictive values of the test based on the possible causes of false positives and false negatives (Table 3).
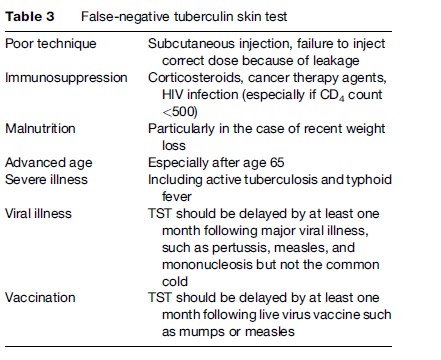
Two important factors can result in a false-positive TST and need to be considered when interpreting the result. The first are non-tuberculous mycobacteria (NTM), also called environmental mycobacteria, which exist in soil and water depending upon the climate and geography. There is antigen cross-reactivity between NTM and M. tuberculosis. The importance of false-positive TST in the setting of exposure to NTM depends upon the prevalence of infection with M. tuberculosis and NTM, as determined by the climate and geography. The second factor is Bacille Calmette-Guerin (BCG) vaccination, which results almost invariably in tuberculin conversion within 4–8 weeks, but this reaction often wanes over time. The timing of the vaccination is also an important consideration (Wang et al., 2002). There is a higher likelihood of a positive skin test within 10 years of the BCG vaccination. For those vaccinated in infancy (less than 1 year), fewer than 1% will have a positive TST related to the BCG vaccine after the age of 10, although more than 20% of persons vaccinated at an older age will remain TST positive after 10 or more years (Wang et al., 2002).
Chest X-Ray
Chest X-ray is often said not to be an effective tool for a diagnosis of latent tuberculosis infection because approximately 90% will have a normal chest X-ray (Table 4). The prevalence of chest radiograph findings consistent with old or healed tuberculosis in the general population is 11% (Barnes et al., 1988).
IFN-G Release Assays
These newly developed ex vivo assays are based on the principle that T-cells previously sensitized to tuberculosis antigens produce high levels of interferon-g (IFN-g) when reexposed to the same antigens. Two IFN-g release assays (IGRA) are currently available commercially. Although approved in many countries, including the USA, Canada, and some European countries, their higher costs, the uncertainty regarding future risk of active TB, and concerns about reproducibility make it difficult to recommend their use for the diagnosis of latent TB infection at the present time (Fietta et al., 2003). These assays may now be considered alternatives to TST in certain settings, such as in previously BCG-vaccinated patients.
Thus, prospective studies are needed to determine whether IFN-g responses are reproducible and predictive of those who have a high risk of progression to active disease. Furthermore, the utility of this test in subgroups of patients such as children and HIV-infected persons needs to be clarified.
Treatment Of Latent Tuberculosis Infection
Isoniazid (INH) remains the drug of choice for treatment of latent TB infection. Its effectiveness has been reported to range from 25 to 92% in patients compliant with the medication; the protective efficacy is approximately 90%.
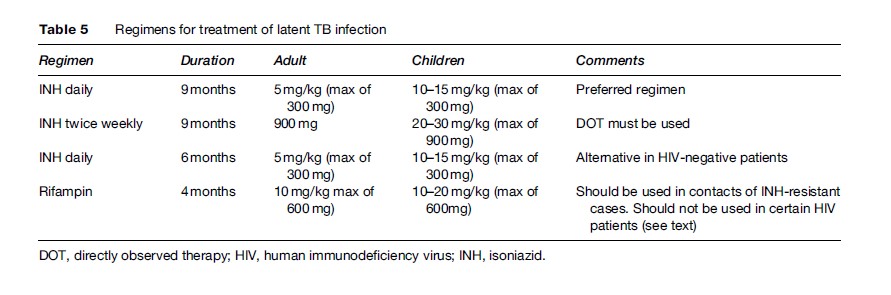
The preferred duration of treatment for latent TB (Table 5) is based on secondary analysis of large trials which suggest that the maximal benefit is achieved by nine months of therapy (Comstock, 1999). Six months of INH is an acceptable alternative in HIV-seronegative adults. The dose recommended is 10–15 mg/kg in children up to a maximal dose of 300 mg/day. In adults, the dose is 5 mg/kg up to 300 mg daily. Intermittent regimens of INH 900 mg twice weekly can be given to children or adults, but directly observed therapy must be used whenever intermittent therapy is given.
