This sample Mercury Research Paper is published for educational and informational purposes only. If you need help writing your assignment, please use our research paper writing service and buy a paper on any topic at affordable price. Also check our tips on how to write a research paper, see the lists of health research paper topics, and browse research paper examples.
The toxicity of mercury has been known since ancient times, and its therapeutic uses have also been explored in the past. In particular, mercurous chloride (calomel) was an important drug for syphilis treatment, and early toxicology evidence derives from patients who inevitably became mercury poisoned. Occupational health risks were described by Bernardino Ramazzini 300 years ago. Risks due to environmental contamination came to the forefront when, around 1960, Minamata disease in Japan was found to be caused by mercury pollution from a local factory. More recently, methylmercury has been discovered as a worldwide environmental hazard. Detailed risk assessments have been published by the UN Environment Programme and the U.S. Environmental Protection Agency.
Chemistry
Mercury exists in three oxidation states: Hg0 (metallic), Hgþ (mercurous), and Hg2þ (mercuric) mercury. In organometallic derivatives, mercuric mercury is covalently bound to one or two carbon atoms, and the organic part of the molecule is often an alkyl group or an alkoxialkyl group. The former compounds are more toxic, because they are more easily absorbed and more slowly metabolized. In its elemental form, mercury is a dense, silvery-white, shiny metal, which is liquid at room temperature and boils at 357 C. At 20 C, the vapor pressure of the metal is 0.17 Pa (0.0013 mmHg), and a saturated atmosphere at this temperature contains a mercury concentration of 14 mg Hg/m3, which is more than 100 times the occupational exposure limit. Mercury compounds differ greatly in their solubility, with metallic mercury being very sparingly soluble, and several salts, such as mercuric chloride, being easily soluble in water. Of relevance for its environmental fate, methylmercuric chloride is also soluble in water, but given the affinity of the methylmercury ion to organic compounds, it bioaccumulates in aquatic and marine species.
Sources Of Exposure
Mercury is emitted to the atmosphere by degassing of the earth’s surface and by resuspension and dissolution of mercury particles previously deposited. Emissions from volcanoes and other natural sources are estimated to constitute about 1000 tons per year, while the total annual emission from anthropogenic sources has been estimated to approximately 2500 tons. The major anthropogenic source is energy production from fossil fuels, especially coals with high mercury contents (UNEP, 2002).
Mercury is produced from cinnabar ore. Elemental mercury has been extensively used as a catalyst in chloralkali plants (for production of chlorine and sodium hydroxide), but modern production technology has now made the large stores of mercury superfluous. Mercury compounds have also been used in paints as preservatives or pigments, in electrical switching equipment and batteries, in measuring and control equipment (thermometers and other medical equipment), in mercury vacuum instruments, as a catalyst in chemical processes, in mercury quartz and luminescent lamps, in the production and use of high explosives using mercury fulminate, in copper/silver amalgams in dental restoration materials, and as fungicides in agriculture (especially as seed dressings). Many of these uses are now being banned or controlled. According to data from the U.S. Environmental Protection Agency, overall mercury emissions from industrial use have dropped 45% since 1990 and are still decreasing.
One of the uses of liquid metallic mercury that has escalated during the last couple of decades is informal gold mining. Alluvial deposits of fine gold particles can be extracted using elemental mercury. The gold particles are caught and dissolved in the mercury as amalgam, and the mercury can subsequently be removed by heating with a gas torch. This practice therefore exposes the gold miner to mercury vapor and also leads to extensive release of mercury to the environment, sometimes into ecologically sensitive areas. The annual consumption of mercury in such mining operations is thought to be about 650 tons, mainly in Asia, Central Africa, and Latin America. Soil contamination may remain for many decades, and sites of previous gold mining operations in the United States (e.g., Carson River, Nevada) are now recognized as being heavily contaminated with mercury, with estimated amounts of mercury exceeding 6000 tons. Deposition of sewage sludge and contamination from other industrial activities often involve mercuric salts with low solubility (i.e., sulfides). The ecological and human health implications depend on the extent of mercury methylation and bioaccumulation.
