This sample Viral Diarrhea Research Paper is published for educational and informational purposes only. If you need help writing your assignment, please use our research paper writing service and buy a paper on any topic at affordable price. Also check our tips on how to write a research paper, see the lists of health research paper topics, and browse research paper examples.
Introduction
Acute gastroenteritis is among the most common illnesses affecting humans and has greatest impact at the extremes of age, severely affecting children and the elderly. The spectrum of disease can range from asymptomatic infections to severe disease with dehydration, which can be fatal. Diarrheal disease continues to be a major cause of mortality in young children, particularly in developing countries. Prior to 1972, the etiology of most episodes of gastroenteritis was unknown, and cases were attributed to a multitude of causes, including teething, weaning, diet, old age, drugs, and malnutrition, as well as infections. Intensive investigation of enteric infections in the past three decades has resulted in the discovery of many new viral agents filling in the ‘diagnostic gap’ in diarrheal disease. With the identification of the Norwalk virus, rotavirus, astroviruses, enteric adenoviruses, and other caliciviruses in the 1970s and subsequently, it has become increasingly clear that viruses cause a significant proportion of the enteric illnesses that did not earlier have a defined etiology. With improvements in sanitation and hygiene, and better standards of living, the proportion of diarrheal disease attributed to bacteria has decreased, resulting in an increase in the proportion of cases associated with viral infections. Developments of new assays to identify viruses have also resulted in the ability to identify the viral etiology of episodes and epidemics of gastroenteritis.
Epidemiology Of Viral Gastroenteritis
Approximately 5 billion episodes of diarrhea occur worldwide annually, with virtually all children infected with the most common agents by the age of 3 years. Widespread use of oral rehydration therapy in the past two decades has resulted in a significant decrease in mortality due to diarrhea, by about 50%, to 2.2 million annual deaths, occurring mainly in developing countries. Infections with gastroenteritis viruses differ from bacterial enteric infections in that they affect children in both developing and developed countries, suggesting that they may also be transmitted by means unrelated to contaminated food or water. Although feco–oral spread is the major route of transmission for all enteric viruses, transmission through contact, fomites, and a respiratory route has been suggested based on the recovery of these viruses from inanimate objects during outbreaks.
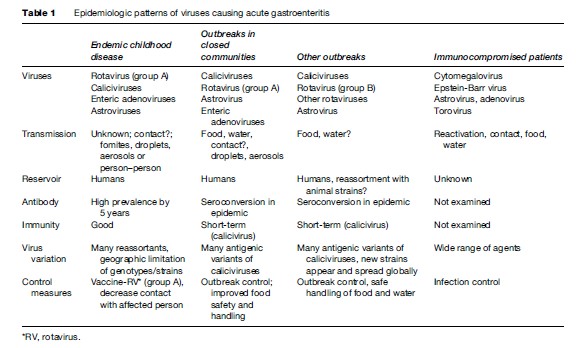
The four distinct patterns of viral gastroenteritis – endemic childhood diarrhea, outbreaks in closed communities, other foodor waterborne outbreaks among wider communities, and viral gastroenteritis in immune compromised patients – reflect the differences in the pathogens, transmission, and host response. These have a direct bearing on strategies for prevention and control (Table 1).
Viral Gastroenteritis In Children
The highest rates of viral gastroenteritis occur between 3 and 24 months of age. Protection in early infancy is believed to be mediated by maternal antibodies, followed by acquisition of protective immunity through repeated exposure in early childhood. This pattern is seen with all viral enteropathogens. However, for the caliciviruses and astroviruses, immunity is not long-lasting, suggesting waning of immunity or lack of cross-protection between different viral strains.
Childhood diarrhea is best exemplified by the group A rotaviruses, but a similar pattern of infection and illness is seen with enteric adenoviruses, astroviruses, and sapoviruses. These agents infect children during the first few years of life, with first infections being symptomatic and protecting against subsequent disease. Disease is caused by a limited number of specific serotypes and incidence decreases with increasing age.
