This sample Oral Cancer Research Paper is published for educational and informational purposes only. If you need help writing your assignment, please use our research paper writing service and buy a paper on any topic at affordable price. Also check our tips on how to write a research paper, see the lists of health research paper topics, and browse research paper examples.
Introduction
Oral cancer is a major public health problem, with 350 000 to 400 000 new cases identified worldwide each year. Worldwide, oral squamous cell carcinoma (OSCC) is the sixth most common cancer for both sexes (Parkin et al., 1999). Incidence varies widely between countries and geographical areas, but it is generally most common in developing countries. Even in the United States, however, it was estimated that oral and pharyngeal cancers would account for 29 800 cases and 8100 deaths in 1999 (Landis et al., 1999). These variations have traditionally been explained by differences in lifestyle exposure to specific risk factors such as tobacco, betel, and alcohol use, although environmental factors (especially infective agents) and socioeconomic factors, genetics, and ethnicity may also be involved.
Oral cancer is a term that usually includes cancers of the lip, tongue, salivary glands, and other sites in the mouth (gum, floor of mouth, and other unspecified parts of the mouth). Approximately 90% of oral cancers are squamous cell carcinomas (OSCCs). Pharyngeal cancer is a term that includes cancers of the nasopharynx, oropharynx, and hypopharynx.
In the developed world, some 42% of OSCC affects the lip, but most intraoral OSCC involve the floor of the mouth or the lateral border of the tongue (the graveyard or coffin area; 22% the tongue, 17% the floor of mouth). In the developing world, cancers of the tongue and buccal mucosa are the most common (Sankaranarayanan, 1990).
Oral cancer is especially common in disadvantaged older people with lifestyle factors of smoking tobacco and drinking alcohol. Incidence increases with age, a steep rise beginning in the age group 60–64 years (Ries et al., 1999). The overall male-to-female ratio is greater than 2:1, but for oropharyngeal cancer the ratio is 4:1 (Ferlay et al., 2001). The excess in older males has largely been attributed to the habits of males, particularly tobacco and alcohol use, and the length of exposure to carcinogens/mutagens.
Among younger individuals, the incidence of OSCC is rising, particularly for tongue cancers. Evidence that traditional risk factors may not be responsible for this trend suggests the need for more definitive and cost-effective means to detect OSCC in populations that until recently have been considered at low risk.
Two-thirds of all OSCC in males and three-quarters in females arise in the developing world (Parkin et al., 1999), where it is the third most common cancer. It is particularly common in South-Central Asia, home to one fifth of the world’s population. Probably 58% of the cases are concentrated in South and Southeast Asia (Nair et al., 2004). In most regions of India, oral cancer is the most common cancer in men and the third most common cancer in women. In Karachi, oral cancer ranks second of all malignancies among both males and females, with one of the highest reported incidences in the world (Bhurgri, 2005). Brazil also has a high incidence in males, and the area including southern Brazil, Uruguay, and Argentina has the highest rates in Latin America. The gender difference in OSCC rates has been decreasing over time, presumably as tobacco and alcohol consumption equalize (Tumino and Vicario, 2004).
The highest incidence of OSCC worldwide, at up to 55 cases per 100 000 per year, has been recorded in the Bas-Rhin de´partement of France (Blot et al., 1996; Levi et al., 1998), but incidence in Hungary and other parts of Eastern Europe is rising (La Vecchia et al., 2004). In Central and Eastern Europe, Hungary, Croatia, Slovenia, Ukraine, Russia, and Lithuania have incidence above 7 per 100 000; intermediate rates at 6–7 per 100 000 are seen in Spain, Switzerland, Germany, Poland, and Italy; and rates below 4 per 100 000 are found in Greece, the Netherlands, some Nordic countries, and England and Wales (Levi et al., 1998). In the United States, OSCC is the tenth most common cancer among males (Moore et al., 1999, 2000).
Oral squamous cell carcinomas are often diagnosed late. Five-year survival is relatively low, at approximately 50%; and this has changed very little in the past 30 years (Swango, 1996). In the United States, 5-year survival is much lower in Blacks (34%) than Whites (55%). Survival is particularly low in black males (28%), compared with 47% in females. In Europe, 5-year survival is about 44% (Berrino et al., 1999). Localized cancers have the highest 5-year relative survival (79%), those with regional disease intermediate (42%), and cancers with distant metastases the lowest (19%) (Gloeckler Ries et al., 1994). OSCC is associated with a very high risk of second primary tumors (SPTs) in the same location (Cooper et al., 1989).