Organomercury compounds used to be produced because of their antimicrobial properties, but are now thought to find only limited use as a fungicide. Methylmercury and other organomercury compounds were extensively used for this purpose in the past, until environmental problems were discovered. The less toxic methoxymethylmercury may still find limited use for wood treatment or in the paper and pulp industry as an anti-slime agent. A related compound, thimerosal or thiomersal (ethylmercury salicylate) has been widely used as a preservative, for example, in vaccines, but is now being phased out.
The various uses of mercury and mercury compounds result in occupational exposures in a range of occupations, in most cases only involving mercury vapor and inorganic compounds. Also, the industrial use of mercury may lead to releases to the environment, due to evaporation or releases via sewage water. Localized problems relating to contamination of river systems and bays have been caused by contamination from chloralkaline plants, paper and pulp industries, and pesticide factories. In Japan, Minamata Bay and the surrounding waters became severely contaminated with methylmercury from a factory that used mercury compounds as catalyst in the production of acetaldehyde and vinyl chloride. In addition, deposition of airborne emissions from coal-fired power plants and incinerators can cause contamination of lakes and rivers. In the United States, coal burning contributes 40% of the mercury emissions, and the prevailing winds cause increased mercury deposition primarily in Eastern Canada and New England (Rice and Hammitt, 2005).
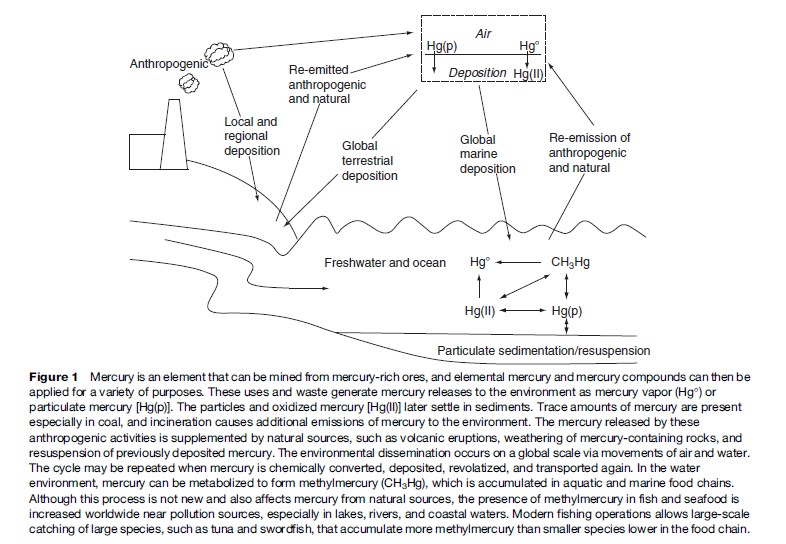
In the aquatic environment, elemental mercury is oxidized to the mercuric mercury ion (Hg2þ), which may become methylated to form methylmercury compounds, either by chemical or microbiologically catalyzed reactions. The intestinal bacterial flora of various animal species, including fish, is also, though to a much lower degree, able to convert ionic mercury into methylmercuric compounds. The opposite process, demethylation, can also occur, for example, in the liver. Methylmercury is accumulated by fish and marine mammals and attains its highest concentrations in large predatory species at the top of the aquatic and marine food chains. By this means, methylmercury enters the human diet. The mercury cycles in the environment are illustrated in Figure 1.
Environmental Exposures
Air
In remote areas, mean concentrations of total mercury in the atmosphere are reported to be in the range of 2–3 ng Hg/m3 in summer and 3–4 ng Hg/m3 in winter. Mercury concentrations in urban air usually average three to fourfold higher levels. Hot spots exceeding 10 000 ng/m3 have been reported close to industrial emissions or in connection with mercury fungicide applications. In general, airborne mercury is of limited relevance and is not of great concern in regard to human respiratory exposures, because concentrations of mercury in air are usually low, except in certain trades or near point sources. The chemistry of atmospheric mercury is complex, and recent research has elucidated some major aspects. The chemical reactions and the partitioning of mercury in gas and aqueous phases appear to determine mercury residence times in the atmosphere and its deposition at various latitudes. Most importantly, during the Arctic spring, the ultraviolet light catalyzes reactions that lead to short-lasting, but extreme levels of mercury deposition (Lindberg et al., 2002).