Group A rotaviruses are the main cause of severe diarrhea in children under 5 years of age, and cause more than 130 million episodes per year throughout the world, and approximately 600 000 deaths annually. Reports from Europe, Australia, and the United States indicate that rotavirus may be responsible for 20–60% of cases of gastroenteritis requiring hospitalization. Recently, from Asia, it has been estimated that 45% of gastroenteritis requiring admission is due to rotavirus, a higher percentage than previously recorded. Of the ‘non-group A’ rotaviruses, group B rotavirus has been identified in epidemic outbreaks of severe diarrhea in adults in China and in symptomatic infections in children. Outbreaks of diarrhea due to group C rotavirus have been identified in Asia, Europe, and South and North America, but are not common. Human caliciviruses, consisting mainly of noroviruses and sapoviruses, are associated mainly with milder cases of gastroenteritis in children, causing greater than 20% of diarrheal disease in children in the community. In many studies, noroviruses are the second most common cause of gastroenteritis in children, following rotaviruses. Enteric adenoviruses cause 10% of diarrheal disease in reports from developed countries and have a variable incidence of 2% to 30% depending on the region in developing countries. Astroviruses were found in approximately 1% of cases, when electron microscopy was employed for detection, but with the availability of a commercial enzyme immunoassay and molecular techniques, the percentage of detection has increased in hospital and community settings to 5% to 10%.
Viral Gastroenteritis Outbreaks In Semi-Closed Or Closed Communities
Outbreaks in closed or semi-closed communities such as old-age homes, cruise ships, and hospitals are mainly due to caliciviruses. Norovirus infections are a significant cause of outbreaks in adults in nursing homes and residential care facilities and can lead to an increased need for hospital care and increased mortality. Nosocomial outbreaks occurring in hospitals have required the closure of wards in order to control infections.
Outbreaks due to noroviruses and to mixed viral infections have been reported among military personnel. Outbreaks of norovirus gastroenteritis are also being recognized and are occurring with increasing frequency on cruise ships. Attack rates as high as 30% have been observed among cruise ship passengers, and repeated outbreaks have continued even after cleaning and disinfection protocols were instituted on successive voyages.
In addition to infections in adult patients, viral agents of gastroenteritis are an important cause of nosocomial infection in pediatric units. Between 20% and 50% of cases of gastroenteritis caused by rotavirus in hospitals are considered to be of nosocomial origin, and nosocomial viral enteric infections have been documented in up to 6% of children admitted for more than 72 hours in both developed and developing countries. Infections in older individuals are usually due to caliciviruses, although adenovirus infections have been documented. The prevalence and transmission of nosocomial infection may be explained by asymptomatic patients who excrete the virus and the relative resistance of these viruses to normal disinfectants.
Food- And Waterborne Disease
The microbiological contamination of food and water is a significant global problem. It is estimated that there are approximately 1.5 billion cases and over 3 million deaths worldwide annually. The microorganisms associated with about 50% of the foodborne disease outbreaks still go unrecognized, particularly those occurring in developing countries. The apparent failure to confirm a viral etiology in such outbreaks has been due largely to the lack of available tests, unavailability of food or water specimens, and the failure to report outbreaks of mild gastrointestinal disease. All of these factors have resulted in a drastic underestimate of the true scope and importance of food- or waterborne viral infection.
The most common types of food- and waterborne viral disease are infectious hepatitis due to hepatitis A virus and acute viral gastroenteritis associated with the human caliciviruses. Noroviruses, transmitted by the fecal–oral and the aerosol routes, are the most common cause of outbreaks of nonbacterial gastroenteritis in industrialized countries, but data from developing countries are lacking. Noroviruses are responsible for an estimated 67% of all illnesses caused by known foodborne pathogens and for 96% of nonbacterial gastroenteritis in the United States. Many outbreaks can be associated with the consumption of primarily or secondarily contaminated foods. Shellfish and fruit implicated in outbreaks have been shown to be contaminated at the site where these foods are harvested or produced, whereas other foods, such as salads, cold foods, and sandwiches, have caused outbreaks after being contaminated by food handlers at the site of food preparation. Shellfish, in particular oysters and clams that are raw or insufficiently cooked, is associated with noroviral outbreaks, frequently occurring because these shellfish filter contaminated seawater to feed and hence result in a concentration of virus. Foodborne outbreaks due to rotaviruses, parvoviruses, and astroviruses are also occasionally reported. Water is also a common source of outbreaks and may include water from municipal supplies, wells, recreational lakes, swimming pools, and ice machines. Rotaviruses, caliciviruses, and some adenoviruses are important causes of waterborne disease outbreaks. Post-recovery and secondary transmission are a particular concern in infections due to these agents.
