This sample West Nile Disease Research Paper is published for educational and informational purposes only. If you need help writing your assignment, please use our research paper writing service and buy a paper on any topic at affordable price. Also check our tips on how to write a research paper, see the lists of health research paper topics, and browse research paper examples.
Introduction
Certain mosquito-borne viruses are known to cause largescale epidemics, often on a recurrent basis. These agents share epidemiological and ecological features that distinguish them within the 800 recognized arboviruses (arthropod-borne) viruses. Those arboviral infections that may be placed in this epidemiological category of recurrent largescale outbreaks include St. Louis encephalitis (United States); Venezuelan equine encephalitis (Latin America); Rift Valley fever (Africa and Middle East); chikungunya fever (Africa, south Asia, Southeast Asia); yellow fever (South America, west Africa); dengue fever (North and South America, south Asia, Southeast Asia); Japanese B encephalitis (south Asia, Southeast Asia); Sindbis virus (northern Europe); and western equine encephalitis (western United States). Tens of thousands of human cases may be recorded from each such outbreak, ranging into the hundreds of thousands. The duration of such explosive episodes may be from months to several years, but all characteristically wane for unknown reasons. Many remain locally endemic but at low transmission levels that signify little human risk. The proximal ecological determinants that serve as the basis for the waxing and waning of such outbreaks largely remain undescribed.
West Nile virus (WNV) has long been classified in such an epidemiological category. Since its description in 1937 from a febrile woman in Uganda, WNV has caused large outbreaks with hundreds or thousands of reported cases in Israel (early 1950s and 2000), France (early 1960s), South Africa (1974), Romania (1996), Russia (1999), and the United States (early 2000s). Its enigmatic introduction into the United States in 1999 serves as a reminder of the potential for transcontinental spread of arboviral agents and the public health burden assumed by a country when there is such an event. We seek to review the history of prior WNV outbreaks as well as the current American one; provide a brief overview of the biology of WNV; and describe its mode of perpetuation. In addition, we shall discuss institutional responses historically and more recently; summarize control efforts and outline other modes of intervention; and examine prospects for the future. The American experience with WNV provides public health practice with lessons in responding to new large-scale outbreaks.
History Of Epidemics Due To WNV
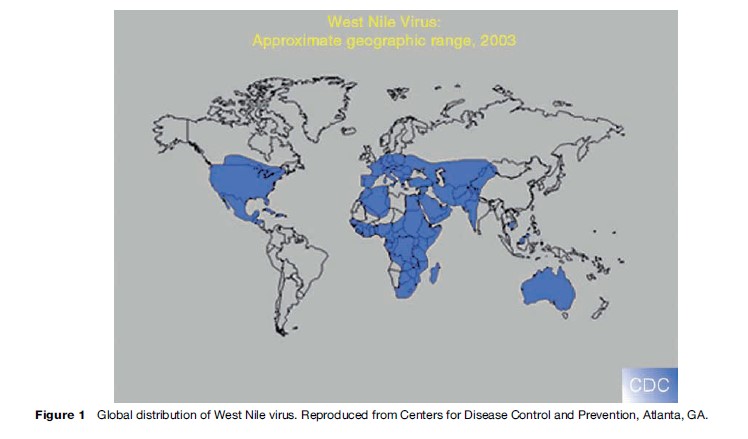
WNV is the most widely distributed arbovirus in the world. The virus was first isolated from a febrile woman in Uganda in 1937, and subsequently was associated with sporadic human cases and infrequent major outbreaks in Africa, Eurasia, Australia, and the Middle East (Figure 1). Early studies conducted during the 1950s in Egypt and the upper Nile delta formed the basis for understanding the ecology of the virus. Large epidemics (with hundreds to thousands of cases) occurring prior to 1996 generally affected rural populations, and few cases of severe neurological disease were noted. Beginning in the 1990s, outbreaks began to occur more frequently. These were focused around the Mediterranean Sea and were associated with increases in the frequency of severe disease, including viral encephalitis and persistent neurological sequelae. For example: An outbreak of West Nile disease in and near Bucharest, Romania, in 1996–97 led to more than 500 clinical cases with a case fatality rate of approximately 10%. Between 1996 and 1999, major WNV epidemics occurred in southern Romania, southern Russia, and the northeastern United States. These outbreaks were notable because they were the first epidemics reported in large urban populations (Zeller and Schuffenecker, 2004). In 2000 in Israel, a countrywide outbreak occurred with a case fatality rate of 8.4%, and neuroinvasive WNV disease was observed in Russia in 2001 and Tunisia in 2003.