Few data are available on average indoor air pollution due to mercury vapor. Fatalities and severe poisonings have resulted from heating metallic mercury and mercury-containing objects at home. Elemental mercury is sometimes used for certain cultural and religious practices that may involve sprinkling mercury inside, burning it in a candle, or mixing it with perfume; such practices can create exposures that may greatly exceed currently permitted occupational exposures (Riley et al., 2001). Release of mercury from dental amalgam fillings, especially copper amalgam, is otherwise the predominant source of human exposure to inorganic mercury in the general population. Due to the availability of modern composite materials, the use of amalgam fillings is decreasing.
With an absorption rate of 80%, respiratory exposure to prevalent levels of atmospheric mercury will result in daily uptakes of up to about 200 ng. Depending upon a subject’s number of amalgam fillings, the mercury concentrations in inhaled air can range up to several thousand ng/m3. Estimated average daily absorption from amalgam fillings is thought to be of the order of 10 000 ng.
Food And Drinking Water
Mercury in drinking water is usually 5–100 ng Hg/l, with average values about 25 ng Hg/l. The mercury speciation in drinking water is poorly known, but Hg2þ is probably the predominant species present as complexes and chelates with various ligands. Its bioavailability depends on the ligand. Mercury in drinking water is usually a minor public health concern, but the presence of mercury in the water phase in the environment can lead to serious problems.
Concentrations of mercury in most foodstuffs are often below the detection limit and likely to be inconsequential. However, freshwater fish, and seafood in general (including marine mammals) constitute the dominant sources, where mercury mainly occurs in the form of methylmercury (70–90% of the total). The mercury concentrations in edible tissues of various fish species cover a wide range, mostly between 50 and 1400 ng/g. The concentration is influenced by the species, the age, and the size of the fish, and environmental factors such as pH and redox potential of the water. Large predatory fish such as pike, swordfish, and tuna as well as shark, seals, and toothed whales contain the highest average concentrations.
Other Sources Of Exposure
Occupational exposures mainly occur in chloralkali plants, mercury mines, thermometer factories, fluorescent light tube production plants, refineries, and in dental clinics. However, such exposures are now becoming rare in most industrialized countries. The most recent estimation of the number of workers exposed to mercury in the United States is 70 000, and this number is decreasing. Despite the phase-out of amalgam fillings, removal of older filling may still cause exposures in dental clinics.
The high gold price and the decreasing price of mercury have spurred an expansion of informal gold mining using mercury for amalgamation. Exposures may exceed those previously occurring in the most hazardous occupations in industrialized countries. Serious mercury exposures may occur when the gold amalgam is heated.
This process is often carried out under field conditions or in small gold vending shops without proper ventilation. An estimated 10 million workers in Africa, Latin America, and Asia are exposed to dangerously high concentrations of elemental mercury through such activities.
Additional exposures can occur from the use of pharmaceuticals, in particular thimerosal, which has been widely applied as a preservative of vaccines and immunoglobulins. With up to 100 mg mercury per injection, this preservative caused substantial bolus doses of mercury, especially on a body weight basis, in connection with childhood immunizations. Skin-lightening lotions are popular in Arabian and African countries and often contain mercury concentrations of about 1000 mg/kg; some products may reach concentrations in the percent range. Although mercury may be absorbed through the skin, consumers are usually not warned about the toxic contents.
Relative Importance Of Mercury Exposures
Human exposure to elemental mercury and inorganic mercury compounds can be substantial in certain trades, but otherwise releases from dental amalgams and the use of skin-lightening lotions constitute the major mercury sources. A small amount of inorganic mercury originates from food, while the amount present in drinking water is insignificant.
Worldwide, the main determinant of mercury exposure is the intake of fish and seafood products, where the mercury mainly occurs in the form of methylmercury. The highest exposures are found in Arctic populations, who eat marine mammals. Increased levels also occur in fishing communities and in, for example, Japanese and Mediterranean populations, who frequently eat fish high in the food chain. Average exposures are lower in countries such as the United States, where NHANES III data suggest that only 40% of Americans consume fish at least once a week, while 1–2% consume fish or shellfish almost daily. From a public health point of view, fish and seafood contain essential nutrients and are recommended as an important part of a varied diet. Thus, dietary advisories need to emphasize the types of fish that are low in mercury and therefore provide the most nutrients with the lowest mercury exposures.