Viral Gastroenteritis In The Immunocompromised Patient
The main viral causes of severe gastroenteritis in immunosuppressed patients are cytomegalovirus (CMV) and Epstein–Barr virus (EBV), which mainly affect patients with AIDS and transplant recipients. CMV is a frequent pathogen in diarrhea associated with AIDS with CD4 counts under 100 cells/mm3. Other viruses that produce HIV-associated gastroenteritis include astrovirus, picobirnavirus, calicivirus, and adenovirus. There is evidence of gastroenteritis due to astrovirus and adenovirus in both child and adult bone marrow transplant recipients. Caliciviruses are now being increasingly recognized as a cause of chronic diarrhea in patients undergoing transplants. Toroviruses have been found in association with diarrhea in immunocompromised children.
Etiologic Agents
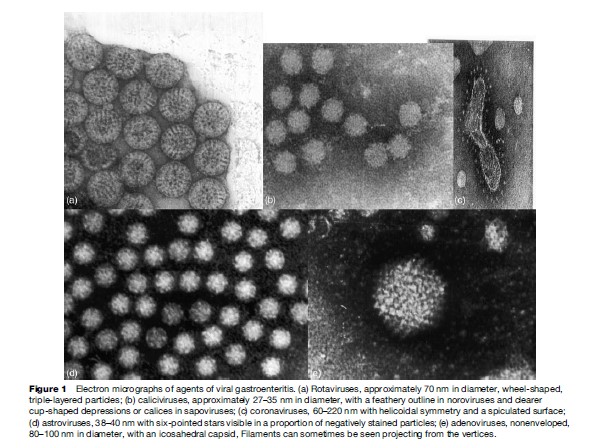
Criteria to define a virus as an etiologic agent of gastroenteritis include (1) the identification of the virus more frequently in study participants with diarrhea than in controls, (2) the demonstration of an immune response to the specific virus, and (3) the demonstration that the beginning and end of the illness correspond to the onset and termination of virus shedding, respectively. So far, these include human caliciviruses, rotaviruses, astroviruses, and the enteric adenoviruses. In immunocompromised patients, gastrointestinal CMV and EBV infections also cause significant morbidity. Coronaviruses, toroviruses, the Aichi virus, and picobirnaviruses have also been found to be associated with diarrhea in some studies, but definitive data are not yet available. Similarly, in conditions such as HIV, it has been difficult to obtain definitive data on the role of enteric viruses in the causation of symptoms. The study of rotaviruses, enteric adenoviruses, and astroviruses has been facilitated greatly by the ability to propagate these viruses in cell culture, which has allowed the production of reagents for use in diagnostic studies, a better understanding of factors correlated with immunity to infection, and the elucidation of each virus’s life cycle. Although human caliciviruses have defied numerous attempts to propagate them in cell culture to date, recent developments in their study by using molecular biology techniques have increased our ability to diagnose and study infections due to these agents (see Figure 1).
Rotaviruses
Structure And Classification
Rotaviruses are double-stranded RNA viruses constituting a genus within the family Reoviridae. The mature virus particles are triple layered, approximately 70 nm in diameter, and possess icosahedral symmetry. The rotavirus genome consists of 11 segments of doublestranded RNA, which code for 6 structural viral proteins and 6 nonstructural proteins. Of the nonstructural proteins, NSP4 is of particular interest, as it has enterotoxin-like activity and can induce diarrhea in mice. The classification of rotavirus into 7 different groups (A–G) is based on the antigenic specificity of the VP6 capsid proteins. Of the 7 groups, only groups A, B, and C are known to infect humans. Severe, life-threatening disease in children worldwide is caused predominantly by group A rotaviruses. Variability in the genes encoding the two outer capsid proteins VP7 and VP4 forms the basis of the current strain typing of group A rotaviruses into G and P genotypes, respectively. All known G serotypes correspond with genotypes; more P genotypes than serotypes have been identified. At least 15 G genotypes and 25 P genotypes have been identified to date. Currently, the strains most commonly reported include G1P[8], G2P[4], G9P[8], G4P[8], and G3P[8], although unusual G and P types have been reported from different parts of the world.
The rapid evolution of rotaviruses by a variety of mechanisms provides one of the major challenges in epidemiological studies. These mechanisms include genetic drift, where an accumulation of point mutations generates genetic lineages leading to the emergence of antibody escape mutants, and genetic shift through gene reassortment during dual infection of a single cell. Hence methods of virus typing need to be regularly monitored and updated to identify emerging novel strains of epidemiological importance.