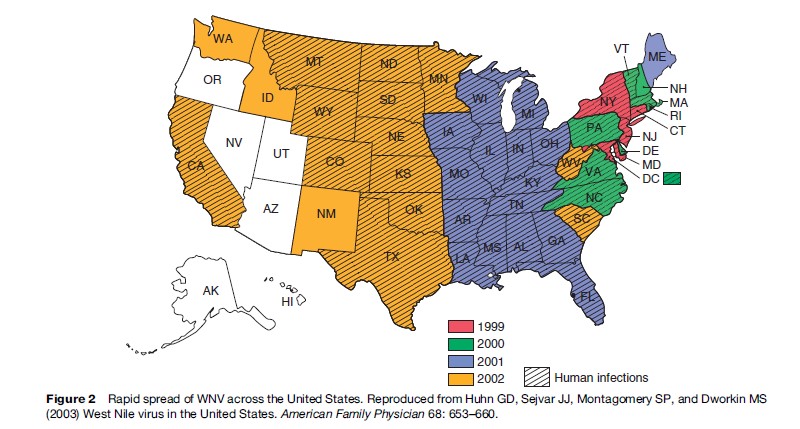
The current epizootic epidemic of WNV in North America was the result of a single-point introduction in the New York City area in 1999. The virus has since undergone a dramatic range expansion, and is currently distributed throughout the temperate and tropical Americas (Figure 2). The mode of transport and rapid expansion of WNV throughout the Americas remains to be fully understood, although north–south movements clearly are related to bird migratory behavior. This disease was previously unrecognized in the western hemisphere. During 1999–2007 (as of September 25, 2007), 26 282 cases were reported in the United States, of which 1020 (3.9%) were fatal (CDC, 2007a). West Nile disease also has been noted in humans in Cuba and the Cayman Islands, and in equines in Argentina, but scant evidence of human, equine, or avian morbidity and mortality has generally been observed in tropical America, possibly due to cross-protection from other flaviviruses circulating in tropical regions, reduced virulence of WNV in the tropics, or less competent arthropod and avian hosts than in temperate regions in concert with the greater diversity of host species in the tropics. The U.S. Centers for Disease Control and Prevention now recognizes that WNV is permanently established in the Americas, and predicts annual seasonal epidemics into the indefinite future.
There have been no overt cases in the United Kingdom, even with evidence of serological conversions in sentinel chickens. However since 2000, after at least 35 years without disease, WNV has been detected regularly in neighboring France in the Camargue region, with high levels of morbidity in equines. The lack of human cases in northern Europe as compared to southern Europe may possibly be attributed to the feeding behavior of local populations of the predominant vector, Culex pipiens, as well as yet undescribed other factors.
Biology
West Nile virus is a member of the Flavivirus genus of the family Flaviviridae, which contains approximately 70 members, most of which are either mosquitoor tick-borne. It is classified within the Japanese encephalitis ( JE) serological complex on the basis of cross-neutralization and molecular genetic studies (Calisher et al., 1989). The WNV virion, like other flaviviruses, is enveloped, spherical, approximately 40–60 nm in diameter, and contains an electron-dense core. Mature virions contain a single copy of the positive sense viral RNA packaged within an icosahedral capsid formed by the capsid protein (C). The genome-containing capsid is surrounded by a hostderived lipid bilayer bearing dimers of the viral envelope protein (E) and the membrane protein (M). Following receptor-mediated endocytic entry, membrane fusion, and uncoating, the RNA genome is transcribed as a single long polyprotein that is coand posttranslationally processed into three structural proteins that form the virus particles and seven nonstructural proteins that replicate the virus genome and interfere with normal host cell function. Thus, the antigenic, genetic, and three-dimensional structure of WNV and its constituent proteins, as well as its replication strategy, are similar to several other flaviviruses, and form the basis for its classification.