Total dietary mercury intake has usually been measured as part of market basket surveys or as part of specific monitoring. Probabilistic analyses based on dietary questionnaire data and fish analyses suggest that small children, on a body weight basis, may receive a higher exposure than adults. Incomplete information is available on the distribution of high-end intakes from seafood diets, especially among vulnerable population groups, such as pregnant women and children.
Metabolic Fate In The Body
Absorption
Approximately 80% of inhaled mercury vapor is absorbed via the lungs and retained in the body. Elemental mercury is poorly absorbed in the gastrointestinal tract, although increased blood-mercury concentrations have been measured in humans after accidental ingestion of several grams of metallic mercury.
The absorption of inhaled aerosols of inorganic mercury will depend on particle size and solubility, similar to other airborne particles. Among few relevant experimental studies, 45% of deposited mercuric oxide aerosols in the airway of dogs were cleared in less than 24 h, and the remainder cleared with a half-life of 33 days. Ten to fifteen percent of an oral, nontoxic dose of mercuric mercury is absorbed from the gastrointestinal tract in adults and retained in body tissues, but considerable individual variations may exist. In children, the gastrointestinal absorption is probably greater. Mercury compounds can also be slowly absorbed through the skin.
With regard to organomercury compounds, human poisoning cases have been caused by inhalation, thus suggesting that a large fraction of these compounds are absorbed into the blood. Alkylmercury compounds are absorbed almost completely in the gastrointestinal tract. Certain methylmercury compounds are probably absorbed through the skin. These pathways can be bypassed when injected into the body, as in the case of ethylmercury in vaccines preserved with thimerosal.
Distribution
After exposure to mercury vapor, the element is found in blood as physically dissolved elemental mercury. Within a few minutes, the mercury is oxidized to mercuric mercury in the erythrocytes, a reaction catalyzed by catalase. Thus, following short-term exposure to mercury vapor, the maximum concentration of mercury in erythrocytes is seen after less than 1 h, whereas plasma levels peak after about 10 h. Before oxidation, elemental mercury readily crosses cell membranes, including the blood–brain barrier and the placental barrier. After oxidation, the Hg2þ ions (or complexes) are distributed in the body via the blood. The kidneys and the brain are the main retention sites after exposure to mercury vapor, whereas absorbed inorganic mercury salts are mainly deposited in the kidneys. The uptake and/or elimination of mercury after exposure to mercury vapor can be altered by a moderate intake of ethanol, possibly due to inhibition of catalase. Thus, the amount of mercury in red blood cells of humans exposed to mercury vapor is significantly reduced in the humans given an alcoholic beverage before the mercury exposure.
The kidneys are the predominant site of inorganic mercury retention. Immediately after oral exposure, some accumulation occurs in the cells of the mucous membranes of the gastrointestinal tract, but most of it is later eliminated due to cell shedding and does not reach the systemic circulation. Mercuric mercury in blood is divided between erythrocytes and plasma in about equal amounts. In erythrocytes, mercury is probably to a large extent bound to sulfhydryl groups on the hemoglobin molecule and possibly also to glutathione. The distribution between different plasma protein fractions varies with dose and time after exposure.
Mercuric mercury crosses the blood–brain and placental barriers only to a limited extent. However, mercuric mercury accumulates in the placenta, fetal membranes, and amniotic fluid, thus suggesting that a slow placental passage occurs. The rate of uptake from blood and different organs varies widely; so does the rate of elimination from different organs. However, the kidneys always constitute the dominating mercury pool in the body after exposure to mercuric mercury. Inorganic divalent mercury can induce metallothionein production in the liver, and a large proportion of the mercury in the kidneys is soluble and bound to metallothionein.
For methylmercury, the pattern of tissue distribution is much more uniform than after inorganic mercury exposure, except in red cells, where the concentration is 10–20 times greater than the plasma concentration. Methylmercury readily crosses the blood–brain and placental barriers. In the fetus, methylmercury is accumulated and concentrated, especially in the brain. As with other forms of mercury, the kidneys retain the highest tissue concentration, but the brain still contains approximately fivefold higher concentrations than blood. Methylmercury accumulates in hair in the process of formation of hair strands, with average concentrations being roughly 250-fold higher than in blood. Methylmercury undergoes biotransformation to inorganic mercury by demethylation, particularly in the gut. Ethylmercury is less stable than methylmercury and it therefore shows similarities with inorganic mercury compounds.