Clinical Features
Rotaviruses induce a clinical illness characterized by vomiting, diarrhea, abdominal discomfort, fever, and dehydration (or a combination of some of these symptoms) that occurs primarily in infants and young children and may lead to hospitalization for rehydration therapy. Fever and vomiting frequently precede the onset of diarrhea. Milder gastroenteric illnesses that do not require hospitalization are common. The highest attack rate is usually among infants and young children 6 to 24 months old. Neonatal infections are largely asymptomatic. Deaths from rotavirus gastroenteritis may occur from dehydration and electrolyte imbalance. The severity of diarrhea is measured by the Vesikari score which includes duration and severity of diarrhea and vomiting, associated fever, and degree of dehydration. In older children and adults, rotavirus gastroenteritis occurs infrequently, although subclinical infections are common.
Rotaviruses also induce chronic symptomatic diarrhea in immunodeficient children. Rotavirus infections can be severe and sometimes fatal in individuals of any age who are immunosuppressed for bone marrow transplantation. Rotavirus infections have also been associated with necrotizing enterocolitis and hemorrhagic gastroenteritis in neonates in special-care units. Recently, rotavirus antigenemia has been described early in infection in children requiring hospitalization and RNA has been extracted from serum of antigenemic children and cerebrospinal fluid (CSF) of children with seizures, but the clinical significance of these findings requires further investigation.
Pathophysiology
Rotaviruses infect the mature enterocytes on the tips of small intestinal villi, leading to villous atrophy with secondary hyperplasia of the crypts. It has been proposed that cellular damage is secondary to villous ischemia. The mechanism that induces the production of diarrhea is not well understood, although it appears to be mediated by the relative decrease of villous epithelium absorption in relation to the secretory capacity of the crypt cells, as well as the possible action of NSP4, the viral enterotoxin that has been shown to cause secretory diarrhea in rodents. There is a loss of intestinal permeability to macromolecules such as lactose, secondary to a decrease in disaccharidase in the intestine. The enteric nervous system is stimulated by this virus, leading to intestinal water and electrolyte secretion.
The immunologic mechanisms responsible for protection against infection by rotavirus are still not well known. Older children and adults usually have asymptomatic or mild infection unless an overwhelming infectious dose is delivered. Several studies have shown that local intestinal immunity produced in response to infection protects against subsequent severe episodes of diarrhea. Studies in children indicate that the antibody response in primary infection is homotypic with subsequent infections producing a broadening of the immune response.
Rotavirus Detection
Laboratory procedures for diagnosis of rotavirus include electron microscopy (EM), passive latex agglutination assays (LA), electropherotyping using polyacylamide gel electrophoresis (PAGE), enzyme-linked immunosorbent assays (ELISA), and reverse transcription–polymerase chain reaction (RT-PCR). In recent years, ELISA has become the method of choice for screening. Early studies on strain surveillance identified rotavirus serotypes using neutralization assays. Monoclonal antibodies to specific serotypes were used. New methods have greatly improved data on circulating rotavirus strains and include multiplex RT-PCR based genotyping based on VP7 and VP4 genes, hybridization assays, and nucleotide sequencing.
Caliciviruses
Structure And Classification
The term ‘calicivirus’ is derived from the Latin calyx, meaning cup or goblet, and refers to the cup-shaped depressions visible by EM. These cuplike depressions are more prominent in some strains, particularly the sapoviruses, leading to the characteristic Star of David appearance from which caliciviruses get their name. Caliciviruses (family Caliciviridae) are a group of nonenveloped, icosahedral viruses with a single-stranded, positive-sense RNA genome. The genome is 7.5 to 7.7 kilobases in length and has three open reading frames (ORFs). Noroviruses can be genetically classified into five different genogroups (GI, GII, GIII, GIV, and GV) which can be further divided into different genetic groups or genotypes. Genogroup II, the most prevalent human genogroup, presently contains 17 genotypes. Genogroups I, II, and IV infect humans, whereas genogroup III is associated with bovine infections and genogroup V has recently been isolated in mice. Noroviruses were named after the places where the outbreaks occurred. Recently a numeric classification system has been proposed based on numbering genogroups with Roman numerals and genotypes with numbers. For example, the genogroup II norovirus, Lordsdale virus, is a member of genotype 4, and therefore classified as a GII.4 norovirus. GII.4 viruses account for the majority of adult outbreaks of gastroenteritis and often sweep across the globe. Sapoviruses, previously called the classical caliciviruses, based on their morphology, have 8 human genotypes in 5 genogroups, and have a similar system of strain designation as noroviruses.