The most important adverse effect of INH is hepatitis. The estimated rate of symptomatic INH-related hepatitis ranges from 1 to 3 per 1000 persons depending on age and up to 5% in people above the age of 65 (Nolan and Buskin, 1999). However, asymptomatic liver enzyme abnormalities are relatively common (Byrd et al., 1979). All persons taking INH should be educated about the symptoms of hepatitis and about avoidance of alcohol, an important co-factor in the development of liver injury with INH treatment. INH should be discontinued if a symptomatic patient’s serum transaminase levels are more than three times the upper limit of normal or five times the upper limit of normal in the absence of symptoms. Neuropathy is also a common adverse effect in populations predisposed to its development, such as patients with diabetes, uremia, poor nutrition, alcoholism, pregnancy, or in HIV-infected persons. In patients with these conditions, 25 to 50 mg/day of pyridoxine is added.
A major limitation of nine months INH treatment for latent TB infection is poor adherence and low completion rates. For this reason shorter durations of therapy are recommended as alternatives. Six months INH provides 70% reduction of risk if taken well, which is significantly less than the 90% reduction of risk with 9 months INH, but some would consider this acceptable. Two months rifampin-pyrazinamide was recommended in 2000 (Gordin et al., 2000), but this recommendation was withdrawn after more widespread use led to unacceptably high rates of hepatotoxicity, which was fatal in some instances. The only remaining alternative is four months of rifampin, although it is not as extensively studied. Four months of therapy with rifampin is currently recommended for patients who are contacts of cases with INH-resistant tuberculosis or in individuals who cannot tolerate INH. Rifampin should not be used in HIV-infected patients who are taking protease inhibitors or certain nonnucleoside reverse transcriptase inhibitors. Rifampin is prescribed at 10 mg/kg/day, up to a maximum of 600 mg/day for adults and children.
Vaccination
Bacille Calmette-Guerin vaccination is a live, attenuated vaccine derived from M. bovis. It is one of the most widely used yet controversial modern vaccines. BCG is routinely given at birth in the majority of countries with an intermediate or high incidence of TB. Approximately 100 million children receive BCG annually throughout the world. Several countries follow their own special policies, either by targeting selected high-risk groups or by giving BCG in infancy with booster or repeat doses at school entry and/or to individuals who either lack a scar or are still considered to be tuberculin ‘negative’ by some criterion. A recently published randomized clinical trial of BCG revaccination of more than 250 000 school children in Brazil showed no additional protection from revaccination (Rodrigues et al., 2005). The WHO has recently issued a statement discouraging the use of BCG booster doses.
The protection imparted by the BCG appears to be more against systemic forms of TB, in particular childhood tuberculous meningitis and military disease, than against pulmonary forms, although the vast majority of studies have shown some degree of protection against pulmonary tuberculosis. This fact, along with consistent protection against severe systemic forms of disease, has supported the use of vaccination in high-prevalence countries (Fine, 2000). This practice is supported by 14 prospective trials and 12 case-control studies on BCG efficacy, which indicated that vaccination reduces the risk of tuberculosis by an average of 50% as documented in a meta-analysis published in 2000 which reviewed all those studies (Brewer, 2000). In that paper, seven trials that used random allocation yielded a relative risk for tuberculosis of 0.37 among those vaccinated, which is equivalent to a protective effect of 63%. In seven trials that studied death from tuberculosis, the combined relative risk for death due to TB among those vaccinated was 0.29 (95% CI 0.16, 0.53), which showed that vaccination had a 71% protective effect against death due to TB.