Elimination
After short-term exposures to mercury vapor, about onethird of the absorbed mercury will be eliminated in unchanged form through exhalation, whereas the remaining mercury will be eliminated as mercuric mercury mainly through feces. Assuming first-order kinetics for the clearance of urinary mercury after exposure to mercury vapor, the median half-life was found to be 41 days. Blood concentrations can serve as indicators of recent mercury vapor exposure, though speciation must be carried out in order to eliminate possible influence of dietary intake of methylmercury from contaminated marine food.
Excretion of absorbed inorganic mercury is mainly via urine and feces, the rates by each pathway being roughly equal. The whole-body half-life in adults is also 30–40 days. The elimination of inorganic mercury follows a complicated pattern with biological half-lives that differ according to the tissue and the time after exposure. Thus, measurement of mercury excretion will not necessarily reflect concentrations of inorganic mercury in the critical organs, that is, the brain or the kidneys, under different exposure conditions. One important consequence is that concentrations of mercury in urine or blood may be low quite soon after exposure has ceased, even though concentrations in the critical organs may still be high.
Methylmercury is slowly demethylated in the gut, and enterohepatic recirculation of methylmercury explains that most, if not all, of the mercury excreted is in the demethylated inorganic form. Some elimination also occurs via urine. The whole-body half-life of methylmercury is generally 45–60 days, and longer retention times may occur in breast-fed infants, who do not have intestinal microorganisms capable of demethylation. Laboratory animal studies have shown that, following acute dosage with methylmercury, blood mercury concentrations will initially reflect organ concentrations reasonably well, but, with time, an increasing fraction of the body burden will be in the brain, muscles, and kidney.
The blood concentration might be a useful indicator of the body burden of mercury, although the erythrocyte mercury concentration is more specific for methylmercury exposure. Thus, if exposure to mercury vapor or other inorganic mercury compounds is suspected, mercury should be speciated or a serum sample analyzed. Mercury in hair, when measured along the length of a hair strand, has also been used as an indicator of past blood levels. However, hair mercury concentrations may be augmented by the binding of exogenous mercury to the surface, and permanent waving may leach mercury from the hair. Prenatal exposure to methylmercury is best determined as the mercury concentration in cord blood, but cord tissue also seems a useful sample for mercury analysis.
Adverse Health Effects
Acute Poisoning
Acute poisoning with mercury vapor may cause a severe airway irritation, chemical pneumonitis, and, in severe cases, pulmonary edema. Ingestion of inorganic compounds may cause gastrointestinal corrosion and irritation such as vomiting, bloody diarrhea, and stomach pains. Subsequently, shock and acute kidney dysfunction with uremia may ensue. Cutaneous exposure to mercury compounds may result in local irritation, and mercury compounds are among the most common allergens in patients with contact dermatitis.
Chronic Effects Of Mercury Vapor And Inorganic Mercury
Chronic intoxication may develop as soon as a few weeks after the onset of a mercury exposure. More commonly, however, the exposure has lasted for several months or years, and an insidious onset may complicate early diagnosis. The symptoms depend on the degree of exposure and the kind of mercury in question. The symptoms mainly involve the oral cavity, the peripheral and central nervous systems, and the kidneys. Elemental mercury present in vapor is oxidized in the blood to mercuric mercury, which does not pass the blood–brain barrier. The non-neurotoxic effects of absorbed mercury vapor and other inorganic mercury compounds are therefore similar.
In exposures to mercury vapor, the central nervous system is the critical organ, and a classic triad of symptoms occurs: Erethism, intention tremor, and gingivitis. Induction of tremor by mercury vapor has been reported when urinary excretion levels exceed 50 mg/l (0.25 mmol/l). The fine intention tremor of fingers, eyelids, lips, and tongue may progress in severe cases to spasms of arms and legs. A jerky micrographia is typical as well. The changes in the central nervous system result in psychological effects known as erethism, that is, restlessness, irritability, insomnia, concentration difficulties, decreased memory and depression, sometimes in combination with shyness, unusual psychological vulnerability, and anxiety. Early stages of erethism have been dubbed micromercurialism, where the main symptoms appear to be decreased memory, dizziness, and irritability. While similar symptoms may be described by patients who attribute their ill health to mercury from their dental fillings, the symptoms are usually nonspecific, and their relation to mercury release from amalgams may be impossible to verify when other causation is possible. A recent study found no adverse effects on neurodevelopment in children with amalgam fillings (Bellinger et al., 2006).