Clinical Features
The incubation period for caliciviral infections is short, about 24 to 48 hours, and the mean duration of illness is 12 to 60 hours. Nausea is prominent, with vomiting, nonbloody diarrhea, and abdominal cramps occurring in most cases. These symptoms are experienced by all age groups, but diarrhea is relatively more prevalent among adults, whereas a higher proportion of children experience vomiting. From 25% to 50% of affected persons also report headache, fever, chills, and myalgias. Adults have died during illness caused by noroviruses, presumably from electrolyte imbalance. Late sequelae have not been reported, but the elderly often report persistence of constitutional symptoms for up to several weeks. Routes of transmission that have been documented include water, food (particularly shellfish and salads), aerosol, fomites, and person-to-person contact. Infectivity can last for as long as 4 days after resolution of symptoms. Presymptomatic shedding has been suspected on epidemiologic grounds but has not been proved in volunteer studies.
Pathophysiology
Other than a murine norovirus, noroviruses have not been grown in culture, making studies of pathogenetic mechanisms difficult. In studies carried out on volunteers, infection by calicivirus produces an expansion of the villi of the proximal small intestine. The epithelial cells remain intact with a shortening of the microvilli. The mechanism by which diarrhea is produced is unknown. In volunteer studies, infection by the Norwalk virus induces a specific IgG, IgA, and IgM serum antibody response, even in persons with preexisting antibodies. After norovirus infection, immunity appears to last for a few months but there is little or no evidence of long-term protection. Volunteer studies conducted in the 1970s also suggest that some people are resistant to Norwalk virus challenge. Recently two host factors have been identified that may contribute to this resistance to infection. In volunteer studies homozygous recessives for the a (1,2) fucosyltransferases gene (FUT2), who do not express H type-1 oligosaccharide were resistant to infection with Norwalk virus (nonsecretors). There is also evidence to suggest that different norovirus strains bind to different blood group antigens. Data on sapovirus infections and immune responses are not yet available.
Calicivirus Detection
Electron microscopy was initially used for identification of these viruses and continues to be used by many laboratories to screen stools for potential viral pathogens. This method is insensitive compared with molecular detection assays. Currently, RT-PCR assays are the most common approach for establishing a diagnosis of norovirus infection. Virus-specific primers are used to amplify conserved regions of the genome, usually in the polymerase or capsid genes. No single primer pair can detect all norovirus or sapovirus strains because of the high sequence diversity, but in most geographic regions, more than 90% of currently circulating strains can be detected using separate primer pairs for genogroups I and II noroviruses and sapoviruses.
Antigen-detection ELISA assays for noroviruses have been established in the last decade, but the first assays had a very narrow reactivity. More broadly reactive assays have been developed using monoclonal antibodies that recognize cross-reactive epitopes or multiple monoclonal antibodies, but are not widely used. Serologic assays also have been developed to detect immune responses to infecting norovirus strains, but are used more in epidemiological studies than for diagnosis in individual patients.
Astroviruses
Structure And Classification
Human astrovirus is the prototype of the Astroviridae, a family of nonenveloped positive-sense RNA viruses, measuring 38–41 nm. By direct EM, astroviruses recovered from stool display a distinctive surface starlike appearance. The genome of astrovirus consists of positive-sense, single-stranded RNA, 6.8 kb in length, organized in three ORFs. All serotypes have at least three capsid proteins, P1, P2, and P3, with the P2 protein carrying the groupreactive epitopes and the P3 protein specifying serotype.
Astroviruses are classified into serotypes based on the reactivity of the capsid proteins with polyclonal sera and monoclonal antibodies. Astroviruses can also be classified into genotypes on the basis of the nucleotide sequence of a 348-bp region of the ORF2, and there is a good correlation with the serotypes. There are eight established genotypes.
Phylogenetic analyses have shown that it is common to find multiple astrovirus strains circulating in one region during a given period of time, and that there are also variations in the prevalent type with time, suggesting either a genetic shift or an introduction of new strains. Serotype 1 is predominant in most studies, followed by 2, 3, 4, and 5. Serotypes 6, 7, and 8 are rarely detected.