Lip Cancer
The incidence of lip cancer in men has decreased in many countries, such as in Finland (Tarvainen et al., 2004) and Pakistan, but the rates in Pakistan have remained level in females (Bhurgri, 2005).
Intraoral Cancer
The incidence of OSCC in U.S. white men stabilized in the 1980s and started to decrease, but incidence in women and especially black men began to increase. About that time, an increased incidence of tongue cancer was noted in young people (under 45 years of age) in many countries (Davis and Severson, 1987; MacFarlane et al., 1987; Moller, 1989; Hindle and Nally, 1991; MacFarlane et al., 1992; Wennerberg et al., 1994; Hindle et al., 1996; Kari et al., 1997; Mork and Glattre, 1998; Myers et al., 2000; Shiboski et al., 2000; Annertz et al., 2002; Schantz and Yu, 2002; Shiboski et al., 2005).
OSCC incidence has also changed in Pakistan and India (Gupta, 1999). Data for 1995–2004 from the Karachi Cancer Registry, Pakistan, showed an earlier onset of disease for all categories (Bhurgri, 2005). The incidence of tongue cancer increased, but a more dramatic increase in OSCC in the cheek was evident in both sexes (Bhurgri, 2005). In males in India, however, a statistically significant decreasing trend in the overall age-adjusted incidence rates of OSCC was observed during the period 1986–2000, which may be attributable to a decrease in the usage of pan and tobacco (Sunny et al., 2004). The high prevalence of smokeless tobacco use among young adult men and women may explain the stable trend in oral cancer incidence in this group.
In Europe during the period 1955–90, several countries in Central and Eastern Europe had at least a twofold increase in OSCC, principally in males under 45 years of age (La Vecchia et al., 2004), and Denmark, Finland, Norway, and Sweden showed more than fivefold increases in young males (Annertz et al., 2002; Tarvainen et al., 2004). Similar increases were also seen in young women in England and Wales (Hindle and Nally, 1991), Scotland (MacFarlane et al., 1992), and Greece (Zavras et al., 2003).
In most European countries, oral cancer mortality had been rising between the 1950s and 1980s, in both genders, and with a twofold increase in men in the Czech Republic, Poland, Romania, and Spain, and a fourfold rise in Hungary and Germany (La Vecchia et al., 1997; Levi et al., 1999). Similar increases were recorded in the United States (Schantz and Yu, 2002), Thailand (Reichart et al., 2003), India and Pakistan (Gupta, 1999), Uruguay (De Stefani et al., 1994a, b), and Brazil. Mortality continued to increase in many European countries during 1980–99, especially in Eastern Europe, although mortality levelled off in Poland and the Czech Republic, and actually fell in some Western European countries including Austria, Germany, and England and Wales (La Vecchia et al., 2004). The disparity in mortality between males and females is largely attributable to differences in incidence.
The increases at younger ages, seen in many countries, indicate cohort effects that suggest that the increase in overall incidence is likely to continue in future decades (Boyle et al., 1992; Macfarlane et al., 1994).
The incidence of tumors of the tongue and tonsil rose in young Whites in the United States over the period 1973–2001 (Shiboski et al., 2005). The incidence of OSCC of the tongue in adults under 40 years of age increased by 60% between 1973–84 and 1985–97 (Schantz and Yu, 2002). Mortality from cancers of the mouth in males increased in 19 of 24 countries between 1950–54 and 1980–85 (MacFarlane et al., 1994). Oropharyngeal cancer incidence rose rapidly in Scotland between 1950 and 1998, particularly among males and females aged 35–64 years during the period 1989–96, although age-standardized mortality appeared to have stabilized (Robinson and Macfarlane, 2003).
These increases in OSCC appear to reflect lifestyle habits that have become common in younger generations, such as the use of tobacco, alcohol, and betel. The rising trend in Central and Eastern Europe appears to reflect the rise in all tobacco-related neoplasms, but the rise in OSCC in Scotland, despite the fall in lung cancer, may reflect increased alcohol use (Levi et al., 2003). The rises in Russia may reflect increasing alcohol use (Zatonski, 1998). The rises in South Asia reflect growing betel use (Nair et al., 2004). However, the increases in other countries have often been seen in the absence of these conventional lifestyle risk factors, particularly in women, which raises questions about the cause of these cancers.