The pathogenesis of WNV has been characterized as a balance between virulence, immunity, and viral adaptation (Samuel and Diamond, 2006). Mouse models have been used extensively to characterize the pathogenesis of WNV in vertebrates. After inoculation by mosquitoes, WNV is thought to replicate in Langerhans cells in the skin. These cells migrate to draining lymph nodes and produce a viremia that allows virus seeding of peripheral tissues. Mice that die of WNV appear to suffer severe central nervous system pathology similar to severe human disease. This pathology includes infection of and damage to spinal cord-, hippocampus-, and brain stem-associated neurons. The precise mechanism of WNV neuroinvasion is currently not clear, but appears to be correlated with increased viral load in blood. A wide array of immune effectors are required for protection from lethal WNV infection in mice, including complement, IFN-a/b, gd T cells, antibodies, and CD8þ and CD4þ T cells. Moreover, an intact immune system is required for WNV clearance in mice. This is consistent with epidemiological and clinical observations that the immunocompromised and elderly are more likely to suffer severe disease.
The human health burden imposed by WNV may be quite severe. WNV causes ‘West Nile fever,’ along with more severe illness, including meningitis, encephalitis, acute flaccid paralysis, coma, and death. In humans, approximately 25% of infected persons develop the febrile illness West Nile fever. In fewer than 1%, neuroinvasive illness develops, with clinical symptoms that include headaches, muscle weakness, cognitive difficulty, and polio-like syndrome. Approximately 10% of neuroinvasive cases, or 0.1% of all infections, terminate fatally (Mostashari et al., 2001). The elderly are significantly more likely to develop neurologic disease; those older than 50 are 10 times more likely, and those 80 years or older are 40 times more likely than those who are younger. Case fatality rates range from 4 to 14% in the recent Romanian, American, and Israeli outbreaks. No specific therapy or vaccine is currently approved for human use. Although heterologous immunity to Japanese B encephalitis ( JE) or yellow fever (YF) reduced the severity of disease due to WNV in a hamster model, such an effect was not observed in humans ( Johnson et al., 2005), suggesting that the many tens of millions of individuals globally who have been vaccinated against JE or YF would not be protected.
Host Associations
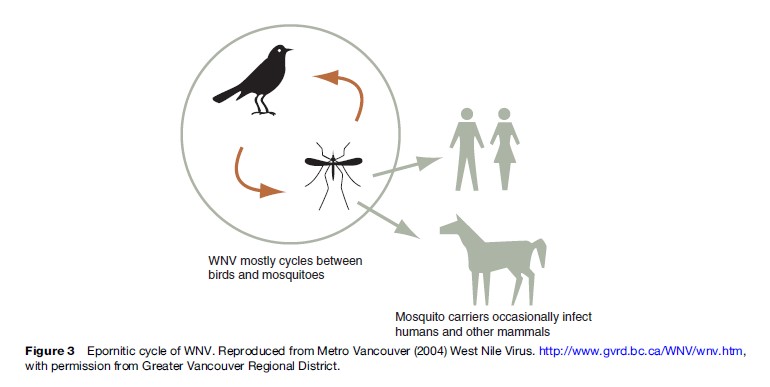
Like other flaviviruses within the Japanese encephalitis antigenic complex, WNV has been isolated from a variety of vectors, but is most commonly associated with Culex mosquitoes and particularly the common house mosquito, C. pipiens, which is cosmopolitan in distribution. WNV is maintained in nature in an enzootic cycle between ornithophilic mosquitoes and birds (Figure 3).
In South Africa, the vector is C. univittatus, a highly ornithophilic mosquito that feeds both at the ground and in the canopy. A variety of avian species there including ducks, coot, ibis, egret, doves, and warblers appear to be most important for WNV maintenance. In Israel, WNV appears to be introduced periodically by migrating white storks (Malkinson et al., 2001). Following introduction, it is maintained locally by C. univitattus, and has caused extensive outbreaks in domestic geese. C. univitattus also is thought to be a major WNV vector in Egypt, although a role for C. pipiens has been suggested. In Australia, WNV is maintained by C. annulirostris and C. sitiens subgroup. During an outbreak in Romania in 1996, C. pipiens was implicated as the main arthropod vector, and several bird species, including house sparrows and various species of domestic fowl, had neutralizing antibodies against WNV.
