This sample Amebiasis Research Paper is published for educational and informational purposes only. If you need help writing your assignment, please use our research paper writing service and buy a paper on any topic at affordable price. Also check our tips on how to write a research paper, see the lists of health research paper topics, and browse research paper examples.
A Deadly Parasitic Disease
Amebiasis is an infection caused by the protozoan parasite Entamoeba histolytica. In contrast, nonpathogenic ameba that infect humans include E. dispar and E. moshkovskii (both morphologically identical to and easily confused with E. histolytica), E. coli, E. hartmanni, and Endolimax nana. Dientamoeba fragilis and E. polecki have been associated with diarrhea and E. gingivalis with periodontal disease. Approximately 50 million illnesses and 100 000 deaths occur annually from amebiasis, making it the third-leading cause of death due to parasitic disease in humans. Long-term consequences of amebiasis in children may include both malnutrition and lower cognitive abilities. Currently there is no vaccine to prevent the childhood morbidity and mortality due to infection with this protozoan parasite. Although amebiasis is present worldwide, it is most common in underdeveloped areas, especially Central and South America, Africa, and Asia.
In the United States and other developed countries, cases of amebiasis are most likely to occur in immigrants from and travelers to endemic regions.
Epidemiology
Infection occurs via ingestion of the parasite’s cyst from fecally contaminated food, water, or hands. This is a common occurrence among the poor of developing countries and can afflict populations of the developed world, as the recent epidemic in Tblissi Georgia due to contaminated municipal water demonstrates (Barwick et al., 2002). Carefully conducted serologic studies in Mexico, where amebiasis is endemic, demonstrated antibody to E. histolytica in 8.4% of the population. In the urban slum of Fortaleza, Brazil, 25% of all people tested carried antibody to E. histolytica; the prevalence of antiamebic antibodies in children aged 6–14 years was 40%. A prospective study of preschool children in a slum of Dhaka Bangladesh has demonstrated new E. histolytica infection in 45%, and E. histolytica-associated diarrhea in 9%, of the children annually. Not all individuals are equally susceptible to amebiasis, with certain HLA DR and DQ alleles associated with resistance to infection and disease (Duggal et al., 2004).
Pathogenesis
The cysts are transported through the digestive tract to the intestine, where they release their mobile, disease-producing form, the trophozoite. E. histolytica trophozoites can live in the large intestine and form new cysts without causing disease. But they can also invade the lining of the colon, killing host cells and causing amebic colitis, acute dysentery, or chronic diarrhea. The trophozoites can be carried through the blood to other organs, most commonly the liver and occasionally the brain, where they form potentially life-threatening abscesses (see Figure 1). Important virulence factors include the trophozoite cell surface galactose and N-acetyl-D-galactosamine (Gal/ GalNAc)-specific lectin that mediates adherence to colonic mucins and host cells, cysteine proteinases that likely promote invasion by degrading extracellular matrix and serum components, and amoebapore pore-forming proteins involved in killing of bacteria and host cells.
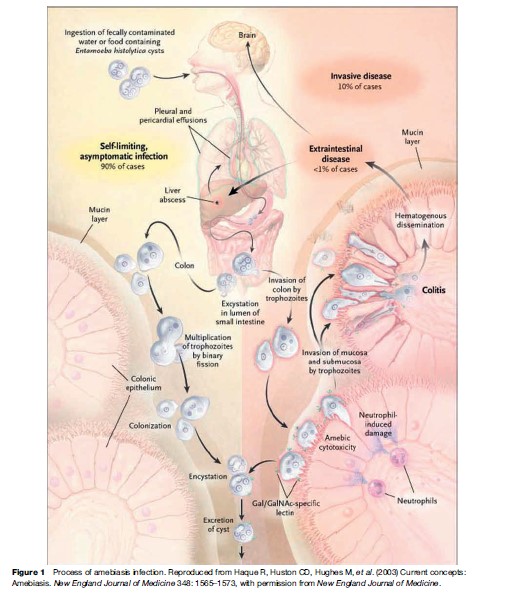
Infection is normally initiated by the ingestion of fecally contaminated water or food containing E. histolytica cysts. The infective cyst form of the parasite survives passage through the stomach and small intestine. Excystation occurs in the bowel lumen, where motile and potentially invasive trophozoites are formed. In most infections, the trophozoites aggregate in the intestinal mucin layer and form new cysts, resulting in a self-limited and asymptomatic infection. In some cases, however, adherence to and lysis of the colonic epithelium, mediated by the galactose and N-acetyl-D-galactosamine (Gal/GalNAc)-specific lectin, initiates invasion of the colon by trophozoites. Once the intestinal epithelium is invaded, extraintestinal spread to the peritoneum, liver, and other sites may follow. Factors controlling invasion, as opposed to encystation, most likely include parasite ‘quorum sensing’ signaled by the Gal/GalNAc-specific lectin, interactions of amebae with the bacterial flora of the intestine, natural immunity, and innate and acquired immune responses of the host.