Carcinogenesis is the result of disturbed growth control arising from DNA damage (mutation) which can arise spontaneously (probably from free radical damage), but may also be precipitated by mutagens (Scully et al., 2000). Tobacco, alcohol, betel, and areca nut are the main risk factors (La Vecchia et al., 1997; Bagnardi et al., 2001; Boeing et al., 2002), but significant numbers of younger patients with OSCC deny exposure to any known risk factors (Ehlinger et al., 1993; Mackenzie et al., 2000; Llewellyn et al., 2003).
A case–control study in Brazil showed the risk for cancer in the head and neck to be raised for those who reported cancer at any site in a first-degree relative, with a greater risk if the relative had cancer in the head and neck (Foulkes et al., 1995). In a UK study, most young OSCC patients had had a relative with cancer (Llewellyn et al., 2003). Some have suggested that familial clustering of cancer points to a genetic component, but so far no study has provided clear-cut evidence to support this view.
Ethnicity
There is an excess mortality from OSCC in minority ethnic and in socially disadvantaged groups (Scully and Bedi, 2000) (Table 1).
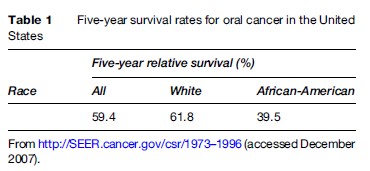
Examination of mortality trends from 1973–87 suggests that the disparity in mortality between Blacks and Whites in the United States was most probably attributable to differences in survival, rather than differences in incidence (Goldberg et al., 1994). In Europe, there is an increased mortality in immigrants from the Indian subcontinent (Swerdlow et al., 1995).
Lifestyle Factors
The results of many studies have been summarized by the International Agency for Research on Cancer (IARC) showing the importance of specific risk factors, i.e., tobacco, betel, and alcohol use (IARC 1986a, b, 1988), which contain known carcinogens.
Tobacco
Tobacco use in all forms appears to carry a risk. Oral cancer risks show a clear decline after stopping tobacco use (La Vecchia et al., 1999). Oral leukoplakia, the most common premalignant oral lesion, is related to tobacco use (Gupta, 1984). Smokers have a sevenfold risk of an oral dysplastic lesion ( Jaber et al., 1999).
Cigarette smokers appear to be about five times more likely to develop OSCC than nonsmokers. Those who abstain (e.g., Mormons and Seventh Day Adventists) have a lower incidence. In one study, the odds ratio for consumption of more than 20 cigarettes a day was double that of smokers consuming fewer cigarettes (Lopez et al., 2000). In one European study, the risk of oral cancer in smokers of low/medium-tar cigarettes was 8.5-fold higher than in nonsmokers, and more than 16-fold for high-tar cigarette smokers (La Vecchia et al., 1990).
Smoking bidi, the unfiltered cigarettes with a small amount of flaked tobacco, which is widely practised in South Asia, increases the risk of OSCC (Rahman et al., 2005). Cigars may predispose to leukoplakia (Baric et al., 1982) and oral and pharyngeal cancers (Shanks and Burns, 1998). Pipe smoking carries a risk of leukoplakia and OSCC (Baric et al., 1982). Reverse smoking (holding the burning end of a cigarette or cigar within the mouth) – seen in India, South America, Taiwan, and the Philippines – is strongly associated with OSCC (Gupta et al., 1980).
Smokeless tobacco (ST) products include paan (or pan), chaalia, gutka, and naswar and are used in all sections of South Asian society. Use of these products increases the risk of OSCC (Gupta et al., 1980).
In India, different forms of chewing tobacco are used such as betel, khaini, pattiwala tobacco, maiwpuri tobacco, zarda, kiwam, and gadakhu. Risks appear to depend on the processing of the particular product, which can markedly affect the level of the carcinogen nitrosamine (Warnakulasuriya, 2004). A wide range of smokeless tobacco products is used worldwide (Table 2). Many migrants to the West continue to use them even several decades after migration. Bangladeshis, in particular, are likely to retain the habit of betel use (Bedi, 1996; Croucher and Islam, 2002). Indian migrants to the UK have a significantly higher incidence of OSCC than native UK populations (Warnakulasuriya et al., 1999), presumably related to the use of smokeless tobacco.