Limited information is available on effects of mercury vapor on early stages of the human life cycle. Effects on pregnancy and birth in women occupationally exposed to mercury vapor have been reported, but insufficient details were available to evaluate dose–response relationships. In children, pink disease may occur, as described later in this section.
Severe exposure to inorganic mercury causes an inflammation of gingiva and oral mucosa, which become tender and bleed easily. Salivation is increased, most obviously so in subacute cases. Often the patient complains of a metallic taste in the mouth. Especially when oral hygiene is bad, a gray border is formed on the gingival edges.
In long-term exposures, the target organ for inorganic mercury compounds is the kidneys. In general, the early renal effects of mercury appear to be reversible after cessation of exposure. The nephrotoxic effects include proximal tubular damage, as indicated by an increased excretion of small proteins in the urine (e.g., beta2microglobulin). Glomerular damage seems to be caused by an autoimmune reaction to mercury complexes in the basal membrane, as demonstrated in experimental studies, although human evidence is inconclusive in this regard.
In children, a different syndrome is seen, the so-called pink disease or acrodynia, diagnosed most frequently in children treated with teething powders, which contained calomel, and also occasionally seen in children who had inhaled mercury vapor (e.g., from broken thermometers). A generalized eruption develops, and the hands and feet show a characteristic, scaly, reddish appearance. In addition, the children are irritable, sleep badly, fail to thrive, sweat profusely, and have photophobia. This condition was extremely common until 30 years ago, when the etiology was finally found and teething powders were phased out.
Organic Mercury Compounds
Intoxications with alkoxialkyl or aryl compounds are similar to intoxications with inorganic mercury compounds, because these organomercurials are relatively unstable. Alkyl mercury compounds, such as methylmercury, result in a different syndrome due to the stability of the mercury–methyl binding. The earliest symptoms in adults are paresthesias in the fingers, the tongue, and the face, particularly around the mouth. Later on, disturbances occur in the motor functions, resulting in ataxia and dysphasia. The visual field is decreased, and, in severe cases, the result may be total blindness. Similarly impaired hearing may progress to complete deafness. This syndrome appeared as Minamata disease in Japan as a result of methylmercury contamination from a local factory. Epidemics also occurred when methylmercurytreated seed grain was used for baking or animal feed in Iraq and elsewhere.
Children and the fetus are more susceptible to the toxic effects of methylmercury than are adults, and congenital methylmercury poisoning may result in a cerebral palsy syndrome, even though the mother remains healthy or suffers only minor symptoms due to the exposure. In populations with a high consumption of fish or marine mammals, methylmercury intakes may approach the levels that resulted in such serious disease in Japan and Iraq. A long-term follow-up study of a Faroese birth cohort has shown that prenatal exposure to methylmercury results in neuropsychological and neurophysiological deficits that are detectable through adolescence. Developmental delays appear to be related to maternal hair mercury concentrations of 1–3 mg/g (Grandjean et al., 1997) (i.e., cord blood concentrations of 4–12 mg/l). Some of the adverse effects on neurodevelopment may be masked by beneficial effects of seafood nutrients. In other words, full benefits from fish and seafood diets require that methylmercury exposures are minimized.
In adults, the clinical effects, such as paresthesias, appear to occur when blood concentrations are above 200 mg/l (1 mmol/l). However, these neurotoxic effects may not be the main concern. Recent epidemiological studies suggest that adverse cardiovascular effects may occur at much lower exposures that are prevalent among people regularly eating seafood (Virtanen et al., 2005). Although the full implications of these findings are not yet clear, they suggest that methylmercury can cause adverse effects in the general population and that benefits of eating seafood must take into account the impact of methylmercury exposures.
With regard to mercury compounds used in vaccines, it seems that thimerosal easily breaks down to ethylmercury, which remains in the body for a shorter time than methylmercury. In 2001, the U.S. Institute of Medicine’s Immunization Safety Review Committee indicated ‘‘that the epidemiological evidence was inadequate to either accept or reject a causal relationship’’ between vaccines containing the preservative thimerosal and neurodevelopmental disorders (autism, attention deficit/hyperactivity disorder, and speech or language delay). It has more recently concluded that the current evidence does not support this theory with regard to thimerosal-containing vaccines and autism, and the evidence on the possible adverse health effects of thimerosal is therefore still unclear (Institute of Medicine, 2004).