Clinical Features
Clinically, these viruses cause similar symptoms to caliciviruses. Like rotaviruses, astrovirus infections occur throughout the year with peaks in the winter months. Infections have been shown to occur mainly in childhood. Other studies showed that most of the cases of infection are detected in children under 5 years of age with the majority of the children being under 1 year of age. Outbreaks of astrovirus infection involving children and elderly patients have been described and prolonged excretion documented in immunosuppressed, immunodeficient, and AIDS patients. Significantly higher seroprevalence rates of astrovirus have been reported in adults exposed to contaminated water compared with a control group.
Pathophysiology
The pathogenesis of the disease induced by astrovirus has not yet been established, although it has been suggested that viral replication occurs in intestinal tissue. In animal studies, atrophy of the intestinal villi is observed, as well as inflammatory infiltrates in the lamina propria leading to osmotic diarrhea.
Symptomatic astrovirus infection occurs mainly in small children and the elderly, which suggests both an acquisition of antibodies with increasing exposure and a reduction in antibodies with advancing age. Studies in adult volunteers indicate that people with detectable levels of antibodies do not develop the illness, although epidemiological observations suggest that human astrovirus infections do not induce heterotypic immunity, as an episode of astrovirus diarrhea is not associated with a reduced incidence of a subsequent episode.
Astrovirus Detection
EM is an insensitive technique, because a high concentration of viral particles is required for detection and the typical five or six-pointed star morphology is seen in less than 10% of particles. Enzyme immunoassays have been developed including streptavidin-biotin assays for increased sensitivity of detection, and are used in most diagnostic laboratories. For epidemiological research, recently astrovirus-specific RT-PCR has been the screening method of choice. While some investigators have used highly sensitive primers targeted to conserved genomic regions coding for the nonstructural proteins and untranslated regions, others prefer to use primers from the capsid coding region which can be less sensitive but provide typing information.
Adenoviruses
Structure And Classification
All adenovirus particles are nonenveloped, 60 to 90 nm diameter, with icosahedral symmetry easily visible in the electron microscope by negative staining, and are composed of 252 capsomers: 240 hexons and 12 pentons bearing fibers at the vertices of an icosahedron. The genome is linear, nonsegmented, double-stranded DNA of 30 to 38 kbp. Based on their immunologic properties, oncogenicity in rodents, genome, and morphology, adenoviruses are classified into six subgroups A through F with 51 serotypes. Serotypes predominantly associated with human infections include h-40 and h-41, which belong to subgenus F, and occasionally h-31 in subgenus A.
Clinical Features
Adenoviruses are widely recognized causes of respiratory, ocular, and genitourinary infections. However, serotypes 40 and 41 (previously called fastidious enteric adenoviruses) primarily affect the gut, contributing to 5% to 20% of hospitalizations for childhood diarrhea in developed countries. Enteric adenoviruses have also been identified in pediatric gastroenteritis in developing countries. Peak incidence is among children under 2 years of age, but older children and adults may be infected, with or without symptoms. Incubation is between 3 and 10 days, with illness lasting 1 week or longer, longer than for other enteric viral pathogens. Diarrhea is more prominent than vomiting or fever, and respiratory symptoms are often present.
Pathophysiology
The lesions produced by serotypes 40 and 41 in the enterocytes lead to atrophy of the villi and compensatory hyperplasia in the crypts, with subsequent malabsorption and loss of fluids. A neutralizing antibody response made in response to infection results in control of disease and protection from reinfection with the same serotype. Asymptomatic virus excretion can continue for prolonged periods even after an antibody response is documented in acute infection. Although adenoviruses can be grown in culture, little data are available on the pathogenetic mechanisms of these agents of viral gastroenteritis.
Adenovirus Detection
Traditionally, adenoviruses have been detected and typed by EM, virus culture, and neutralization assays. These assays are time-consuming, and more rapid serological assays including immunofluorescence, enzyme immunoassays, and latex agglutination have been developed. The rapid assays are useful in the diagnostic laboratory, but do not generally distinguish between serotypes. PCRbased techniques are more sensitive and relatively rapid, but have been shown to give discrepant results when compared with serotyping by neutralization.
Other Viruses
Torovirus is a genus within the Coronaviridae family, and toroviruses are known causes of diarrhea among cattle. These viruses have an envelope of 100–140 nm, with a helicoidal capsid and a single-stranded positive-sense RNA genome. Torovirus was detected for the first time in human gastroenteritis in 1984. They are associated with persistent and acute diarrhea in children, and may represent an important cause of nosocomial diarrhea.