In North America, C. pipiens and C. restuans maintain WNV in their enzootic cycle in the northeast (Apperson et al., 2004), while C. quinquefasciatus and C. tarsalis are major vectors in the southern and western United States, respectively. Approximately 317 species of birds have been found infected in North America (CDC, 2007b). WNV has been isolated from large domestic animals (horses, donkeys, goats, buffalo, sheep, pigs, cows), rodents (squirrels, among others), and bats. The number of vertebrate and invertebrate species implicated in WNV transmission is an unusual feature of its ecology that has emerged as its geographic range has spread.
Several aspects of the WNV enzootic transmission cycle require further study. For example, the importance of highly visible species such as crows and other corvids is not well characterized, and may be largely dependent upon site-specific ecological parameters such as mosquito and bird density or overall biome species richness. It is also unclear why the high avian mortality that has been the hallmark of WNV since its introduction into the United States was not observed in the Old World and has not extended to tropical America. Finally, the importance of animals other than birds in WNV transmission continues to be debated. Evidence has been presented that other animals (e.g., some mammals and reptiles) are competent hosts for WNV.
Recent observations have challenged the view that bridge vectors are required for transmitting WNV to humans and other nonavian hosts (Kilpatrick et al., 2006). Typically, a vector-borne zoonotic agent is maintained in nature (‘enzootic’) by an arthropod with great host specificity feeding on a few species of reservoir host. The zoonotic condition (infection of humans) relies on the participation of an indiscriminately feeding arthropod that bridges the infection from the enzootic cycle to people. Both population-based studies and examinations of host feeding preferences have suggested that C. pipiens are responsible for most human infections in the northeastern and north central United States, as well as in eastern Europe and Russia. Early studies by Spielman (1964) hypothesized that there were C. pipiens populations that almost never fed on blood (autogenous), some that required blood meals (anautogenous) from birds, and hybrid offspring that formed late in the summer (i.e., in August and later months) that fed indiscriminately on either birds or mammals. It has thus been known for years that C. pipiens possess atypical feeding patterns marking two behaviorally and physiologically different forms, autogenous (C. pipiens form molestus) and anautogenous (C. pipiens form pipiens) populations. C. pipiens may therefore be involved in both early season amplification of WNV in enzootic cycles, and as bridge vectors when autogenous–anautogenous hybrids become more common. In support of this observation, a series of field studies has recently shown a strong temporal association between shifts in mosquito feeding behavior from a strong to a weak focus on American robins, with concomitant increases in human disease. Two other significant enzootic vectors in the United States, C. tarsalis and C. nigripalpus, are similarly important as bridge vectors because of similar shifts in host feeding patterns. Two other species, C. quinquefasciatus and C. salinarius, feed frequently on both avian and mammalian hosts, including human beings.
The reasons that WNV appears to be increasingly epizootic across large areas of the world remain speculative, but themes seem apparent. Agricultural irrigation was associated with WNV endemicity in the Nile River delta. In Pakistan, irrigation was associated with great densities of C. tritaeniorhynchus in a WNV enzootic site. Interestingly, treated wastewater used for agriculture (an increasing practice) created breeding sites for C. univittatus in South Africa (McIntosh et al., 1976), and the crops attracted dense infestations of birds, thereby juxtaposing the two most important elements of the WNV life cycle. Urban sites infested with pigeons may promote great densities of C. pipiens, although pigeons themselves appear irrelevant as reservoirs of WNV. Thus, WNV outbreaks may have a largely anthropogenic basis.