There is a trend toward discontinuation of routine BCG in Europe. In 1976 Sweden discontinued routine BCG in infancy. This was followed by an increase in tuberculous meningitis and lymphadenopathy among children, which provided weak evidence of the efficacy of the vaccine (Brewer, 2000). In the fall of 2005, the United Kingdom announced that routine BCG would be stopped. Instead BCG vaccination would be targeted to infants in communities with an average incidence of tuberculosis of at least 40 per 100 000 and to unvaccinated individuals who come from, or whose parents or grandparents come from, countries where the incidence exceeds 40 per 100 000. This means that TST will become increasingly efficient as a means of identifying people exposed to and latently infected with TB.
Prevention At The Community Level
Contact Tracing, Outbreak Management, And Public Health Intervention
The most important means to prevent TB is to prevent transmission (primary prevention). This is best achieved by prompt diagnosis and effective treatment of individuals with active contagious forms of TB. The next most important method is the identification and investigation of contacts – namely individuals who were exposed to cases recently diagnosed with active TB disease. It has been estimated that in the United States approximately nine contacts are identified for each case of active TB. Of these 21 to 40% are infected and another 1 to 3% have already progressed through active disease (Dasgupta et al., 2000). Because of the high prevalence of infection and active disease as well as the increased risk of progression of infection to disease, infected contacts are high-priority candidates for prophylactic therapy. The examination of TB contacts is therefore one of the most important methods of case finding.
Contact tracing may also identify a TB outbreak when more newly infected persons or more TB cases are discovered during the investigation than were anticipated based on previous epidemiological data.
In resource-poor, high-incidence countries the first priority in TB prevention is to assure effective therapy for all cases diagnosed through passive case finding. In these countries the role of contact investigation is considered secondary to the treatment of active cases, due to concerns over the yield, feasibility, and cost effectiveness of this approach. One study demonstrated skepticism with regard to the usefulness of contact examinations in eight different territories in sub-Saharan Africa, since the yield of active contact tracing was not higher than the rate in the general population; another study conducted in Kenya showed that the investigation of household contacts yielded an additional 15% of sputum smear-positive cases and high tuberculin reactor rates compared to the general population (Andersen and Gesser, 1960; Devadatta et al., 1970).
Prevention Of Emergence Of Drug Resistance, Including Multi-Drug-Resistant Tuberculosis
Soon after streptomycin was introduced in the 1940s, it was recognized that patients who had initially responded to monotherapy with streptomycin later relapsed with disease that was resistant to the new agent (Chiba et al., 1964). Subsequent work demonstrated that spontaneous mutation by the tubercle bacillus accounted for the emergence of resistance and could be largely prevented by simultaneous treatment with two or three effective antibiotics. As a result modern therapy consists of a minimum of three effective anti-TB drugs initially and at least two effective drugs at all times. An additional factor that leads to emergence of drug resistance is when patients prematurely discontinue therapy, leading to relapse with drugresistant organisms. In a given individual with active tuberculosis, drug resistance can emerge either because of physician error – prescribing inadequate treatment regimens – or because of poor compliance. Patients who develop drug resistance may transmit these organisms to other patients who then present with drug-resistant active TB without having ever been treated. This is called initial or primary drug resistance and is considered an indicator of regional or national TB control programs. To prevent the development of drug resistance, compliance must be improved by using directly observed therapy, and a single drug must never be added to a failing regimen (Ormerod, 2005). Another important method is the use of fixed dose combinations (FDC) of first-line anti-TB drugs – at minimum this prevents mono-therapy.
Multi-drug-resistant tuberculosis (MDRTB) is defined as resistance to at least INH and rifampin, the two most powerful anti-TB agents and the basis of short-course chemotherapy regimens used in DOT programs. Patients with disease due to MDR strains are more difficult to treat, remain infectious for longer, pose a public health hazard, and are more likely to die. Spread of MDRTB within institutions such as prisons, homeless shelters, and hospitals has been well documented and is a particular concern when many highly vulnerable individuals, for example those infected with HIV, congregate.
