This sample Venous Thromboembolism (VTE) Research Paper is published for educational and informational purposes only. If you need help writing your assignment, please use our research paper writing service and buy a paper on any topic at affordable price. Also check our tips on how to write a research paper, see the lists of health research paper topics, and browse research paper examples.
Venous Thromboembolism: Overview
Deep venous thrombosis (DVT) and its most important sequel, pulmonary embolus (PE), are collectively referred to as venous thromboembolism (VTE). VTE is the third most common cause of cardiovascular death after myocardial infarction and stroke, and it is thought to be the most preventable cause of hospital-acquired mortality.
VTE is initiated by the formation of a thrombus (blood clot) in a larger vein, usually in the lower extremities, but occasionally in the upper extremities or in unusual venous beds such as the mesenteric circulation in the abdomen or venous sinuses within the cranium. Although more commonly unilateral, it may present with bilateral limb involvement. The potentially life-threatening embolic complications occur when a part of or the whole clot dislodges, travels proximally via the inferior vena cava and heart to obstruct blood flow in the main pulmonary artery or one of its branches connecting the right side of the heart to the lungs. Because the patient presenting with the symptoms and signs of PE may be asymptomatic for a DVT (and vice versa), the two disorders are generally considered to be part of the same disease continuum, collectively known as VTE. Apart from the short-term risk of death from PE, VTE is a significant cause of morbidity that can result in prolonged hospitalization, and delayed disability due to post-thrombotic syndrome (in up to 20% of patients) and/or pulmonary hypertension (in 2–3% of patients).
The treatment of VTE with long-term anticoagulants or ‘blood thinners’ carries with it a significant risk of hemorrhage. Major bleeding complications occur in 2–3% of patients per annum on vitamin K antagonists (the most commonly used long-term therapy for VTE), and mortality – primarily due to intracranial bleeding – may annually account for the death of 1 in 200 to 1 in 400 elderly patients on these agents. Since the incidence rate for VTE in most populations has been estimated to be between 1 and 2 per 1000 person-years, with a 30-day case fatality rate of about 10%, this debilitating and potentially deadly disease is appropriately considered a major public health problem.
Pathophysiology
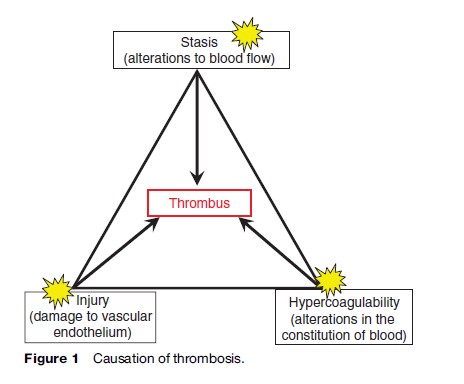
In 1856, Rudolf Virchow, a German pathologist, proposed that changes in blood flow (stasis and/or turbulence), changes in the composition of the blood (now often referred to as thrombophilia), and changes in the vessel wall all contribute in some proportion to the causation of thrombosis (Figure 1). While this hypothesis remains a useful framework on which to construct an understanding of VTE, there remain many gaps in our knowledge regarding the etiology of venous thrombosis. For example, it is not known what stimuli are responsible for initiating VTE in most patients, especially when it does not occur in association with recent surgery or trauma. Unlike arterial thrombosis, in deep vein thrombosis the vessel wall beneath the thrombus – including its delicate lining cellular layer known as the endothelium – is usually morphologically intact. However, it is theorized, although not yet conclusively demonstrated, that the endothelium loses its potent repertoire of antithrombotic molecular mechanisms at that point in time, thereby failing to resist the prothrombotic influence of perturbed blood flow and/ or inherited or acquired thrombophilia(s).
