This sample Leishmaniasis Research Paper is published for educational and informational purposes only. If you need help writing your assignment, please use our research paper writing service and buy a paper on any topic at affordable price. Also check our tips on how to write a research paper, see the lists of health research paper topics, and browse research paper examples.
Introduction
The leishmaniases are parasitic diseases caused by flagellate protozoa of the genus Leishmania (Kinetoplastida, Trypanosomatidae), which infect numerous mammalian species, including humans, and are transmitted through the infective bite of an insect vector, the phlebotomine sand fly. In humans, the disease may be visceral (VL), cutaneous (CL), of localized (LCL) or diffuse (DCL) type, or mucocutaneous leishmaniasis (MCL). The leishmaniases are widely distributed around the world. In numerous countries, increasing risk factors are making leishmaniasis a major public health problem.
General Epidemiology Of Leishmaniasis
Parasite
Leishmania are dimorphic parasites that present as two principal morphological stages: the intracellular amastigote, in the cells of the mammalian host mononuclear phagocyte system, and the flagellated promastigote, in the intestinal tract of the insect vector.
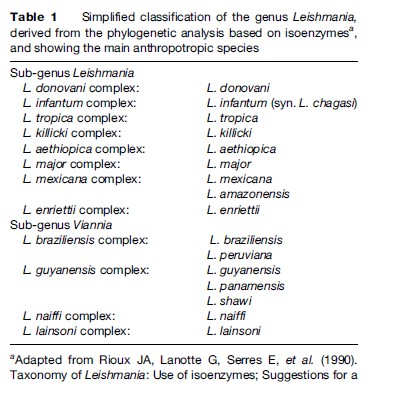
Since the first Leishmania species was described (Laveran and Mesnil, 1903), the number of species has increased steadily, and currently stands around 30. As the different species are morphologically indistinguishable, other characteristics have been used for their taxonomy. Although DNA-based methods are increasingly being used, isoenzyme electrophoresis remains the gold standard technique for identification and is the basis for the current classification (Table 1).
Vector
Sandflies are psychodid Diptera of the subfamily Phlebotominae. Their life cycle includes two different biological stages: the free-flying adult and the developmental stages, which include egg, four larval instars, and pupa, and occur in damp soil rich in organic material.
The adults are small flying insects about 2–4 mm in length, with a yellowish hairy body. During day, they rest in dark, sheltered places. They are active at dusk and during the night. Both sexes feed on plant juices, but females also need a blood meal before they are able to lay eggs. It is during this blood meal that Leishmania parasites are transmitted between the mammalian hosts.
Among the 800 known species of sand flies, about 70, belonging to the genera Phlebotomus in the Old World and Lutzomyia in the New World, are proven or suspected vectors of Leishmania, and a certain level of specificity exists between Leishmania and sand fly species.
Reservoir
Various species of seven different orders of mammals are the reservoir hosts responsible for long-term maintenance of Leishmania in nature. Depending on the focus, the reservoir host can be either a wild or a domestic mammal, or even in particular cases human beings. In visceral leishmaniasis, these different types of reservoir host represent different steps on the hypothetical path toward ‘anthropization’ of a ‘wild’ zoonosis (Garnham, 1965). Rodents, hyraxes, marsupials, and edentates are common reservoirs of wild zoonotic CL. Dogs are currently considered as true reservoirs of L. infantum and L. peruviana, two species that have peri-domestic or even domestic transmission. Humans are the commonly recognized reservoir of L. donovani VL and L. tropica CL.
Life Cycle And Transmission
In nature, Leishmania parasites are alternately hosted by the insect (flagellated promastigote) and by mammals (intracellular amastigote stage). Leishmaniasis is normally transmitted to humans by the inoculation of metacyclic promastigotes through the sand fly bite. Other routes remain exceptional. Exchange of syringes is thought to explain the high prevalence of L. infantum/human immunodeficiency virus (HIV) co-infection in intravenous drug users in southern Europe (Alvar and Jimenez, 1994).