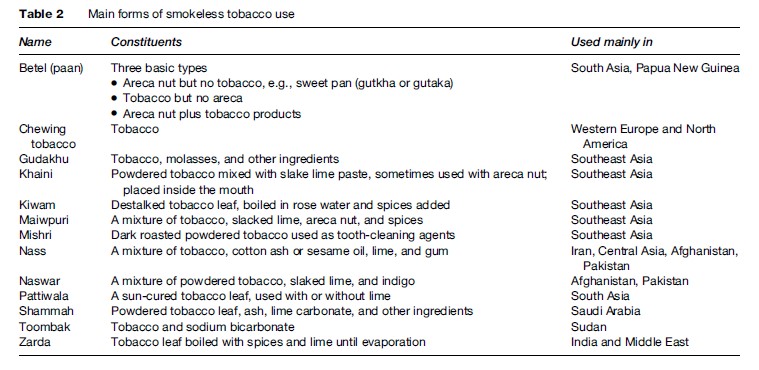
Betel quid is probably used by 20% of the world’s population. In some areas (e.g., Pakistan), up to 70% of primary school children use it on a daily basis (Shah et al., 2002). The way that the quid of betel is prepared and used varies widely. It may contain tobacco, areca (betel) nut, and lime or other products. The quid may be left in the mouth for long periods, even overnight, in close contact with the oral mucosa. The habit can cause premalignant lesions ( Jacob et al., 2004), submucous fibrosis, and invasive squamous cell carcinoma (Seedat et al., 1988; Nandakumar et al., 1990; Sankaranarayanan et al., 1990; Van Wyk et al., 1993).
Snuff is finely powdered plant material, principally tobacco. It is used orally or nasally, and is a risk factor for OSCC (Axell et al., 1978; Winn et al., 1991; Hirsch, 2002). The risks are mainly in snuff users who also use tobacco (Accortt et al., 2005).
Increased consumption of any alcohol-containing beverages is associated with a risk of OSCC (WRCF, 1997; WHO, 2002; Ogden, 2005). The risk decreases after stopping alcohol use, but the effects appear to persist for several years (Franceschi et al., 2000). The risk is highest among the heaviest drinkers of alcohol (Barra et al., 1990; Petti and Scully, 2005). The type of alcoholic beverage appears to influence the risk: Hard liquors confer higher risks (Barra et al., 1990; Day et al., 1994; De Stefani et al., 1998; Gronbaek et al., 1998; La Vecchia et al., 1999; Huang et al., 2003; Lissowska et al., 2003). Ecological studies suggest that the impact of alcohol on OSCC mortality has increased in recent years (Hindle et al., 2000; Petti and Scully, 2005).
The use of mouthwash with a high alcohol content (25% or higher) increases the risk of OSCC by 40% in men and by 60% in women (Winn et al., 1991). However, it appears as if the effect of alcohol from mouthwash could be similar to that of alcohol used for drinking, although in terms of attributable risk, the contribution of mouthwash use to OSCC must be very small (Elmore and Horwitz, 1995; Morse et al., 1997).
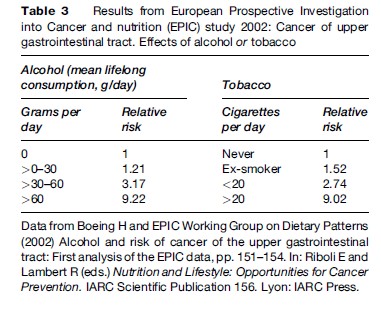
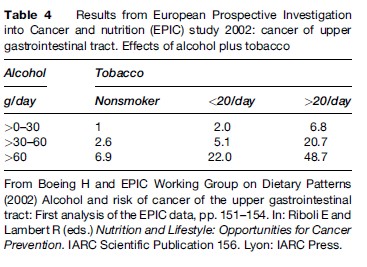
The combined effect of alcohol and tobacco on the risk of OSCC is multiplicative, not simply additive (Blot et al., 1988; Franceschi et al., 1990; Rodriguez et al., 2004) (Tables 3 and 4).