Immunity
Acquired immunity to infection and invasion by E. histolytica is associated with a mucosal IgA antibody response against the carbohydrate recognition domain (CRD) of the parasite Gal/GalNAc lectin (Haque et al., 2001, 2006). The average duration of protection afforded by anti-CRD IgA is under 2 years. Cell-mediated immunity in protection from invasive amebiasis, but not infection per se, has also been demonstrated. There is substantial evidence from in vitro animal model and most recently human studies revealed an important role for IFN-g in protection from amebic colitis, acting in part by activating macrophages to kill the parasite. Invasive amebiasis rarely occurs in individuals with HIV/AIDS, even in areas where amebiasis is common, suggesting an important role also for natural resistance and/or innate immune responses in protection from infection.
Diagnosis
Historically, diagnosis of amebiasis was complicated because several areas of the body can be affected, symptoms may be similar to other conditions such as inflammatory bowel diseases, and diagnostic tests were not highly specific. Before the development of new antigen detection and polymerase chain reaction (PCR) tests, diagnosis of amebiasis was performed by examining a stool sample through a microscope to determine whether E. histolytica cysts were present (Figure 2(a)). However, this method often requires more than one specimen because the number of cysts in the stool is highly variable. In addition, stool microscopy has limited sensitivity and specificity. The body’s own immune system produces macrophages that can look like the ameba. Moreover, three different amebas – E. histolytica, which causes amebiasis, and E. dispar and E. moshkovskii, which do not cause disease – look identical under a microscope (Diamond and Clark, 1993).
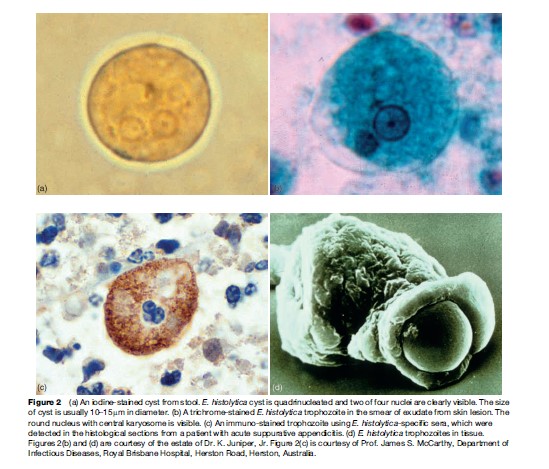
Amebiasis outside the intestine has been even more difficult to diagnose. Clinical manifestations of extraintestinal disease vary widely, and less than 10% of person with amebic liver abscesses have identifiable E. histolytica in their stools. Noninvasive diagnostic procedures such as ultrasound, computer tomographic (CT) scan, and magnetic resonance imaging (MRI) can detect liver abscesses but cannot distinguish between abscesses caused by ameba and those caused by bacteria, thus hampering proper treatment of the condition. Until recently, the most accurate diagnostic test involved examining a sample of the abscess tissue obtained by needle aspiration (Figure 2(b)), a procedure that is painful, potentially dangerous, and relatively insensitive, identifying amebic trophozoites only 20% of the time.
A stool antigen diagnostic test using polyclonal anti-bodies to adhesin of E. histolytica that allows specific and sensitive diagnosis of E. histolytica infection is manufactured by TechLab, Inc. This FDA-approved test is 80–90% sensitive and nearly 100% specific compared to real-time PCR. The E. histolytica antigen test can be performed rapidly and cheaply, and can detect infection before symptoms appear. Early presymptomatic treatment can prevent the development of invasive amebiasis and minimize the spread of infection. Moreover, follow-up tests can be performed to confirm eradication of intestinal infection. In addition, immunohistochemical staining of ameba is useful in a case difficult to diagnose (Figure 2(c)).
Serologic tests for antiamebic antibodies are also a very useful tool in diagnosis, with sensitivity of 70–80% early in disease and approaching 100% sensitivity upon convalescence. The combined use of serology and stool antigen detection test offers the best diagnostic approach.
What Are The Symptoms Of Amebic Colitis?