Epidemiology
Incidence And Prevalence
Numerous studies have evaluated the epidemiology of VTE in the general population. As up to 70% of cases of VTE discovered at autopsy are asymptomatic antemortem, the estimated incidence may be influenced by autopsy rates and the diagnostic approach used. Notably, autopsy rates continue to progressively decline in the United States and other Western countries. Overall, however, the incidence of VTE is estimated to be in the range of 1–2 per 1000 per year. Specifically, White (2003) estimated the incidence of VTE to be approximately 1 in 1000 person-years. In published reports without and with corroborating autopsy data, Hansson et al. (1997) found the incidence of DVT and PE to be 182 and 205 cases per 100 000 person-years, respectively. Silverstein et al. (1998) also used correlative data to describe a reverse in the usual DVT to PE ratio, with 48 cases per 100 000 of DVT and 69 cases of PE per 100 000 person-years. In a study that did not include autopsy data, Anderson et al. (1991) found the population incidence of DVT (69 per 100 000 personyears) to be more common than PE (48 per 100 000 person-years), a relative frequency that is more reflective of the majority of current literature.
In the general population and in the subgroup of pregnant women, the overall incidence of PE has been reported to be decreasing over the past 30 years, even though the incidence of DVT has remained constant.
Age
It has been conclusively demonstrated that the risk of VTE increases exponentially with age. Silverstein et al. (1998) reported an incidence of 1 case per 10 000 per annum in individuals aged less than 40 years old, rising to approximately 5 cases per 1000 annually by age 80; these observations have been corroborated in numerous other studies. Tsai et al. (2002) reported that the risk of VTE is 13-fold greater in individuals aged 85 years old compared to those aged 50. It can therefore be anticipated that an increasingly aging population in Western societies will be associated with an increasing prevalence of VTE in the future.
Ethnicity
In the few studies that have evaluated the epidemiology of VTE in ethnic/racial groups in the United States, the incidence has been noted to be relatively low in the Hispanic and Asian-American communities, with an incidence risk ratio of 0.60 and 0.27 (respectively) compared to a reference Caucasian population; conversely, the incidence risk ratio of VTE in African-Americans is 1.27. The biological reasons for these discrepancies are unclear, and studies that have evaluated possible socioeconomic factors (such as access to care) have failed to provide a satisfactory explanation. Therefore, it seems likely that certain haplotypes predisposing to – or perhaps protecting from – VTE are more or less represented in the various ethnic/racial communities.
Sex
Most studies have shown that the incidence of VTE is similar in both genders, albeit with an increased risk in women of childbearing age (probably related to an increased risk associated with pregnancy and the use of hormonal therapies) offset by an increased risk in men over the age of 75.
Worldwide Differences In VTE Incidence
The incidence of VTE in Western countries is relatively comparable. One study from the United Kingdom demonstrated an incidence of VTE of 74.5 per 100 000 person-years. In a Norwegian, population-based study, the incidence was 1.43 per 1000 person-years. Both studies also revealed that the incidence rate increased with age, following surgery, and with underlying cancer. In Asian countries, fewer studies have been published. However, the majority of these have reported a decreased incidence of VTE compared to Western populations. For example, in Hong Kong, the incidence of VTE was found to be 16.6 per 100 000 person years. This estimate is in good agreement with the study by White, in which the reported incidence of VTE in Asian-Americans living in California was 19 per 100 000 person years.
Risk Factors
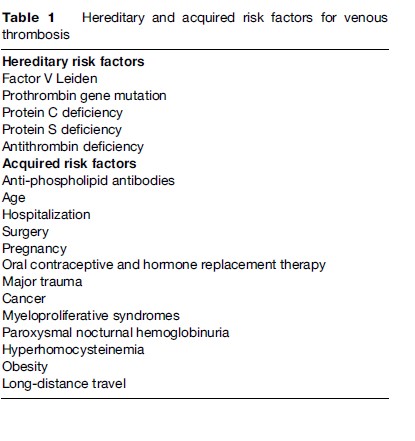
As predicted by Virchow, both genetic and environmental risk factors have been demonstrated to be associated with an increased risk of VTE. In general, it is the combination of multiple risk factors that appears to underlie most events, with more than one risk factor identified in 65% of VTE cases. A number of thrombophilias, which may be either hereditary or acquired (Table 1), have been described in the past four decades. As a rule, these thrombophilias, some of which are uncommon, are relatively strong risk factors. Although more recently described genetic variants are more prevalent, they are weak risk factors that only cause VTE when other factors are operative.
Hereditary
In general, the inherited forms of thrombophilia described below are associated with an increased risk of first VTE, and sometimes, recurrent VTE. These disorders are not associated with any significantly increased risk of arterial thrombosis in adults.