Institutional Responses
WNV serves as the prototype for a rapid institutional response to an emerging infection. Following the definitive identification of the causative agent for the encephalitis cases detected in 1999, investigators from the CDC and New York State undertook comprehensive epizootiologic and epidemiologic investigations in and around the Bronx, New York City, and Long Island. In 2000, the CDC published guidelines for surveillance to assist state and local health agencies and developed ArboNET, an electronic surveillance and reporting system. Annual conferences sponsored by CDC also facilitated a concerted public health response by state and local authorities. Congressional budget appropriations began in 1999, and in particular, $23.8 million was made available through the CDC to states through cooperative agreements. These funds were designated to help state public health departments initiate and carry out surveillance, diagnosis, and prevention of WNV. Such activities included active human case detection, reporting of dead birds, surveys of mosquito infection, public education on personal protective measures against mosquitoes, and serologic and virologic diagnostics. The National Institutes of Allergy and Infectious Diseases of the NIH funded at least 16 grant proposals focusing on WNV during 2000–07, totaling about $4 million, primarily to develop new therapeutics or vaccines. This extraordinary degree of funding support certainly allowed for rapid expansion of the capacity for public health officials to respond and for academic scientists to undertake basic research.
Cases of WNV infection have been acquired from blood transfusion, leading to an FDA mandate to screen all donations for evidence of virus. Diagnostic assays to detect WNV within blood donations were placed on accelerated FDA review and approval. During 2003, 818 infected units were detected from about 6 million donations. A formal cost–benefit analysis of various screening modes suggested, however, that universal screening was not economically justifiable given the overall low risk, but that targeted screening (within high transmission areas and of those units that may be provided to immunocompromised individuals) may have public health utility (Korves et al., 2006).
The urgency with which federal authorities acted requires explanation. From 1933–80, an estimated 10 000 cases of St. Louis encephalitis (SLE) (with 1000 fatalities) occurred in the United States. SLE virus is maintained in a similar epornitic cycle with Culex spp. as the maintenance vectors. Indeed, the first human neurologic cases of WNV disease from New York City were first thought to be due to SLE. However, the institutional responses to SLE outbreaks have largely been locally driven, with considerably less available funding. The specter of a foreign disease invading the United States largely influenced the vigorous institutional response, but it seems likely that political pressure from a public alarmed in part by the exotic name of the infection helped drive the federal response. Regardless of motives, the exemplary response to the WNV outbreak strongly suggests that the U.S. infrastructure is capable of effectively responding to other acute infectious disease outbreaks.
Interventions
Surveillance
In 1999, recognition of human cases was presaged by weeks by reports of dead exotic and domestic birds in the New York City area. This observation provided the basis for a cornerstone of WNV surveillance: Reports of dead birds, particularly crows, and the testing of select specimens for evidence of virus, serves as a very sensitive early warning of local transmission, as well as allowing the mapping of transmission activity (CDC, 2003). Use of sentinels such as live captive birds, attempting to document seroconversion, or morbidity/mortality of horses can be useful in rural sites where dead birds are less likely to be detected. Mosquito sampling (particularly larval counts) can provide evidence for trends in the density of important vector species, and testing adult mosquitoes for WNV infection may provide the earliest possible warnings of an outbreak. ‘Gravid’ traps, in particular, will provide samples with a greater probability of infection than those taken by light traps because Culex spp. ovipositing in the fetid water that is used as attractant will by definition have taken a blood meal, whereas light traps will sample mosquitoes that have never fed as well as those that have.
The surveillance case definition (Table 1) demonstrates the difficulty with which incidence may be measured. The case definition, in fact, is for arboviral disease in general. Laboratory confirmation can be achieved by direct evidence (isolation of virus or, more commonly, detection of viral RNA by RT-PCR) or by seroconversion. Seroconversion requires the use of specific reagents such as recombinant WNV proteins, inasmuch as there is much cross-reactivity among flaviviruses; even YF vaccination (common for international travelers or immigrants from South America) will induce antibody that may cross-react.

Active case detection for meningoencephalitis serves to better define the public health burden of WNV but plays little role if any in predicting zoonotic risk or need for intervention. Interestingly, enhanced surveillance for neurologic disease has increased our detection of other arboviral infections endemic to North America such as Powassan encephalitis (tickborne) or Jamestown Canyon virus (mosquitoes). Ensuring that physicians report suspect neurologic cases in real time, as well as requesting the cooperation of diagnostic laboratories in reporting results or forwarding specimens of cerebrospinal fluid for further analysis can help state departments of public health determine the appropriate allocation of resources for predictive surveillance as well as better define the public health burden.