Geographical Distribution
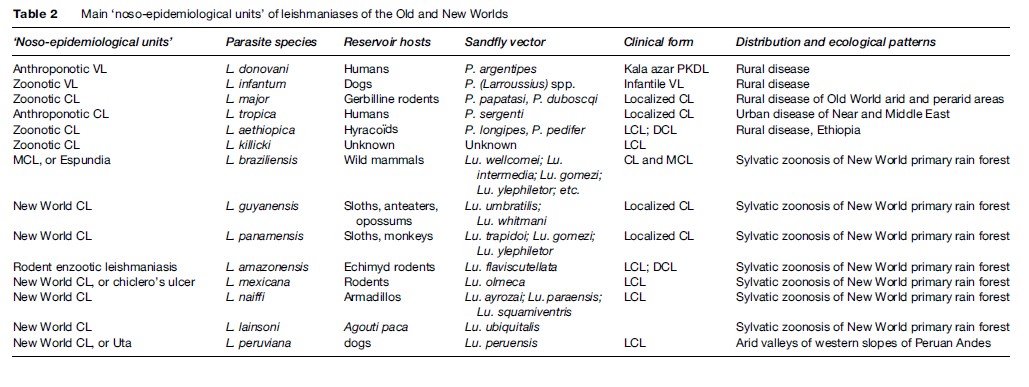
Leishmaniasis occurs in more than 88 countries, ranging over the intertropical zones of America and Africa, and extending into temperate regions of South America, southern Europe, and Asia. The limits of the disease are latitudes 45 north and 32 south. The geographical distribution is governed by those of the mammal or sand fly host species, their ecology, and their own distribution area. The leishmaniases are diseases with natural focality. They include several ‘noso-epidemiological units,’ which can be defined as the conjunction of a particular Leishmania species circulating in specific natural hosts, evolving in a natural focus with specific ecological patterns, and having a particular clinical expression (see Table 2).
Visceral Leishmaniasis
The two viscerotropic species of Leishmania have distinct life cycles and geographic distribution. The anthroponotic species L. donovani is restricted to India and East Africa. The disease is known as ‘kala-azar’ and can be complicated by a chronic CL form called ‘post-kala-azar dermal leishmaniasis.’ The zoonotic species L. infantum extends from China to Brazil, and is responsible for infantile VL. The main historical foci of endemic VL are located in China, India, Central Asia, East Africa, the Mediterranean basin, and Brazil.
Old World Cutaneous Leishmaniasis
The large majority of Old World CL cases are due to the species L. major or L. tropica and occur mainly in countries of the Near and Middle East: Afghanistan, Iran, Saudi Arabia, and Syria. L. major is responsible for zoonotic CL, of which reservoir hosts are gerbilline rodents, and vectors are sand flies of the subgenus Phlebotomus, principally Phlebotomus papatasi and P. duboscqi. This species has a wide distribution, including west, north, and east Africa, the Near and Middle East, and Central Asia.
L. tropica is an anthroponotic species with humansacting as reservoir hosts and the vector being P. (Paraphlebotomus) sergenti. It occurs in various cities of the Near and Middle East, but extends also to Morocco, where the dog is suspected to be a reservoir host in some foci.
Other species have restricted distributions: L. aethiopica in Ethiopia and Kenya, and L. killicki in Tunisia.
New World Tegumentary Leishmaniasis
In the New World, L. braziliensis is the species responsible for MCL. It has a wide distribution, extending from southern Mexico to northern Argentina. L. amazonensis has a wide South American distribution, but human cases of this rodent enzootic species are unusual. Other species have more restricted distributions: L. guyanensis (north of the Amazon basin), L. panamensis (Colombia and Central America), L. mexicana (Mexico and Central America), and L. peruviana, which is restricted to Andean valleys of Peru. With the exception of this last species, all American dermotropic species are responsible for sylvatic zoonoses occurring within the rain forest.
Disease Burden
An estimated 350 million people in more than 88 countries on four continents are ‘at risk’ of leishmaniasis. The annual incidence of new cases, including all clinical forms, is estimated between 1.5 and 2 million (Desjeux, 1999). Differences in morbidity, or even in mortality, depend on the form of the disease. The clinical expression of leishmaniasis depends not only on the genetically determined tropism of the different Leishmania species (viscero-, dermo-, or mucotropisms) but also on the immunological status of the infected patient.