Activated Protein C (APC) Resistance
Activated protein C (APC) is a ‘natural’ anticoagulant molecule that is derived from its circulating precursor molecule protein C. APC is instrumental in the inactivation of coagulation factors Va and VIIIa, essential cofactor proteins in the formation of a thrombus. In 1993, Dahlback described a familial form of VTE in which the proband’s plasma was relatively resistant to the anticoagulant action of APC added to patient plasma ex vivo. Subsequent studies revealed that almost 90% of patients with inherited APC resistance had a point mutation of an allele of factor V (factor V R506Q , G1691A), now commonly referred to as factor V Leiden, after the Dutch city in which the mutation was discovered. This mutation is found in approximately 5% of the Caucasian population, and in about 10% of allcomers with VTE. In the spectrum of hereditary thrombophilias leading to a VTE, factor V Leiden accounts for approximately 50–60% of these cases.
The relative risk of first VTE in patients who are heterozygous for factor V Leiden is 4–7, compared to a relative risk of 50–80 for homozygotes. However, by age 65, only 6% of heterozygotes have developed VTE, with most of these occurring in the presence of coexisting risk factors (hospitalization, etc.).
Factor V Leiden is rare in individuals descended from ancestors originating in sub-Saharan Africa or the Far East, although when present, it confers the same risk of VTE.
Prothrombin Gene Mutation
This point mutation (G20210A) in the gene for coagulation factor II (prothrombin) was first discovered by Poort et al. (1996) by studying candidate genes in families with idiopathic VTE. This mutation, located in the regulatory domain of the prothrombin gene, results in a relatively modest increase in plasma levels of prothrombin, a circulating procoagulant that is converted to thrombin (factor IIa) during the activation of coagulation. The mutation is found in 2–3% of the Caucasian population in the United States, and in approximately 7% of those with VTE. The relative risk of first VTE in heterozygous carriers is increased by a factor of 2–3, and thus, as is the case with heterozygotes for factor V Leiden, VTE when it occurs is usually multicausal. Also in common with factor V Leiden, this mutation is rare in African-Americans, Native Americans, and those of Asian descent.
Antithrombin Deficiency
Antithrombin (formerly known as antithrombin III) is a naturally occurring regulatory protein that acts as an inhibitor of thrombin and other procoagulant enzymes including factors Xa, IXa, and XIa. The therapeutic efficacy of heparin as an anticoagulant is dependent on its ability to accelerate the inhibitory effects of antithrombin. A partial deficiency of this protein leads to a prothrombotic state, as now the balance is geared toward more thrombin generation, that is, more thrombus formation. In 1965, antithrombin deficiency was noted in a family with a history of VTE. Homozygous deficiency of antithrombin is probably incompatible with life. Heterozygous deficiency of antithrombin is a relatively uncommon thrombophilia, occurring in about 0.2% of the general population and 0.5% of younger patients with VTE.
Protein C And Protein S Deficiency
Protein S is an important cofactor for activated protein C (discussed earlier). The (partial) loss of either protein leads to a prothrombotic state. Thrombophilic families with protein C or protein S deficiency were first described in the 1980s. A large number of mutations have been documented in each deficiency state. Again, both conditions are relatively rare, occurring in <1% of the general population and approximately 2 to 5% of younger cases of VTE.
Acquired
In this section, a number of established risk factors, many of which can be considered acquired or environmental in origin, are reviewed.
Antiphospholipid Antibodies (APLs)
Antiphospholipid antibodies (APLs) are a group of autoantibodies that are directed against phospholipids (cell wall components) associated with certain coagulation proteins. Most relevant to thrombosis risk are lupus anticoagulants (or lupus inhibitors) and anticardiolipin antibodies. When either or both are found in association with arterial or venous thrombosis, or with recurrent pregnancy loss, the term antiphospholipid antibody syndrome is applied. This syndrome most commonly occurs in isolation, as primary antiphospholipid antibody syndrome, but it may also be encountered as a secondary manifestation of another autoimmune disorder, most notably systemic lupus erythematosus. APLs in the absence of a thrombotic history, and generally of lower titer, are relatively prevalent in the general population, having been described in 2–3% of healthy blood donors.