In 2004, the World Health Organization published its third report on surveillance of anti-tuberculosis drug resistance. The prevalence of resistance to at least one anti-TB drug ranged from 0% in some high-income countries to 56.4% in Kazakhstan. Median prevalence of resistance to specific drugs in new cases was as follows: SM 6.3%, INH 5.9%, rifampin 1.4%, and ethambutol 0.8%. Prevalence of MDRTB ranged from 0% in eight countries to 14.2% in Kazakhstan and Israel. Other locations of high prevalence of drug resistance were Tomsk Oblast of the Russian Federation (13.7%), Karakalpakstan (Uzbekistan) 13.2%, Estonia 12.2%, Liaoning Province of China 10.4%, Lithuania 9.4%, Latvia 9.3%, Henan Province of China 7.8%, Ecuador 6.6%, and South Africa 2.4%.
Rapid and early diagnosis of MDRTB is thought to improve survival and is of public health benefit. Early identification of these patients would permit rapid isolation and initiation of appropriate treatment with second-line therapy, thus avoiding acquisition of additional resistance, until results of further drug susceptibility testing are available.
Extensively drug-resistant tuberculosis (XDRTB) is a newly recognized type of multi-drug-resistant tuberculosis (MDRTB), which is relatively rare but of great concern. It is resistant to almost all drugs used to treat TB, including isoniazid, rifampin, fluoroquinolones, and at least one of the three injectable drugs (amikacin, kanamycin, or capreomycin). Because of this resistance to the most powerful first-line and second-line drugs, patients are left with treatment options that are much less effective. This often results in worse treatment outcomes. XDRTB is of special concern for persons with HIV infection or other conditions that can weaken the immune system.
Conclusion
Two million people die every year of tuberculosis, and a new infection develops every second. Prevention is the key to controlling this disease. The major challenges are early diagnosis and ensuring treatment compliance. Ensuring treatment completion will ensure cure of individuals while minimizing the risk of emerging multi-drug-resistant strains, all-important issues in the worldwide control of tuberculosis infection.
Bibliography:
- Andersen S and Gesser A (1960) The distribution of tuberculosis infection among households in African communities. Bulletin of the World Health Organization 22: 39–60.
- Barnes PF, Verdegem TD, Vachon LA, Leedom JM, and Overturf GD (1988) Chest roentgenogram in pulmonary tuberculosis. New data on an old test. Chest 94: 316–320.
- Brewer TF (2000) Preventing tuberculosis with Bacillus Calmette-Guerin vaccine: A meta-analysis of the literature. Clinical Infectious Diseases 31: S64–S67.
- Byrd RB, Horn BR, Solomon DA, and Griggs GA (1979) Toxic effects of isoniazid in tuberculosis chemoprophylaxis: Role of biochemical monitoring in 1000 patients. Journal of the American Medical Association 241: 1239–1241.
- Chiba YTT, Kondo K, and Nagashima A (1964) Chemoprophylaxis of tuberculosis for adults in Japan. Bulletin of the International Union against Tuberculosis 35: 91–93.
- Comstock GW (1999) How much isoniazid is needed for prevention of tuberculosis among immunicompetent adults? International Journal of Tuberculosis and Lung Disease 3: 847–850.
- Comstock GW, Edwards LB, Philip RN, and Winn WA (1964) A comparison in the United States of America of two tuberculins, PPD-S and PPD-RT 23. Bulletin of the World Health Organization 31: 161–170.
- Dasgupta K, Schwartzman K, Marchand R, Tennenbaum TN, Brassard P, and Menzies D (2000) Comparison of cost-effectiveness of tuberculosis screening of close contacts and foreign-born populations. American Journal of Respiratory and Critical Care Medicine 162: 2079–2086.
- Devadatta S, Dawson JJ, Fox W, et al. (1970) Attack rate of tuberculosis in a 5-year period among close family contact of tuberculosis patients under domiciliary treatment with isoniazid plus PAS or isoniazid alone. Bulletin of the World Health Organization 42: 337–351.