Morbidity And Mortality Of VL
VL is the most severe form of leishmaniasis, with a high untreated fatality rate (around 90% in fully developed disease, though a high proportion of people may become immune without becoming sick). It is found in 47 countries, and the mean annual incidence is estimated around 500 000 new cases. At the present time, 90% of the VL cases in the world are in Bangladesh, India, Nepal, Sudan, and Brazil. The Bihar state of India experienced a dramatic epidemic with more than 300 000 cases reported between 1977 and 1990, with a mortality rate over 2%. In western Upper Nile Province in southern Sudan, an outbreak was responsible for 100 000 deaths from 1989 to 1994, in a population of less than 1 million.
In endemic VL countries, asymptomatic Leishmania infections are numerous, which do not lead to clinical outcome except in immunosuppressed individuals.
Morbidity Of CL
Whatever the species responsible, localized CL is normally a self-healing disease with spontaneous cure occurring within between 6 months and a few years, depending on the Leishmania species. Cured lesions are accompanied by permanent scars. Multiple lesions, or those located on the face, may be disabling. Some species, such as L. tropica, are responsible for long-lasting recidivans leishmaniasis.
In patients with a specifically defective cell-mediated immune response, some species (Table 2) are responsible for diffuse cutareous leishmaniasis (DCL), a severe form of the disease characterized by nodular disseminated skin lesions, subject to relapses and resistant to treatment.
Morbidity Of MCL
After a primary cutaneous self-healing lesion, the species L. braziliensis can cause later secondary involvement of facial mucosae. This form led to disfiguring and mutilating facial lesions, greatly affecting life conditions.
Risk Factors
Environmental Risk Factors
Human intrusion into a leishmaniasis natural focus represents the major risk factor of infection by exposure to sand fly vectors. For example, in the case of sylvatic zoonotic New World CL, leisure or work activities in tropical rain forests expose individuals or groups to infection (see Table 2). Population movements, such as rural to suburban migration, returnees, and refugees due to civil unrest, are factors for the spread of leishmaniasis or for dramatic epidemic outbreaks by exposing thousands of nonimmune individuals to infection (Desjeux, 2001). In several endemic countries, a dramatic increase in the number of leishmaniasis cases has occurred in the last decade: Brazil, several countries of the Middle East, North Africa, and sub-Saharan Africa (Desjeux, 2004).
Immunosuppression As An Individual Risk Factor
Immunosuppression is the major individual risk factor facilitating the development of disease from infection, particularly that caused by HIV infection. The spread of HIV to rural areas where VL is endemic, and the spread of VL to suburban areas, has resulted in a progressively increasing overlap between the two diseases, initially in the Mediterranean basin, and, more recently, in other historical foci of VL, such as East Africa, India, and Brazil. In southern Europe, between 1990 and 1998, 1616 cases were reported, 87% of which occurred in the Mediterranean area: Spain, southern France, Italy, and Portugal (Desjeux, 1999). In Spain, the prevalence of VL during HIV infection was around 2% (Alvar, 1994).
A potential health problem is the increase of organ transplantations in endemic VL countries. So far, according to a recent literature review (Basset et al., 2005), the number of reported VL cases related to organ transplantation is limited to about 50, but this is a gross underestimate and will increase with multiplication of organ transplantation programs, principally renal transplants.
Control Strategies
Intervention strategies for prevention or control are hampered by the diversity of the structure of leishmaniasis foci, with many different reservoir hosts of zoonotic forms and a multiplicity of sand fly vectors, each with a different pattern of behavior. In 1990, a WHO Expert Committee described 11 distinct eco-epidemiological entities and defined control and etoparasiticides strategies for each one (WHO, 1990).
Prevention
The aim of prevention is to avoid host infection (human or canine) and subsequent disease. It includes means to prevent intrusion of people into natural zoonotic foci and protection against infective bites of sand flies. Prevention can be at an individual or collective level. It includes the use of repellents, pyrethroid-impregnated bed nets, self-protection insecticides, indoor residual spraying, and forest clearance around human settlements. For dog protection, insecticide collars and etoparasiticides have been available for a few years.
Control
Control programs are intended to interrupt the life cycle of the parasite, to limit or, ideally, eradicate the disease. The structure and dynamics of natural foci of leishmaniasis are so diverse that a standard control program cannot be defined and control measures must be adapted to local situations. The strategy depends on the ecology and behavior of the two main targets, the reservoir hosts and the vectors, which are not mutually exclusive.