Obesity
Obesity, defined as a body mass index (BMI) > 30 kg/m2, is in general associated with an increased predilection to VTE. The Nurses’ Health Study (Goldhaber, 1997) reported an increased relative risk of PE of 3.2 in those with BMI >29 kg/m2 compared to the relative risk of 1.7 in the reference group (patients with a BMI of 25 kg/m2 or less). Similarly, Tsai et al. (2002) reported a relative risk of VTE of 1.5 in those with a BMI 25–30 kg/m2, and 2.01 for those >30 kg/m2.
Obesity as a contributing cause of VTE will undoubtedly continue to increasingly contribute to the incidence of VTE as it becomes more prevalent in the United States and other Western nations. One retrospective study concluded that the contribution of obesity to the incidence of VTE between 1990 and 2000 could have accounted for an excess of 38 000 new cases of VTE in the United States. However, prospective trials should be completed in the future to better estimate the increasing cost of this health epidemic.
Hospitalization And Surgery
It has been well established that the risk of developing VTE is increased during hospitalization. It has been estimated that >12 000 000 inpatients, constituting about 30% of hospital discharges annually in the United States, are potentially at risk of VTE, and that approximately 10% of hospital deaths are directly attributable to PE, with the majority occurring in nonsurgical inpatients.
Surgical patients are at risk for VTE, but the magnitude of the risk varies according to the type of surgery as well as comorbidities such as age, BMI, and underlying cancer. In the absence of prophylactic anticoagulation, the risk of thrombosis is reasonably well established, and consensus guidelines on the appropriate use of prophylaxis in surgical and nonsurgical patients have been published and are updated regularly (Buller, 2004; Geerts, 2004). In general, the risk of VTE is greatest in patients undergoing surgical procedures for cancer and in major orthopedic procedures, such as a total hip or knee replacement.
Cancer
Patients with cancer are known to have an increased risk of VTE. Certain cancers (of the brain, ovary, pancreas, and lymph system, as in lymphoma) have been correlated with a more marked propensity to VTE. Although many postulated mechanisms have been described, the molecular mechanism of cancer-induced thrombosis remains largely unknown. The prevalence of VTE in cancer patients is approximately 10–20%, and in patients presenting with idiopathic VTE, the prevalence of occult cancer may be in the range of 5–10%. The risk of VTE is further accentuated in patients treated with chemotherapy. Randomized controlled trials have demonstrated that anticoagulation therapy (especially using low-molecular-weight heparins) can prolong survival in patients with cancer – especially those with nonmetastatic disease at the time of presentation.
Pregnancy
The risk of VTE is increased in pregnant patients, and its importance is emphasized because PE is the most common cause of maternal mortality in the United States and much of Western Europe. The etiology of pregnancy-induced VTE includes a combination of hormonal and vascular changes, but also other risk factors, such as obesity, bed rest, age, and preeclampsia. James et al. (1996) determined that the prevalence of VTE in pregnant patients is approximately 1 in 1000 pregnancies. The risk of VTE is increased throughout pregnancy, but the risk of PE is highest during the last trimester and in the puerperium. The combination of pregnancy and congenital thrombophilia remains a controversial issue in regards to the need for prophylactic anticoagulation to prevent maternal VTE and/or pregnancy loss, presumably due in major part to placental thrombosis. To date, the only thrombophilic state in which randomized controlled trial level evidence supports the use of prophylactic anticoagulation to prevent pregnancy loss is antiphosphoplipid antibody syndrome. Anticoagulation with the use of unfractionated heparin and aspirin compared to aspirin alone demonstrated a significant improvement in the live birth rate of 70% with combined therapy, as opposed to 41% with aspirin alone.
Oral Contraceptive Therapy (OCT)
Many studies have focused on the risk of VTE during hormonal contraceptive usage. Several factors contribute to this risk, including the precise estrogen content, the type of component progestagen in combined estrogenprogestagen therapies, and other comorbidities. The reported risk ratio of VTE in women taking non-third-generation, combined, oral contraceptives ranges from 1.0 to 5.0, although the vast majority of studies demonstrate some significantly increased risk. Unfortunately, as the side effect profile of oral contraceptive therapy (OCT) has improved, so too has the risk of VTE. Thus, the risk of VTE appears to be a further two to threefold higher in patients taking OCT containing third-generation progestagens compared to those containing second-generation progestagens. The use of OCT is preferably avoided in patients with a known history of VTE and/or thrombophilia.