Sufficient evidence exists that methylmercury chloride is carcinogenic to experimental animals. In the absence of comprehensive epidemiological data, methylmercury is therefore considered a possible human carcinogen (class 2B). The U.S. Environmental Protection Agency has classified both inorganic mercury compounds and methylmercury as possible human carcinogens.
Prevention
Prevention should preferably start at the source. Anthropogenic releases of mercury clearly exceed natural sources, and they often occur in the proximity of human populations and sensitive ecosystems. Worldwide, coal-fired power plants and incinerators are important point sources. When mercury emissions from such sources were controlled in Florida and Massachusetts, methylmercury contamination of local freshwater fish significantly decreased after a few years. However, on a global scale, much still remains to be done, especially with regard to pollution from burning of mercury-containing coals in East Asia.
Uses of mercury can be better controlled, and such efforts will also decrease the amount of mercury that may reach incinerators. The European Union has enacted a ban on mercury exports, an action that was important because of the large surplus mercury available from outdated chloralkali plants. A variety of mercury uses are being phased out, such as mercury in household thermometers, and some countries and some communities have initiated household collection of old thermometers, and used batteries and fluorescent light bulbs for recycling or safe storage. Dental health care emphasizes caries prevention, and amalgam fillings are now used much less than in the past. Some countries advise pregnant women not to have dental work performed. Suitable alternative restorative materials are now available for most purposes, although some may carry a risk of allergy development.
With regard to occupational exposures, the World Health Organization has recommended that long-term mercury vapor exposures should be limited to a time weighted average limit of 25 mg/m3. The corresponding limit for inorganic mercury is 50 mg/m3.
Biological monitoring is crucial in the diagnosis of mercury exposure and in the control of occupational exposure levels. Although blood concentrations are highly useful, they do not reflect mercury retained in the brain, where mercury from vapor inhalation has a half-life of several years. Urine levels are usually preferred as an indicator of occupational exposures to inorganic mercury species, and a limit of 50 mg mercury/g creatinine (28 mmol/mol creatinine) has been recommended.
Few countries monitor the mercury exposures of the general population. The mercury concentration in whole blood reflects recent exposures, and in fish-eating populations, the blood level indicates the level of methylmercury exposure. Hair mercury analyses have proved very useful for screening as fairly reliable indicators of individual exposures during past months, although the analytical result is sometimes imprecise due to effects of external factors on the cumulated hair concentration. In order to protect the most sensitive populations against methylmercury toxicity, blood-mercury concentrations should be kept below 4 mg/l, and hair-mercury below 1 mg/g. These levels correspond to the Reference Dose (RfD) used by the U.S. EPA as an estimate of a daily exposure to the human population (including sensitive subgroups) that is likely to be without an appreciable risk of deleterious effects during a lifetime. Data on blood-mercury concentrations in the general population of the United States suggest that between 5% and 10% of different population sections exceed the Rf D, although the fraction is higher in certain subpopulations who depend on seafood or freshwater fish. Average concentrations are also higher in countries such as Japan.
These limits are based on results from three prospective cohort studies in the Faroe Islands, New Zealand, and the Seychelles Islands, where increased exposures to methylmercury occur due to frequent seafood consumption (National Research Council, 2000). The limit has also been translated to recommendations concerning total dietary intakes of methylmercury. The reference dose has been calculated to 0.1 mg/kg body weight per day (National Research Council, 2000). This level was based on the results of the prospective study in the Faroe Islands and also on a joint analysis of three major studies. Using a slightly different calculation and a smaller uncertainty factor, the Joint FAO/ WHO Expert Committee on Food Additives proposed a slightly higher provisional tolerable weekly intake (PTWI) at 1.6 mg/kg body weight per week.
Limits have been in existence for many years for mercury in food, in particular for (methyl)mercury in fish and seafood products. They are not based on any specific risk assessment, but aims at excluding, for example, particularly contaminated swordfish from the market if it exceeds a concentration of 1.0 mg/g. Most other fish must satisfy a limit of 0.5 mg/g, although some countries use a lower limit. In the European Union, it was discovered that several species of fish often exceeded the 0.5 limit, and a revised directive was then issued that they needed to comply with the 1.0 limit only. This pragmatic step was taken to protect the interests of the fishing industry, while recognizing that these limits by themselves do not constitute a useful tool to control mercury accumulation in food chains.