Control measures will be very different depending on whether the disease is anthroponotic or zoonotic. In the New World, almost all the leishmaniases are sylvatic, and control is not usually feasible. Even removal of the forest itself may not be effective, as various Leishmania species have proved to be remarkably adaptable to environmental degradation.
Case detection and treatment are recommended when the reservoir host is human or dog, while destruction may be the chosen intervention if the reservoir host is a wild animal. The reduced efficacy of the current antileishmanial drugs and their toxicity limit their use for systematic treatment of cases. The high level of asymptomatic infection both in human and canine hosts affects the efficiency and the feasibility of systematic case detection and treatment programs.
As far as vectors are concerned, control of breeding sites is limited to the few instances where they are known (rodent burrows for P. papatasi and P. duboscqi). Antiadult measures consist of insecticide spraying. Malaria control programs, based on indoor residual insecticide spraying, have had a side benefit for leishmaniasis incidence in several countries where a resurgence of leishmaniasis was observed after the ending of these campaigns: India, Italy, Greece, the Middle East, and Peru.
In practice, control programs include several integrated measures targeted not only at the reservoir host and/or vector but also at associated environmental changes. Health education campaigns can considerably improve the efficiency of control programs. National leishmaniasis control programs have been developed in various countries to face endemics or epidemics (India, China, and Brazil for VL; Central Asian republics of the former USSR and Tunisia for CL).
In conclusion, the leishmaniases are widely distributed and are an important public health problem in various countries. Despite progress in understanding of most facets of their epidemiology, control of leishmaniasis remains unsatisfactory. There is much still to be done.
Bibliography:
- Alvar J (1994) Leishmaniasis and AIDS co-infection: The Spanish example. Parasitology Today 10: 160–163.
- Alvar J and Jimenez M (1994) Could infected drug users be potential Leishmania infantum reservoirs? AIDS 8: 854.
- Basset D, Faraut F, Marty P, et al. (2005) Visceral leishmaniasis in organ transplant recipients: 11 new cases and review of literature. Microbes and Infection 7: 1370–1375.
- Desjeux P (1999) Global control and Leishmania/HIV co-infection. Clinics in Dermatology 17: 317–325.
- Desjeux P (2001) The increase in risk factors for leishmaniasis worldwide. Transactions of the Royal Society of Tropical Medicine and Hygiene 95: 239–243.
- Desjeux P (2004) Leishmaniasis: Current situation and new perspectives. Comparative Immunology, Microbiology and Infectious Diseases 27: 305–318.
- Garnham PCC (1965) The Leishmanias, with special reference of the role of animal reservoirs. American Zoologist 5: 141–151.
- Laveran A and Mesnil F (1903) Sur un protozoaire nouveau, Piroplasma donovani Lav. et Mesn, parasite d’une fie` vre de l’Inde. Comptes rendus de l’ Acade´mie des Sciences 137: 957–961.
- Rioux JA, Lanotte G, Serres E, et al. (1990) Taxonomy of Leishmania: Use of isoenzymes; Suggestions for a new classification. Annales de Parasitologie humaine et compare´e 65: 111–125.
- World Health Organization (1990) Control of the leishmaniases. Technical Report Series 793. Geneva, Switzerland: WHO.
- Chang KP and Bray RS (1985) Leishmaniasis. Amsterdam, the Netherlands: Elsevier.
- Dedet JP and Pratlong F (2003) Leishmaniasis. In: Cook GC and Zumla AA (eds.) Manson’s Tropical Diseases, 21st edn., pp. 1339–1371. London: Saunders.
- Farrell JP (2002) Leishmania, Vol. 4: World Class Parasites. Boston, MA: Kluwer Academic Publishers.
- Killick-Kendrick R (1990) Phlebotomine vectors of the leishmaniases: A review. Medical and Veterinary Entomology 4: 1–24.
- Molyneux DH and Ashford RW (1983) The Biology of Trypanosoma and Leishmania, Parasites of Man and Domestic Animals. London: Taylor and Francis.
- Murray HW, Berman JD, Davies CR, and Saravia NG (2005) Advances in leishmaniasis. Lancet 366: 1561–1577.
- Peters W and Killick-Kendrick R (1987) The Leishmaniases in Biology and Medicine, 2 vols. London: Academic Press.
See also:
Free research papers are not written to satisfy your specific instructions. You can use our professional writing services to buy a custom research paper on any topic and get your high quality paper at affordable price.