Mosquito Control
When infected mosquitoes are documented, intervention should be considered. Local and state public health boards may differ on when intervention must occur; the choice of a threshold for action is arbitrary but is based on the density and distribution of infected mosquitoes. Two complementary modes of intervention are typical: public education campaigns to promote personal protection (limiting activities at dusk; ensuring that houses have intact window screens; use of repellants when outdoors activity cannot be avoided), and reduction of adult mosquitoes (‘adulticiding’).
Adulticiding targets those older mosquitoes that have fed at least once, thereby providing the opportunity to have acquired infection, as well as reducing those newly emerged mosquitoes that may feed on hosts that are currently viremic. Misperceptions exist among the lay public regarding aerial or truck-mounted ultra-low volume (ULV) spraying for mosquito adulticiding, leading to ‘environmental’ activism that hinders public health efforts. Concerns regarding human exposure, nontarget species kill (‘‘Beneficial insects such as dragonflies that eat mosquitoes also die’’), and the limited value of the exercise (‘‘Spraying doesn’t work – mosquitoes just come back in a week or so’’) appear to be mistakenly based on perceptions of agricultural crop dusting, which is a crude approach and one for which such concerns are valid.
ULV spraying for public health intervention utilizes precisely engineered nozzles and atomizers such that the droplet size that is delivered is 50 mm or less, and occurs during dusk or nighttime hours to target flying host-seeking mosquitoes. Wind speed, direction, and temperature are also critically evaluated before undertaking aerial ULV spraying for mosquitoes. The droplet size for agricultural applications ranges from 100–400 mm, which allows delivery in a variety of weather conditions. The precision of aerial ULV spraying for public health purposes allow targeting for flying mosquitoes during their activity period, reducing effects on nontarget species (Boyce et al., 2007). The pyrethroid or organophosphate compounds that are used are typically delivered at a rate of 3 oz. per acre, compared with 50–90 oz. per acre for crop-dusting. Thus, adulticiding is a highly targeted action with minimal environmental effect. In fact, mosquitoes quickly reappear within sprayed sites, often within a week or two, demonstrating the absence of lingering environmental toxicity but also confusing the public, who infer that the action was unsuccessful in eradicating mosquitoes. What is unappreciated is that older, infected mosquitoes were targeted and killed; new mosquitoes that emerge subsequent to spraying are less likely to be infected.
The utility of aerial ULV has been questioned with respect to intervening against WNV because the main vectors (C. pipiens) in the northern United States tend to be in urban habitats and may be protected by buildings and other infrastructure. However, C. pipiens seek out birds in urban tree canopies as well as those roosting on or within buildings and thus would be vulnerable to ULV spraying. In addition, C. tarsalis, the main western U.S. vector, and C. nigripalpis, an important southern U.S. vector, would be found in habitats amenable to ULV spraying (irrigated agricultural fields for the former; virtually any habitat for the latter).
Preemptive mosquito control may comprise source reduction, in which potential breeding sites are identified and cleaned. For C. pipiens, which tends to breed in semipermanent foul water such as sewers, larvicides such as the growth regulator methoprene or BTI (Bacillus thuringiensis israelensis, which secretes a toxin that is active only within the alkaline midguts of mosquitoes) may be effective by delivering it to such bodies of water. Periodic flushing of sewers with large volumes of water may dislodge C. pipiens larvae and wash them away: paradoxically, it may be that floods due to heavy rains may actually be detrimental to C. pipiens breeding because of this flushing effect.
Personal Protection
Public education about mosquito breeding and biting habits (Figure 4) may help to reduce risk at the individual and community levels. Recommendations for avoiding mosquito bites include reducing outside personal activity during dusk and dawn as well as ensuring that screens on windows are intact. Repellants are the main form of personal protection, and a variety of products are commercially available. The CDC has evaluated many such products and generally continues to recommend DEET (diethyltoluamide)-based repellants given their long record of efficacy and general safety record. Rare reports of neurotoxicity, due to overapplication to infants by overanxious parents, have raised some concerns regarding DEET use, but with tens of millions of applications to humans over the years with only a handful of adverse events, there cannot be any doubt about the public health utility of this repellant. Efficacy is said to be related to DEET concentration, with current recommendations for using formulations containing only up to about 30% active ingredient, which appears nearly as effective as those with greater concentrations; the lower concentrations further reduce the very small risk of an adverse event. DEET needs to be reapplied frequently, depending on formulation. Microencapsulated or liposome-based products tend to control volatilization and provide more extended protection per application.