Patients present with several days to weeks of gradual onset of abdominal pain and tenderness, diarrhea and occasionally bloody stools (Figure 3(a)). This is different from bacterial causes of dysentery, where patients usually only have 1 to 2 days of symptoms. Surprisingly, fever is present in only the minority of patients with amebic colitis. Colonic lesions can vary from only mucosal thickening to flask-shaped ulcerations to necrosis of intestinal wall (Figures 3(b)–3(f )). Unusual manifestations of amebic colitis include toxic megacolon (0.5% of cases, usually requiring surgical intervention), ameboma (granulation tissue in colonic lumen mimicking colonic cancer in appearance), and a chronic nondysenteric form of infection that can present as years of waxing and waning diarrhea, abdominal pain, and weight loss (easily misdiagnosed as inflammatory bowel diseases).
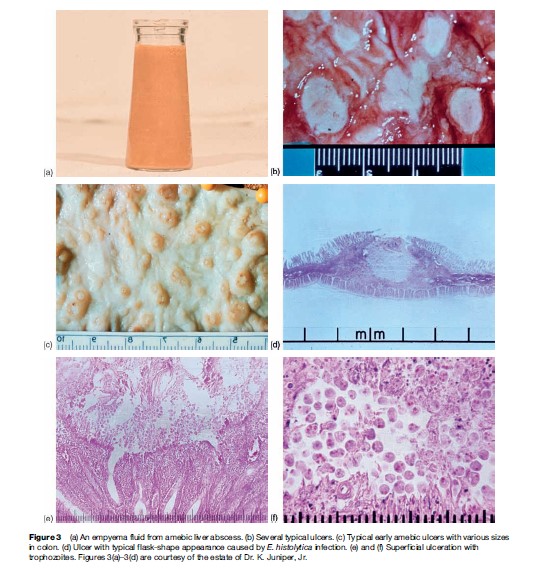
A heightened suspicion of amebiasis should be present if the patient has been in a developing country in the last year (as a resident or traveler). In a patient with diarrhea, if blood is present in the stool (grossly bloody or occult blood positive; Figure 3(a)), then infectious (Shiga toxin-producing E. coli, Salmonella, Shigella, Campylobacter, and E. histolytica) and noninfectious (inflammatory bowel disease, diverticulosis, arteriovenous malformations, cancer) causes should be considered. The diagnosis of amebic colitis is best made by antigen detection in stool (not widely available), by colonoscopy and biopsy, and by detection of antiamebic antibodies in serum (present in most but not all patients).
How Does A Patient With Amebic Liver Abscess Present?
The typical patient with an amebic liver abscess in the United States is an immigrant, usually a Hispanic male, 20–40 years old, who presents with fever, right upper quadrant pain, leukocytosis, abnormal serum transaminases and alkaline phosphatase, and a defect on hepatic imaging study. Roughly 90% of patients with liver abscess are males. The abscess is usually single and is in the right lobe of the liver 80% of the time (Figure 4(d)) (Katzenstein et al., 1982).
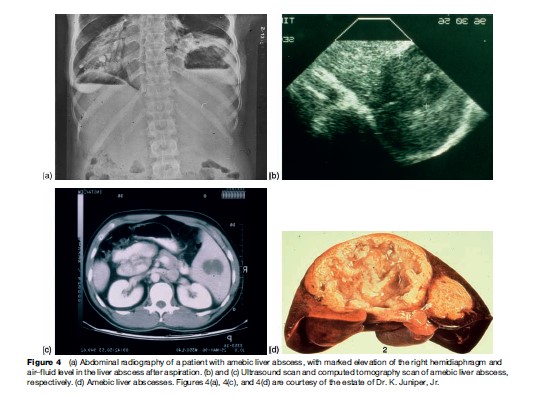
Most frequently, patients will present with liver abscess without concurrent colitis, although a history of dysentery within the last year can often be obtained. Ameba are infrequently seen in the stool at the time of diagnosis of liver abscess (Adams and MacLeod, 1977). Liver abscess can present acutely with fever, right upper abdominal tenderness, and pain, or subacutely with prominent weight loss and, less frequent, fever and abdominal pain. The peripheral white blood cell count is elevated, as is the alkaline phosphatase level in many patients. Early evaluation of the hepatobiliary system with ultrasound or CT is essential to demonstrate the abscess in the liver (Figure 4). The differential diagnosis of the lesion in the liver would include pyogenic abscess (less likely if the gallbladder and ducts appear normal), hepatoma, and echinococcal cyst. Aspiration of the abscess is occasionally required to diagnose amebiasis. (Although ameba are visualized in the pus in only the minority of cases, if the abscess is pyogenic the responsible bacteria will be seen and/or cultured.) Antibodies to E. histolytica are present in the serum of 92–97% of patients upon acute presentation with amebic liver abscess, and therefore are very useful diagnostically. Unusual extraintestinal manifestations of amebiasis include direct extension of the liver abscess to pleura or pericardium, and brain abscess.