Coronaviruses are well-established causes of diarrhea in animals and respiratory disease in humans. These viruses are between 60 and 220 nm, with helicoidal symmetry, a spiculated envelope which gives them the appearance of a crown, and a genome with positive-sense single-stranded RNA. They have been identified in the stool of persons with gastroenteritis (usually children under 2 years of age), but human controls have been found to shed them with higher frequency, raising doubt about their etiologic role in human diarrhea.
Picobirnaviruses are small viruses, without an envelope, 30–40 nm in diameter, with an icosahedral capsid and a genome made up of two or three segments of bicatenary RNA. Reports from Brazil documented human cases of diarrhea caused by picobirnavirus, which had been thought to be a cause of diarrhea only in animals. The importance of this pathogen is unknown, but it has been found in association with HIV and Cryptosporidium-infected individuals.
Aichi virus, in the genus Kobuvirus, was first recognized in 1989 in oyster-associated nonbacterial gastroenteritis in humans. Aichi virus appears to morphologically resemble astroviruses when examined by EM. Recently Aichi virus was isolated from Pakistani children and from Japanese travelers with gastroenteritis returning from tours of South-East Asian countries.
Twenty-three percent of children under 2 years of age with gastroenteritis of unknown etiology were antigenpositive for pestivirus, compared with 3% of controls, in a study on an American Indian reservation. No further studies have been done on the role of this agent in gastroenteritis.
Parvovirus-like particles have been identified by EM in stool specimens of both well and ill persons in Britain. The relationship of these particles to disease is unclear, but they have been associated with shellfish-related outbreaks of gastroenteritis.
Enteroviruses cause a wide spectrum of disease, in which gastroenteritis plays a minor role. Although the entry of polio, Coxsackie, echo, or other enteroviruses through the gut may cause incidental mild diarrheal symptoms, the spread of the virus through the bloodstream to other organs (e.g., central nervous system, heart, pleura, pancreatic islets) produces major disease manifestations. Reports have linked some enteroviruses to illnesses in which diarrhea was the sole symptom; nevertheless, an outbreak or case of gastroenteritis should not be attributed to an enterovirus merely because it was isolated in the stool of an affected person.
Management Of Viral Gastroenteritis
Treatment of viral gastroenteritis is symptomatic, and its aim is to prevent or treat the dehydration secondary to the disease. Dehydration is assessed using blood pressure, pulse, heart rate, skin turgor, fontanelle depression, mucous membranes, eyes, extremities, mental status and activity, urine output, and thirst. Assessment of dehydration, particularly in children in community studies or by field workers, relies on lethargy, restlessness, appearance of eyes, skin turgor, and feeding/thirst.
Fluid and metabolic imbalances must be assessed and corrected. The most important factor predicting adverse outcome of viral gastroenteritis is delay in fluid and electrolyte therapy. Clinically significant dehydration can occur within 6 hours of onset of illness, especially during primary infection in children. Malnutrition, malignancy, and immunodeficient states predispose to a more severe episode of illness or unremitting diarrhea that can persist until the underlying condition is corrected.
Oral rehydration therapy is recommended for preventing and treating early dehydration and continued replacement therapy for ongoing losses. Intravenous therapy is required in severe dehydration, shock, and decreased consciousness. In children, age-appropriate diet should be continued during oral rehydration and following intravenous rehydration. Anti-emetics, anti-diarrheal agents, and antibiotics should not be given to children, although anti-emetics and anti-diarrheals may be used in adults. Studies have shown that antirotavirus immunoglobulin as bovine hyperimmune colostrum or human milk may decrease the frequency and duration of rotavirus diarrhea. Probiotics such as Lactobacillus casei GG and Saccharomyces boulardii reduce the frequency and duration of diarrhea. Racecadotril, an enkephalinase inhibitor, has been shown in some studies to be useful in treating rotaviral diarrhea in children. Zinc supplements have been suggested to reduce severity and duration of illness.
Preventive measures can limit the number of episodes of viral gastroenteritis both within the home and in institutions. Diaper-changing areas should be separate from food preparation areas. Diapers should be disposed of directly in the changing area and should be placed in closed bags. Hands should be washed after contact with soiled diapers and clothing. Interruption of transmission of the infection is extremely important, especially in hospitals and centers that care for small children. Therefore, it is necessary to reinforce hygiene measures, such as hand washing, and clean all surfaces with suitable disinfectants. As viruses do not replicate outside a host, decreasing the potential inoculum is key to preventing further infection of susceptible hosts. Further preventive measures in childcare facilities and hospitals include isolation and cohorting of ill children. Asymptomatic infections probably also play an important role in the spread of infection.