- Fietta A, Meloni F, Cascina A, et al. (2003) Comparison of a whole-blood interferon-gamma assay and tuberculin skin testing in patients with active tuberculosis and individuals at high or low risk of Mycobacterium tuberculosis infection. American Journal of Infection Control 31: 347–353.
- Fine P (2000) BCC vaccines and vaccination. In: Reichman LB and Herschman EM (eds.) Tuberculosis: A Comprehensive International Approach vol. 144, pp. 503–524. (Lung Biology in Health and Disease). New York: Marcel Dekker.
- Furcolow ML, Watson KA, Charron T, and Lowe J (1967) A comparison of the Tine and Mono-vacc tests with the intradermal tuberculin test. American Review of Respiratory Disease 96: 1009–1027.
- Gordin F, Chaisson RE, Matts JP, et al. (2000) Rifampin and pyrazinamide vs isoniazid for prevention of tuberculosis in HIV-infected persons: an international randomized trial. Journal of the American Medical Association 283: 1445–1450.
- Guld J (1954) Quantitative aspects of the intradermal tuberculin test in humans. Acta Tuberculosis Scandinavica 30: 16–36.
- Landi S, Held HR, and Gupta KC (1975) The multi-facets of tuberculin standardization. Developments in Biological Standardization 29: 393–411.
- Nolan CM, Goldberg SV, and Buskin SE (1999) Hepatotoxicity associated with isoniazid preventive therapy: A 7-year survey form a public health tuberculosis clinic. Journal of the American Medical Association 281: 1014–1018.
- Ormerod L (2005) Multidrug-resistant tuberculosis (MDR-TB): Epidemiology, prevention and treatment. British Medical Bulletin 73–74: 17–24.
- Rodrigues LC, Pereira SM, Cunha SS, et al. (2005) Effect of BCG revaccination on incidence of tuberculosis in school-aged children in Brazil: The BCG-REVAC cluster-randomised trial. Lancet 366: 1290–1295.
- Wang L, Turner MO, Elwood RK, Schulzer M, and FitzGerald JM (2002) A meta-analysis of the effect of Bacille Calmette Guerin vaccination on tuberculin skin test measurements. Thorax 57: 804–809.
- Pai M, Riley LW, and Colford JM Jr. (2004) Interferon-gamma assays in the immunodiagnosis of tuberculosis: a systematic review. Lancet Infectious Disease 4(12): 761–776.
- Streeton JA, Desem N, and Jones SL (1998) Sensitivity and specificity of a gamma interferon blood test for tuberculosis infection. International Journal of Tuberculosis and Lung Disease 2(6): 443–450.
- WHO (2007) International, Standards for Tuberculosis, Care (TBCTA), and the Patients, Charter for Tuberculosis, Care (World Care, Council). Available at: http://www.who.int/tb/publications/en/ (accessed August 2007).
- WHO (2006) WHO Report 2006: Global Tuberculosis Control: Surveillance, Planning, Financing. Available at: http://www.who.int/ tb/publications/en/ (accessed August 2007).
- WHO (2007) TheStop, TB Strategy. Available at: http://www.who.int/tb/features_archive/stop_tb_strategy/en/index.html (accessed August 2007).
- WHO (2007) Tuberculosis, Air Travel: Guidelines for Prevention and Control, 2nd edn. Available at: http://www.who.int/tb/publications/ en/ (accessed August 2007.
- WHO (2007) Guidelines for the Programmatic Management of Drug- resistant Tuberculosis. Available at: http://www.who.int/tb/publications/en/ (accessed August 2007).
- WHO (2006) The Global Plan to Stop TB 2006–2015: Actions for Life Towards a World Free of Tuberculosis. Available at: http://www.who. int/tb/strategy/en/ (accessed August 2007).
- http://www.thoracic.org – American Thoracic Society.
- http://www.respdiv.mcgill.ca – Respiratory Division, McGill University.
See also:
Free research papers are not written to satisfy your specific instructions. You can use our professional writing services to buy a custom research paper on any topic and get your high quality paper at affordable price.