In a patient who presents with right upper quadrant pain, an ultrasound, CT, or MRI should be performed to examine the liver and gallbladder. If a space-filling defect in the liver is observed, the differential diagnosis includes: (1) amebiasis (most common in adult males with a history of travel or residence in a developing country), (2) pyogenic or bacterial abscess (suspect in women, patients with cholecystitis, the elderly, individuals with diabetes, and in patients presenting with jaundice), (3) echinococcal cysts (this would be an incidental finding as echinococcal cysts should not cause pain or fever, unless secondarily infected), and (4) cancer. Most patients with amebic liver abscess will have detectable circulating antigen in serum, as well as serum antiamebic antibodies.
How Should Amebiasis Be Treated?
Invasive amebiasis should be treated with metronidazole or tinidazole plus a ‘luminal’ agent such as diloxanide furoate, paromomycin, or diiodohydroxyquin. Metronidazole or tinidazole alone does not eliminate intestinal colonization in up to 50% of patients with invasive amebiasis, leaving patients open to the real possibility of a relapse of invasive infection months later. The majority of patients defervesce after less than 3 days’ treatment with metronidazole. Chloroquine, dehydroemetine, and needle aspiration of the abscess have all been successfully used for the rare patient not responding to metronidazole.
Metronidazole is concentrated in the ameba probably via reduction of its nitro group by ferredoxin or flavodoxinlike electron transport proteins, which maintain a gradient for the entry of the unchanged drug. Metabolic intermediates of metronidazole damage DNA and possibly other macromolecules, and deprive the organism of reducing equivalents by acting as an electron sink.
Future
Providing safe food and water for all children in developing countries would be ideal for prevention of the disease but require massive societal changes and investments. An effective vaccine is less costly and would be a desirable and feasible goal. Continued support of developed countries is essential for the development of effective vaccines and the control of infectious diseases in developing countries.
Bibliography:
- Adams EB and MacLeod IN (1977) Invasive amebiasis. I. Amebic dysentery and its complications. II. Amebic liver abscess and its complications. Medicine 56: 315–334.
- Barwick R, Uzicanin A, Lareau S, et al. (2002) Outbreak of amebiasis in Tbilisi, Republic of Georgia, 1998. American Journal of Tropical Medical and Hygiene 67: 623–631.
- Diamond LS and Clark CG (1993) A redescription of Entamoeba histolytica Schaudinn 1903 (emended Walker 1911) separating it from Entamoeba dispar (Brumpt 1925). Journal of Eukaryotic Microbiology 40: 340–344.
- Duggal P, Haque R, Roy S, et al. (2004) Influence of human leukocyte antigen class II alleles on susceptibility to Entamoeba histolytica infection in Bangladeshi children. Journal of Infectious Diseases 189: 520–526.
- Haque R, Ali IKM, Sack RB, et al. (2001) Amebiasis and mucosal IgA antibody against the Entamoeba histolytica adherence lectin in Bangladeshi children. Journal of Infectious Diseases 183: 1787–1793.
- Haque R, Mondal D, Duggal P, et al. (2006) Entamoeba histolytica infection in children and protection from subsequent amebiasis. Infection and Immunity 74: 904–909.
- Katzenstein D, Rickerson V, and Braude A (1982) New concepts of amebic liver abscess derived from hepatic imaging, serodiagnosis, and hepatic enzymes in 67 consecutive cases in San Diego. Medicine 61: 237–246.
- Haque R, Huston CD, Hughes M, et al. (2003) Current concepts: Amebiasis. New England Journal of Medicine 348: 1565–1573.
- Hughes M and Petri WA Jr (2000) Amebic liver abscess. Infectious Diseases Clinics of North America 14: 565–582.
- Stanley SL Jr (2003) Amoebiasis. Lancet 361: 1025–1034.
- WHO (1997) Amoebiasis. WHO Weekly Epidemiologic Record 72: 97–100.
See also:
Free research papers are not written to satisfy your specific instructions. You can use our professional writing services to buy a custom research paper on any topic and get your high quality paper at affordable price.