Vaccines
Vaccine development has been on an accelerated track because of the perceived failure of anti-mosquito interventions in limiting the incidence and geographic spread of WNV. Currently, a successful phase I (safety) clinical trial has been undertaken with a live attenuated chimeric virus based on the yellow fever 17D vaccine (Monath et al., 2006). The YF premembrane and envelope genes have been replaced with those of WNV, with the envelope gene itself being modified to reduce neurovirulence in mouse models. The rapid development of this vaccine candidate reflects the fact that YF 17D is generally recognized as safe, with nearly half a billion persons globally having been vaccinated over its half-century of use. Modifying such a well-known product would facilitate regulatory approval. The phase I trial demonstrated that all of the 45 subjects receiving the chimeric vaccine (Chimerivax WN, Acambis Inc.) developed neutralizing antibody after 1 dose. Neutralizing antibody is critical in protection against arboviral infections.
Effective vaccines for yellow fever, Japanese B encephalitis, and tickborne encephalitis have greatly reduced the public health burden of these flaviviral infections, and it is likely that a WNV vaccine will soon become available. The costs and benefits of its routine use for public health purposes, however, need to be formally stated.
Prospects For The Future
The American experience with WNV has provided a lesson on the lacunae that still exist in responding to explosive outbreaks of vector-borne infection. Although a large pool of well-trained basic researchers and public health workers existed and financial resources were directed to risk reduction and basic research, WNV marched inexorably across the United States from east to west, crossing what appeared to be large geographical barriers in ways that are poorly understood. This east-to-west spread is not readily attributable to transport by birds because few birds undergo migration in such a direction. The distances across which WNV moved are too great to be due to mosquito movements. Without fully understanding the mechanism of such spread, the United States cannot effectively limit the potential for new public health burdens due to other mosquito-transmitted infections that might be introduced as was WNV, such as chikungunya, Rift Valley fever, Japanese encephalitis, Venezuelan equine encephalitis, or dengue. Their eventual arrival into the United States, Europe, or any other continental landmass may be predicted, but institutional capacity to preempt invasion appears to be minimal.
Bibliography:
- Apperson CS, Hassan HK, Harrison BA, et al. (2004) Host feeding patterns of established and potential mosquito vectors of West Nile virus in the eastern United States. Vector-Borne and Zoonotic Diseases 4: 71–82.
- Boyce WM, Lawler SP, Schultz JM, et al. (2007) Nontarget effects of the mosquito adulticide pyrethrin applied aerially during a West Nile virus outbreak in an urban California environment. Journal of the American Mosquito Control Association 23: 335–339.
- Calisher CH, Karabatsos N, Dalrymple JM, et al. (1989) Antigenic relationships between flaviviruses as determined by cross-neutralization tests with polyclonal antisera. Journal of General Virology 70 (pt. 1): 37–43.
- Johnson BW, Kosoy O, Martin DA, et al. (2005) West Nile virus infection and serologic response among persons previously vaccinated against yellow fever and Japanese encephalitis viruses. Vector-Borne and Zoonotic Diseases 5: 137–145.
- Kilpatrick AM, Kramer LD, Jones MJ, et al. (2006) West Nile virus epidemics in North America are driven by shifts in mosquito feeding behavior. PLoS Biology 4: e82.
- Korves CT, Goldie SJ, and Murray MB (2006) Cost-effectiveness of alternative blood-screening strategies for West Nile Virus in the United States. PLoS Medicine 3: e21.
- Malkinson M, Banet C, Weisman Y, et al. (2001) Intercontinental transmission of West Nile virus by migrating white storks. Emerging Infectious Diseases 7: 540.