Vaccines
Studies with vaccines against group A rotavirus began in 1982. The first vaccine developed was the tetravalent human–rhesus reassortant vaccine, which induces protection against the four main rotavirus serotypes, G1–G4. Efficacy studies showed a reduction in the appearance of severe gastroenteritis caused by rotavirus in vaccinated children, and the vaccine was approved in the United States in 1998. However, the detection of an increase in the risk of intussusception after vaccination led to its suspension. In 2006, two licensed vaccines became commercially available.
These are Rotateq, a live oral attenuated pentavalent vaccine from Merck which is recommended by the CDC Advisory Committee on Immunization Practices to be given at 2, 4, and 6 months with the last dose administered no later than 32 weeks, and Rotarix from GlaxoSmithKline, a human strain derived monovalent live oral vaccine given in two doses at 2 and 4 months. Rotateq is licensed in the United States and Rotarix in many countries in Europe and South America. These vaccines need to be evaluated in settings where there is marked diversity of rotaviruses and infection occurs at a younger age, as in developing countries. Owing to the high cost of these vaccines, vaccine manufacturers in developing countries have initiated the process of formulating and testing vaccines based on local strains, which may address the issues of cost and heterotypic immunity in settings where different viruses circulate. DNA-based and virus-like particle vaccines may also provide an alternate method of prevention in the future.
Studies on virus-like particle-based vaccines against Norwalk virus have also been initiated with capsid proteins expressed in plants inducing an antibody response in experimental models. However, these potential vaccines are still in preclinical development.
In summary, viral gastroenteritis is a major cause of morbidity in developed countries and mortality in developing countries. Hygiene is the first preventive step in viral gastroenteritis and rehydration is the key to management of clinical illness. The range of organisms that can cause gastroenteritis is immense and vaccines will be important in reducing the impact of childhood gastroenteritis.
Bibliography:
- Cheng AC, McDonald JR, and Theilman NM (2005) Infectious diarrhea in developed and developing countries. Journal of Clinical Gastroenterology 39: 757–773.
- Desselberger U and Gray J (eds.) (2003) Viral Gastroenteritis: Perspectives in Medical Virology vol. 9). Amsterdam, the Netherlands: Elsevier Science B. V.
- Duizer E and Koopmans M (2004) Foodborne viruses: An emerging problem. International Journal of Food Microbiology 90: 23–41.
- Fong TT and Lipp EK (2005) Enteric viruses of humans and animals in aquatic environments: Health risks, detection, and potential water quality assessment tools. Microbiology and Molecular Biology Reviews 69: 357–371.
- Glass RI, Bresee J, Jiang BM, et al. (2001) Gastroenteritis viruses: An overview. Novartis Foundation Symposium 238: 5–19.
- Guandalini S (2006) Probiotics for children: Use in diarrhea. Journal of Clinical Gastroenterology 40: 244–248.
- Guix S, Bosch A, and Pinto RM (2005) Human astrovirus diagnosis and typing: Current and future prospects. Letters in Applied Microbiology 41: 103–105.
- Hutson AM, Atmar RL, and Estes MK (2004) Norovirus disease: Changing epidemiology and host susceptibility factors. Trends in Microbiology 12: 279–287.
- Kapikian AZ (ed.) (1994) Viral Infections of the Gastrointestinal Tract. 2nd edn. New York: Marcel Dekker.
- Sartor RB (2005) Probiotic therapy of intestinal inflammation and infections. Current Opinion in Gastroenterology 21: 44–50.
- Vesikari T, Giaquinto C, and Huppertz HI (2006) Clinical trials of rotavirus vaccines in Europe. Pediatric Infectious Diseases Journal 25 (supplement 1): S42–S47.
- http://www.cdc.gov/ncidod/dvrd/revb – Centers for Disease Control and Prevention (CDC).
- http://www.emedicine.com/MED – eMedicine World Medical Library.
- http://www.digestive.niddk.nih.gov/ddiseases/pubs/viralgastroenteritis – National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (NIDDKD/NIH).
See also:
Free research papers are not written to satisfy your specific instructions. You can use our professional writing services to buy a custom research paper on any topic and get your high quality paper at affordable price.