However, if a dietary intake of two fish dinners (400 g) per week is considered optimal, then the reference dose for mercury may be exceeded, if the average mercury concentration in fish is higher than 0.1–0.2 mg/g. Accordingly, the U.S. Environmental Protection Agency has recommended fish advisories at freshwater lakes and waterways, and mercury is now the most common reason for fish advisories in the United States. Likewise, in countries such as Sweden, consumption of sports fish is banned from many lakes that were severely polluted in the past, and where the mercury concentration in fish has not yet decreased to a safe level. Food safety authorities in various countries advise pregnant women to avoid species that are known to contain high methylmercury concentrations, and the general population to limit the frequency of such meals.
Such recommendations may stimulate a controversy between nutrition, toxicology, and commerce interests. However, while recognizing that seafood and freshwater fish constitute an important source of energy and essential nutrients, full benefits of these foods will require that exposures to toxic methylmercury be minimized. It will take many decades to reduce the ecological impact of anthropogenic mercury pollution, and in the meantime, dietary advisories must be accepted as an important tool for prevention.
Bibliography:
- Bellinger DC, Trachtenberg F, Barrega˚ rd L, et al. (2006) Neuropsychological and renal effects of dental amalgam in children: A randomized clinical trial. Journal of the American Medical Association 295: 1775–1783.
- Grandjean P, Weihe P, White RF, et al. (1997) Cognitive deficit in 7-year-old children with prenatal exposure to methylmercury. Neurotoxicology and Teratology 19: 417–428.
- Institute of Medicine (2004) Immunization Safety Review: Vaccines and Autism. Washington, DC: National Academy Press.
- Lindberg SE, Brooks S, Lin CJ, et al. (2002) Dynamic oxidation of gaseous mercury in the Arctic troposphere at polar sunrise. Environmental Science and Technology 36: 1245–1256.
- National Research Council (2000) Toxicological Effects of Methylmercury. Washington, DC: National Academy Press.
- Rice G and Hammitt JK (2005) Economic valuation of human health benefits of controlling mercury emissions from U.S. coal-fired power plants. Northeast States for Coordinated Air Use Management (NESCAUM). Boston, MA: Harvard center for Risk Analysis.
- Riley DM, Newby CA, Leal-Almeraz TO, and Thomas VM (2001) Assessing elemental mercury vapor exposure from cultural and religious practices. Environmental Health Perspectives 109: 779–784.
- United Nations Environment Programme (UNEP) (2002) Global Mercury Assessment Report. Geneva, Switzerland: UNEP chemicals.
- Virtanen JK, Voutilainen S, Rissanen TH, et al. (2005) Mercury, fish oils, and risk of acute coronary events and cardiovascular disease, coronary heart disease, and all-cause mortality in men in eastern Finland. Arteriosclerosis, Thrombosis, and Vascular Biology 25: 228–233.
- Budtz-Jørgensen E, Grandjean P, and Weihe P (2007) Separation of risks and benefits of seafood intake. Environmental Health Perspectives 115: 323–327.
- Debes F, Budtz-Jørgensen E, Weihe P, White RF, and Grandjean P (2006) Impact of prenatal methylmercury toxicity on neurobehavioral function at age 14 years. Neurotoxicology and Teratology 28: 363–375.
- Grandjean P and Nielsen JB (2007) Mercury. In: Lippman M (ed.) Environmental Toxicants: Human Exposures and Their Health Effects, 3rd edn. New York: Wiley.
- Murata K, Weihe P, Budtz-Jørgensen E, Jørgensen PJ, and Grandjean P (2004) Delayed brainstem auditory evoked potential latencies in 14-year-old children exposed to methylmercury. The Journal of Pediatrics 144: 177–183.
- US Environmental Protection Agency (2001) Water Quality Criterion for the Protection of Human Health: Methylmercury. Washington, DC: US Environmental Protection Agency.
See also:
Free research papers are not written to satisfy your specific instructions. You can use our professional writing services to buy a custom research paper on any topic and get your high quality paper at affordable price.