Hormone Replacement Therapy (HRT)
Estrogen-based hormone replacement therapy (HRT) is also associated with an increased risk of VTE, with a relative risk of 2–3 in several large-scale studies. Unlike the case with OCT therapy in premenopausal women, HRT administered transdermally (using slow-release skin patches) does not appear to be associated with the same increased risk of VTE that may be seen with orally administered preparations.
Homocysteine
Homocysteine is an amino acid that is derived from dietary methionine. Plasma levels are influenced by genetic factors as well as relative dietary deficiencies of folic acid, vitamin B6, and/or vitamin B12, which are cofactors in the metabolic recycling of homocysteine. Numerous case-control studies have demonstrated that homocysteine levels may be mildly elevated in numerous pathological conditions (peripheral vascular disease, diabetes, cardiovascular disease, etc.) in addition to VTE. However, the clinical utility of screening for hyperhomocysteinemia is unclear. In several prospective studies, patients that had a documented reduction in their homocysteine levels by dietary vitamin supplementation failed to experience any reduction in recurrent VTE. In addition, the molecular mechanism(s) by which homocysteine might exert its prothrombotic effects remain(s) unknown.
Long-Distance Air Travel
The possible association between prolonged (>6 hours) air travel and VTE has attracted considerable media attention in recent years. Lapostolle et al. (2001) found that the incidence of fatal PE is approximately 1.5 per 1 million travelers. Perez-Rodriguez et al. (2003) reported that traveling for 4 or more hours approximately doubles the risk of VTE for several weeks following the trip. In contrast, several other studies failed to demonstrate any increased risk of thrombosis with air travel. Reconciling these discrepancies with other available literature, the most likely interpretation is that a subset of higher-risk individuals (for example, those with thrombophilia, and/ or taking OCT) is uniquely susceptible to the coagulation-activating effects of the hypoxic hypobaric conditions during airline travel. In the general population, however, the risk is considered to be very low to negligible.
Interaction Of Risk Factors
The interaction of these genetic and environmental risk factors may be either additive or multiplicative. For example, among young women on OCT, the risk of VTE is increased about fourfold compared to women not using OCT. Similarly, women who are heterozygous for the factor V Leiden mutation are estimated to have about a sevenfold increased risk of VTE. The interaction between these two risk factors appears to be multiplicative rather than additive; thus, women who are heterozygous for factor V Leiden and are on OCT have a risk of VTE that is increased by approximately 30to 35-fold.
Clinical Presentation
The clinical presentations of DVT may include leg pain, tenderness, swelling, prominence of superficial veins, and skin discoloration. Patients presenting with PE may complain of chest pain (typically worse on inspiration, and due to infarction of a portion of the lung), shortness of breath, hemoptysis, and tachycardia. As already mentioned, PE may also present as a sudden loss of consciousness or sudden death due to acute hypoxia. In general, the symptoms and signs of both DVT and PE are nonspecific, so that a high index of suspicion, and initiation of the appropriate laboratory and radiological investigation is required to make the diagnosis. Various validated clinical scoring systems (such as the Wells score), which take into account the patient’s profile and risk factors, have been devised to increase the pretest probability of a diagnosis of VTE. However, it has been demonstrated that the diagnosis is not confirmed by more objective criteria in 50–80% of patients with a clinical suspicion of DVT.
Diagnosis
Laboratory
Currently, the most common laboratory assay in use to exclude the diagnosis of VTE is the measurement of plasma D-dimer. D-dimer is the product of the breakdown of intravascular cross-linked fibrin (the insoluble protein product of coagulation that stabilizes a thrombus). Most centers use an enzyme-linked immunosorbent assay (ELISA) or a variant thereof for the measurement of D-dimer levels. The challenge with this test lies in its interpretation. An elevated level is nonspecific, as many conditions (sepsis, trauma, etc.) can also lead to activation of coagulation and therefore a positive D-dimer. However, a D-dimer within a previously validated normal reference range for a given institution/instrument combination has a negative predictive value of 95% or greater for VTE.