- McIntosh K, Jupp PG, Dos Santos I, and Meenehan GM (1976) Epidemics of West Nile and sindbis viruses in South Africa with Culex (Culex) univittatus Theobald as vector. South African Journal of Science 72: 295–300.
- Monath TP, Jian L, Kanesa-Thasan N, et al. (2006) A live, attenuated recombinant West Nile virus vaccine. Proceedings of the National Academy of Science of the United States of America 103: 6694–6699.
- Samuel MA and Diamond MS (2006) Pathogenesis of West Nile Virus infection: A balance between virulence, innate and adaptive immunity, and viral evasion. Journal of Virology 80: 9349–9360.
- Spielman A (1964) Studies on autogeny in Culex pipiens populations in nature: I. Reproductive isolation between autogenous and anautogenous populations. American Journal of Hygiene 80: 175–183.
- S. Centers for Disease Control and Prevention (2003) Epidemic/ Epizootic West Nile Virus in the United States: Guidelines for Surveillance, Prevention, and Control. Ft. Collins, CO: US Dept of Health and Human Services. http://www.cdc.gov/ncidod/dvbid/ westnile/resources/wnvguidelines2003.pdf (accessed January 2008).
- S. Centers for Disease Control and Prevention (2007a) West Nile Virus: Statistics, Surveillance, and Control. http://www.cdc.gov/ncidod/dvbid/westnile/surv&control.htm (accessed January 2008).
- S. Centers for Disease Control and Prevention (2007b) West Nile Virus: Vertebrate Ecology. http://www.cdc.gov/ncidod/dvbid/westnile/birdspecies.htm (accessed January 2008).
- Zeller HG and Schuffenecker I (2004) West Nile virus: An overview of its spread in Europe and the Mediterranean basin in contrast to its spread in the Americas. European Journal of Clinical Microbiology and Infectious Diseases 23: 147–156.
- Edman JD and Taylor DJ (1968) Culex nigripalpus: Seasonal shift in the bird-mammal feeding ratio in a mosquito vector of human encephalitis. Science 161: 67–68.
- Hayes CG (1989) West Nile fever. Monath TP (ed.), The Arboviruses: Epidemiology and Ecology, vol. 5, pp. 59–88. Boca Raton, FL: CRC Press.
- Hayes EB, Komar N, Nasci RS, et al. (2005) Epidemiology and transmission dynamics of West Nile virus disease. Emerging Infectious Diseases 11: 1167–1173.
- Kuno G, Chang GJ, Tsuchiya KR, et al. (1998) Phylogeny of the genus Flavivirus. Journal of Virology 72: 73–83.
- Lanciotti RS, Roehrig JT, Deubel V, et al. (1999) Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science 286: 2333–2337.
- Marfin AA and Gubler DJ (2001) West Nile encephalitis: An emerging disease in the United States. Clinical Infectious Diseases 33: 1713–1719.
- Monath TP (1980) St. Louis Encephalitis. Washington, DC: American Public Health Association.
- Peterson LR and Marfin AA (2002) West Nile virus: A primer for the clinician. Annals of Internal Medicine 137: 173–179.
- Smithburn KC, Hughes TP, Burke AW, and Paul JH (1940) A neurotropic virus isolated from the blood of a native of Uganda. American Journal of Tropical Medicine and Hygiene 20: 471–473.
- Taylor RM, Work TH, Hurlbut HS, and Rizk F (1956) A study of the ecology of West Nile virus in Egypt. American Journal of Tropical Medicine and Hygiene 5: 579.
- mosquito.org/mosquito-information/virus.aspx – The American Mosquito Control Association.
- cdc.gov/westnile/ – Centers for Disease Control and Prevention (CDC).
- http://environmentalrisk.cornell.edu/WNV/ – Cornell University, Department of Communications Environmental Risk Analysis Program.
- niaid.nih.gov/topics/westnile – National Institute of Allergy and Infectious Diseases.
- http://www.nwhc.usgs.gov/disease_information/west_nile_virus/ – US Geological Survey, National Wildlife Health Center.
See also:
Free research papers are not written to satisfy your specific instructions. You can use our professional writing services to buy a custom research paper on any topic and get your high quality paper at affordable price.