There have been a number of case reports of oral cancer in marijuana smokers but any relationship has yet to be supported by full epidemiological studies (Firth et al., 1997; Fung et al., 1999). A review of two cohort studies and 14 case–control studies showed a possible association between marijuana use and cancer risk (Hashibe et al., 2005), but regular users of marijuana are often heavy cigarette smokers (Firth, 1997; Fung et al., 1999).
Charcoal-grilled red meat (Franco et al., 1989) and fried foods (Galeone et al., 2005) have been implicated as risk factors. Increased consumption of fruits and vegetables is associated with a lower risk of OSCC (Potter et al., 1997).
Socioeconomic status plays an important role, even if it is indirect. Most premalignant lesions (Hashibe et al., 2003) and most squamous cell carcinomas of the oral cavity occur in people of low socioeconomic status (Edwards and Jones, 1999; Greenwood et al., 2003; Bhurgri, 2005).
Raised risks of oral cancer have been found in a number of occupations (Table 5). Candida albicans and viruses such as herpesviruses and human papillomaviruses may be implicated in at least some cases (Gillison et al., 1999; Scully, 2005).
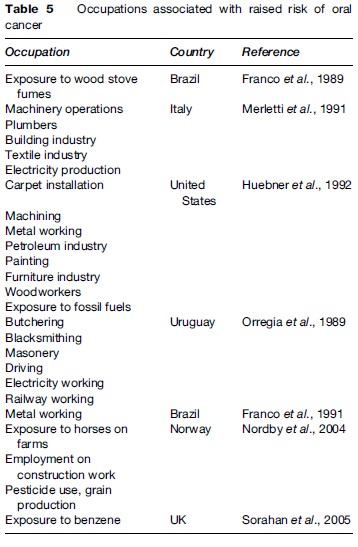
Lip cancer is seen mainly in males with chronic sun exposure and in smokers (Luna-Ortiz et al., 2004). Ionizing radiation exposure is a possible risk factor for second primary cancers of the oral cavity (Hashibe et al., 2005).
Persons with poor oral hygiene appear to have increased risk of OSCC, independent of any effect of tobacco, alcohol, or other proven risk factors, but not all workers agree, so further studies are required.
Potentially Malignant (Precancerous) Clinical Lesions
Some potentially malignant (precancerous) clinical lesions that can progress to OSCC include especially:
- Erythroplasia (erythroplakia): The most likely lesion to progress to severe dysplasia or carcinoma. Erythroplastic lesions are velvety red plaques, which in at least 85% of cases show frank malignancy or severe dysplasia. Carcinomas are seen 17 times more frequently in erythroplakia than in leukoplakia (but leukoplakias are far more common).
- Leukoplakia, particularly where admixed with red lesions, as in speckled leukoplakia, and proliferative verrucous leukoplakia, sublingual leukoplakia, candidal leukoplakia, syphilitic leukoplakia (now exceptionally rare).
- Lichen planus.
- Oral submucous fibrosis ( Jeng et al., 2001).
Apart from these lesions, most other potentially malignant lesions or conditions have only a very low incidence of dysplasia or malignant change (Table 6).
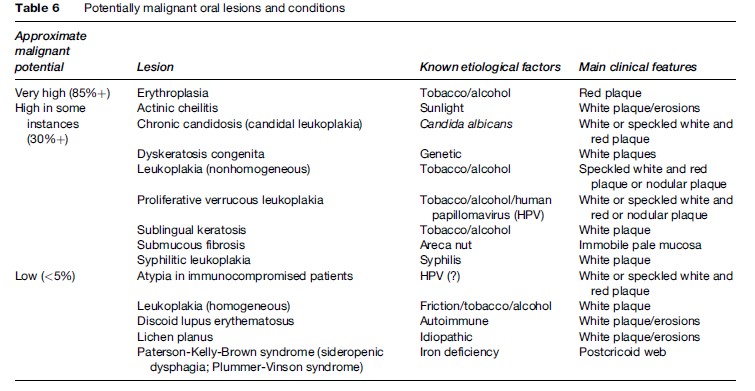
There is a highly significant increase in the incidence of OSCC of the tongue in systemic sclerosis (Derk et al., 2005) and an increase of OSCC in transplant recipients (Scheifele et al., 2005).