Imaging
Compression Ultrasound
Compression ultrasound is a quick, noninvasive method of evaluating for DVT. In essence, deep or superficial veins are compressed using the ultrasound probe at specific sites in the affected extremity. The inability to compress these veins is indirect evidence of occlusion by thrombus, with a specificity of >95%. In a symptomatic individual, the sensitivity of the method for the diagnosis of proximal DVT (popliteal vein or above) is 95% or greater, and, in combination with a negative D-dimer, its sensitivity in ruling out DVT approaches 100%. However, the sensitivity to distal DVT is not as high. Although distal thromboses are unlikely to embolize, a fraction of them may extend proximally and place the patient at risk for PE. One validated clinical strategy that has been recommended to rule out this possibility is repeating the ultrasound in 5 to 7 days. Compression ultrasound may remain abnormal for 1 year in 50% of patients after the original thrombus due to fibrotic organization of the thrombus.
Radionuclide Lung Scan (V/Q Scan)
A radionuclide lung scan (V/Q scan) is used to evaluate for a lack of vascular perfusion (Q scan) in a ventilated portion (V scan) of the lung(s) during evaluation for PE. An interpretation of a ‘normal’ V/Q scan is seen in only 10% of patients with suspected PE. While the interpretation of a ‘high probability’ mismatched defect has a positive predictive value of about 85%, it is only found in 10% of symptomatic patients. The remaining interpretations of intermediate and low probability for PE are seen in 70% of patients but in themselves do not exclude or establish the diagnosis of PE. For these reasons, the V/Q scan, although of great historical significance, is being used with increasingly lower frequency in clinical practice.
Spiral Computed Tomography (Spiral CT)
The spiral computed tomography (spiral CT) test has become the most commonly employed method of evaluating for pulmonary embolus in the United States. The test uses CT angiography to evaluate the pulmonary vascular system. In the presence of vascular obstruction, the thrombus is visualized by a ‘filling defect’ (absence of intravenously administered contrast). This finding is indicative of approximately a 90% probability of pulmonary embolus.
Pulmonary Angiography
Angiography is still considered the gold standard in the diagnosis of PE. The procedure entails insertion of a catheter into the pulmonary artery through which radiocontrast media is administered to visualize vessel patency. While pulmonary angiography has a high positive and negative predictive value in screening for PE, the technical difficulty and invasiveness in performing this procedure limits its value to those cases in which other approaches have proven to be inconclusive.
Treatment
The mainstay of treatment for VTE is systemic anticoagulation therapy. Anticoagulant agents do not directly induce clot breakdown or removal, but they do inhibit thrombus extension and embolization. While the intensity of anticoagulation is reasonably standard regardless of the presentation, the duration of treatment will depend on several factors. Foremost among these is the mode of presentation. Patients developing VTE after a clear inciting event (e.g., surgery or trauma) are at lower risk of recurrence than those presenting with idiopathic (i.e., apparently unprovoked) VTE, and therefore require a shorter duration of therapy (usually limited to 3 months). Other factors that may influence the duration of therapy include patient comorbidities, risk of bleeding on anticoagulation, (some forms of ) thrombophilia, prior history of VTE, age, pregnancy, patient preference, and the type of VTE (DVT vs. PE).
Heparin And Low-Molecular-Weight Heparins (LMWH)
As previously stated, heparin and low-molecular-weight heparins (LMWH) work by accelerating antithrombinmediated inactivation of coagulation proteases. Both forms can be given intravenously or subcutaneously. LMWH has the added benefit of providing a longer duration of therapy and does not require routine monitoring under usual circumstances. Both forms of heparin have the advantages that their onset of action is immediate, and they are relatively rapidly reversible. They are therefore used as the mainstay of early treatment in the management of VTE. In a meta-analysis performed by Quinlan et al. (2004), after reviewing at least 12 randomized controlled trials, the major risk of bleeding was 1.4% among patients receiving LMWH compared to 2.3% among patients receiving unfractionated heparin (odds ratio, 0.67 [confidence interval, 0.36 to 1.27]).
Oral Vitamin K Antagonists
Longer-term oral anticoagulation can be achieved using one of the vitamin K antagonists; in the United States, warfarin is the most commonly used agent. It is estimated that almost 1% of the population is being treated with warfarin, for various indications. By inhibiting the vitamin K epoxide reductase enzyme, warfarin prevents the essential recycling of vitamin K that is required to process the final steps in the synthesis of several coagulation factors in the liver. The cumulative effect of reduced circulating levels of coagulation factors II, VII, IX, and X partially inhibits the coagulation system to prevent extension or recurrence of thrombosis. The major risk of any form of anticoagulation therapy is bleeding, estimated to be 2% during the first 3 months of therapy, and 1–3% per annum thereafter, with the risks being particularly high among the elderly. The case-fatality rate from bleeding is approximately 13%. Therefore, the risk/ benefit ratio of anticoagulation therapy needs to be carefully considered, especially in patients at higher risk of recurrence, where long-term anticoagulation therapy is being considered.
Inferior Vena Cava (IVC) Filter
An inferior vena cava (IVC) filter is a mechanical device that allows blood and soluble elements to pass, but obstructs the passage of thrombi that may result in symptomatic PE. IVC filters are placed in situations when necessary anticoagulation cannot be continued (e.g., due to serious bleeding complications), or when recurrent PE is documented despite seemingly adequate intensity anticoagulation. However, a high incidence of recurrent DVT distal to the filter has been recognized in patients with permanent IVC filters, itself necessitating longer-term anticoagulation therapy. More recently, several types of temporary (removable) IVC filters have been introduced. These devices may provide the necessary short-term alternative to anticoagulation without themselves precipitating late distal DVTs.
VTE Recurrence
The cumulative risk of recurrent VTE in patients with idiopathic VTE is approximately 5–7% at 6–12 months after the initial event, and approximately 20–30% after 5 years. These rates are substantially higher than in patients presenting with an event after an inciting episode. As already discussed, the assessment of the duration of anticoagulation needs to take into account all the factors that contributed to the event. The D-dimer assay has been used to evaluate for the risk of recurrence. Specifically, when measured in patients after 3 months of anticoagulation for DVT, an elevated level is predictive of a higher risk of recurrent DVT and may indicate a need to continue anticoagulation for a more prolonged period.
Prevention
Because the ability to diagnose VTE by clinical (nonradiologic) means is so poor, emphasis should be placed on the prevention of VTE in hospitalized patients. Evidence-based guidelines, updated and published every few years by the American College of Chest Physicians’ consensus conference participants, recommend the use of mechanical or pharmacological methods to prevent DVT in a large proportion (about one-third) of hospitalized patients. In most of these situations, the risk of low-intensity anticoagulation prophylaxis has to be balanced against the risk of bleeding due to (as well as cost of) anticoagulant medications. High-quality evidence has gradually accumulated showing that some types of surgery (e.g., total knee replacement) are at particularly high risk of postoperative VTE. In this, and in most other at-risk situations, prophylactic anticoagulation reduces the relative risk of VTE by about two-thirds.
Aside from short-term intervention using anticoagulation in these types of situations, preventive strategies should also be linked to controlling comorbid illnesses – that is, better implementation of proven screening strategies for cancer, improved control of intercurrent illnesses such as diabetes to avoid hospitalization. Lastly, given the link between risk factors – such as obesity, immobility, and dietary habits – and VTE, the opportunity exists to reduce the burden of disease by appropriately implemented prevention strategies. For example, the frail elderly – who are at particularly high risk of VTE – may benefit from appropriately implemented physical therapy programs. Similarly, a diet enriched in fish, fruit, and vegetables has recently been demonstrated to reduce the risk of VTE. More work is required to understand the relationship between diet and dietary supplements in the etiology and/or prevention of VTE.
Mortality
VTE remains a significant cause of mortality in our society. Cushman et al. (2004) reported that the 28-day mortality for those presenting for DVT was 9%, while those presenting with PE was 15%. Similarly, White (2003) reported a 6-month fatality rate of 10% from DVT, compared to a rate of 14% from PE. The International Cooperative Registry (Goldhaber, 1999) for PE revealed that the 3-month overall mortality rate was approximately 15% for PE. Mortality rates are increased with age, and in the presence of cancer. These findings are fairly similar to the rates noted in Western Europe. Although mortality rates from PE have been reported to have decreased over the past 30 years, the declining autopsy rates may be part of the explanation.