Prevention Of Oral Cancer
There is remarkably little good-quality evidence on the prevention of OSCC. However, OSCC shares several risk factors with other diseases, and is thus potentially amenable to prevention using the principles of the Common Risk Factor Approach (Sheiham and Watt, 2000). Health promotion campaigns centered on cessation of tobacco use in particular have been advocated and directed either at primary prevention or at preventing the malignant transformation of potentially malignant lesions and conditions. Most notably, Warnakulasuriya et al. (1991) used primary health-care workers in Sri Lanka not only to screen for oral cancer and precancer, but also to deliver tobacco cessation advice to high-risk subjects.
However, despite a reduction in tobacco product consumption in many developed countries, oral cancer incidence and mortality has not always fallen in these countries, leading some researchers to hypothesize that alcohol consumption (Hindle et al., 2000), genetic factors, and environmental factors such as infective agents (Scully et al., 2005) are increasing in relative importance.
Chemopreventive clinical trials aim to prevent the potentially malignant lesions or attempt to prevent malignant transformation or aim to cause regression of lesions in high-risk patient groups (Zaridze et al., 1993; Mathew et al., 1995).
Oral Cancer Awareness By The General Public And Health Professionals
Many studies have reported variable, and frequently suboptimal, levels of knowledge of oral cancer, risk factors, and diagnosis among both the public and health-care professionals. Although it is among the ten most common cancers worldwide, OSCC is rarely mentioned in the popular press, and public awareness is dismal (Canto et al., 1998). Significant numbers of the public are unaware of risk factors or even that OSCC exists, sometimes even after high-profile public education campaigns (Papas et al., 2004; Stahl et al., 2004). The reported trend among healthcare professionals is, unsurprisingly, that the knowledge and confidence of appropriate practice is related to education and experience, being greater in specialists than general dental practitioners, who in turn tend to be better informed than general medical practitioners and other health-care professionals (Reed et al., 2005; Sohn et al., 2005; Kujan et al., 2006).
From the perspective of improving the prognosis, there are two major stumbling blocks for a preventive approach. First, although many groups have attempted to educate both public and professionals about the signs and symptoms of OSCC, there is no robust evidence that these campaigns have produced a significant long-term change in the knowledge, attitudes, or behaviors of all the targeted groups or shortened delays in diagnosis (McLeod et al., 2005). Second, the probability of being diagnosed with advanced disease does not relate well to recorded delays, since variations in disease stage at presentation may also reflect underlying differences in the speed of progression of the lesion, and symptomatology does not always correlate with tumor stage (Scott et al., 2005).
Diagnostic Delays
Delays in diagnosis may occur as a result of patient delay in presentation or professional delay in referral to an appropriate specialist center, or both (Onizawa et al., 2003; Llewellyn et al., 2004).
Oral Cancer Screening Programs
Oral cancer fulfills many of the criteria proposed by Wilson and Junger (1968) for a disease to be suitable for screening (Speight et al., 1993), but there are many logistical hurdles and costs to be overcome in order to assess the feasibility of screening and to implement a program. As a consequence, most of the research has reported on process measures such as diagnostic test performance (Moles et al., 2002; Downer et al., 2004) or uptake or compliance with the screening (Speight et al., 2006). Where outcomes have been reported, they have mainly been proxy outcomes such as absolute survival. Survival is an unreliable outcome for this purpose because it is subject to the effects of both lead-time bias and length bias.
A Cochrane systematic review (Kujan et al., 2003) found only one cluster-randomized controlled clinical trial using population-based mortality as the primary outcome measure (Sankaranarayanan et al., 2005). This trial, based in Kerala, India, randomized nearly 200 000 people to 13 clusters to either receive periodic screening (seven clusters) in the form of a visual examination at 3-year intervals or no screening (six clusters). The results indicate no appreciable difference in OSCC mortality between the screened (21.2/100 000 person-years) and nonscreened groups (21.3/100 000 person-years).
In the absence of reliable data on effectiveness, it is difficult to justify the expense of setting up large-scale randomized controlled trials in either developed or developing countries. In an attempt to provide such data, Speight et al. (2006) undertook a computer simulation modeling exercise of the cost-effectiveness of oral cancer screening in primary care in the UK, based on data from primary research, systematic reviews, published health service costs, and – for model parameters where no robust data were available – expert opinion. The model indicated that opportunistic screening of high-risk individuals might be cost-effective in either general dental practice (because dentists performed relatively well in screening) or general medical practice (because of a greater throughput of at-risk patients). However, there was considerable uncertainty in some of the model parameters used.