Future Directions
Over the past decade, considerable effort has been invested in the development of novel anticoagulant therapies. Some agents have recently been approved by the regulatory authorities, although as yet, no orally available agent to replace warfarin has been successfully developed. However, this situation is likely to change within the next 5 years. One limitation of all newer therapies is the avail- ability of a specific antidote to be given in the event of urgent reversal due to bleeding; therefore, it is certainly not a fait accompli that warfarin will become obsolete.
A better understanding of the reasons underlying the ethnic/racial differences in the incidence of VTE would seem to be a research priority, as would the availability of improved clinical algorithms to predict the risk of recurrence in individual patients. Similarly, an improved understanding of the scientific basis underlying the association between VTE and increasing age and obesity is a high priority. Finally, because of the difficulties in the diagnosis of early VTE, especially in hospitalized patients, efforts will continue to focus on the prevention of VTE by the appropriate use of prophylactic dose anticoagulation in high-risk patient groups.
Bibliography:
- Anderson FA Jr., Wheeler HB, Goldberg RS, et al. (1991) A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study. Archives of Internal Medicine 151(5): 933–938.
- Buller HR, Agnelli G, Hull CD, et al. (2004) Antithrombotic therapy for venous thromboembolic disease: The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 126(supplement 3): S401–S428.
- Cushman M, Tsai AW, White RH, et al. (2004) Deep vein thrombosis and pulmonary embolism in two cohorts: the longitudinal investigation of thromboembolism etiology. American Journal of Medicine 117(1): 19–25.
- Geerts WH, Pineo GF, Heit JA, et al. (2004) Prevention of venous thromboembolism. The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 126(supplement 3): S338–S400.
- Goldhaber SZ, Grodstein F, Stanpfer MJ, et al. (1997) A prospective study of risk factors for pulmonary embolism in women. Journal of the American Medical Society 277(8): 642–645.
- Goldhaber SZ, Visani L, De Rosa M, et al. (1999) Acute pulmonary embolism: Clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). The Lancet 353(9162): 1386–1389.
- Hansson PO, Welin L, Tibblin G, et al. (1997) Deep vein thrombosis and pulmonary embolism in the general population. ‘‘The Study of Men Born in 1913.’’ Archives of Internal Medicine 157(15): 1665–1670.
- James KV, Lohr JM, Deshmukh RM, et al. (1996) Venous thrombotic complications of pregnancy. Cardiovascular Surgery 4(6): 777–782.
- Lapostolle F, Surget V, Borron SW, et al. (2001) Severe pulmonary embolism associated with air travel. New England Journal of Medicine 345(11): 779–783.
- Perez-Rodriguez E, Jimenez D, Diaz G, et al. (2003) Incidence of air travel-related pulmonary embolism at the Madrid-Barajas airport. Archives of Internal Medicine 163(22): 2766–2770.
- Poort SR, Rosendaal FR, Reitsma PH, et al. (1996) A common genetic variation in the 30 -untranslated region of the prothrombin gene is associated with elevated plasma prothrombin levels and an increase in venous thrombosis. Blood 88(10): 3698–3703.
- Quinlan DJ, McQuillan A, Eikelbcom JW, et al. (2004) Low-molecular-weight heparin compared with intravenous unfractionated heparin for treatment of pulmonary embolism: A meta-analysis of randomized, controlled trials. Annals of Internal Medicine 140(3): 175–183.
- Silverstein MD, Heit JA, Mohr DN, et al. (1998) Trends in the incidence of deep vein thrombosis and pulmonary embolism: A 25-year population-based study. Archives of Internal Medicine 158(6): 585–593.
- Tsai AW, Cushman M, Rosamond WD, et al. (2002) Cardiovascular risk factors and venous thromboembolism incidence: The longitudinal investigation of thromboembolism etiology. Archives of Internal Medicine 162(10): 1182–1189.
- White RH (2003) The epidemiology of vein thromboembolism. Circulation 107(supplement 23): 14–18.
- Crowther MA and Kelton JG (2003) Congenital thrombophilic states associated with venous thrombosis: A qualitative overview and proposed classification system. Annals of Internal Medicine 138(2): 128–134.
See also:
Free research papers are not written to satisfy your specific instructions. You can use our professional writing services to buy a custom research paper on any topic and get your high quality paper at affordable price.