Opportunities And Barriers To Progress
The randomized controlled trial in Kerala is still in progress. Since it is the only trial to examine the impact of population-based screening for OSCC with mortality as the primary outcome, the long-term results are eagerly awaited. The transferability of the results to other countries or health-care settings will be a source of debate.
Bibliography:
- Accortt NA, Waterbor JW, Beall C, and Howard G (2005) Cancer incidence among a cohort of smokeless tobacco users (United States). Cancer Causes Control 16(9): 1107–1115.
- Annertz K, Anderson H, Biorklund A, et al. (2002) Incidence and survival of squamous cell carcinoma of the tongue in Scandinavia, with special reference to young adults. International Journal of Cancer 101: 95–99.
- Axell T, Mornstad H, and Sundstrom B (1978) Snuff and cancer of the oral cavity: A retrospective study (in Swedish). Laekartidningen 75: 1224–1226.
- Bagnardi V, Blangiardo M, La Vecchia C, and Corrao G (2001) A meta-analysis of alcohol drinking and cancer risk. British Journal of Cancer 85: 1700–1705.
- Baric JM, Alman JE, Feldman RS, and Chauncey HH (1982) Influence of cigarette, pipe, and cigar smoking, removable partial dentures, and age on oral leukoplakia. Oral Surgery, Oral Medicine, Oral Pathology 54(4): 424–429.
- Barra S, Franceschi S, Negri E, Talamini R, and La Vecchia C (1990) Type of alcoholic beverage and cancer of the oral cavity, pharynx and oesophagus in an Italian area with high wine consumption. International Journal of Cancer 46: 1017–1020.
- Bedi R (1996) Betel-quid and tobacco chewing among the United Kingdom’s Bangladeshi community. British Journal of Cancer 74 (supplement XXIX): S73–S77.
- Berrino F, Capocaccia R, Esteve J, et al. (1999) Survival of Cancer Patients in Europe: The EURO-CARE 2 Study. IARC Scientific Publication No. 151. Lyon, France: IARC.
- Bhurgri Y (2005) Cancer of the oral cavity – Trends in Karachi South (1995–2002). Asian-Pacific Journal of Cancer Prevention 6(1): 22–26.
- Blot WJ, McLaughlin JK, Devesa SS, and Fraumeni JF (1996) Cancers of the oral cavity and pharynx. In: Schottenfeld D and Fraumeni JF (eds.) Cancer Epidemiology and Prevention, 2nd edn., pp. 666–680. New York: Oxford University Press.
- Blot WJ, McLaughlin JK, Winn DM, et al. (1988) Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Research 48: 3282–3287.
- Boeing H and EPIC Working Group on Dietary Patterns (2002) Alcohol and risk of cancer of the upper gastrointestinal tract: First analysis of the EPIC data. In: Riboli E and Lambert R (eds.) Nutrition and Lifestyle: Opportunities for Cancer Prevention, pp. 151–154. IARC Scientific Publication 156. Lyon, France: IARC Press.
- Boyle P, Macfarlane GJ, Zheng T, et al. (1992) Recent advances in epidemiology of head and neck cancer. Current Opinions in Oncology 4: 471–477.
- Canto MT, Kawaguchi Y, and Horowitz AM (1998) Coverage and quality of oral cancer information in the popular press: 1987–98. Journal of Public Health Dentistry 58: 241–247.
- Cooper JS, Pajak TF, Rubin P, et al. (1989) Second malignancies in patients who have head and neck cancer: incidence, effect on survival and implications based on the RTOG experience. International Journal of Radiation, Oncology, Biology, Physics 17: 449–456.
- Croucher R and Islam S (2002) Socio-economic aspects of areca nut use. Addiction Biology 7: 139–146.
- Davidson BJ, Root WA, and Trock BJ (2001 Apr) Age and survival from squamous cell carcinoma of the oral tongue. Head Neck 23(4): 273–279.
- Davis S and Severson R (1987) Increasing incidence of cancer of the tongue in the United States among yoiung adults. Lancet 2: 910–911.
- Day GL, Blot WJ, McLaughlin JK, and Fraumeni JF (1994) Carcinogenic risk of dark vs light liquor. International Journal of Cancer 59: 319–321.
- De Stefani E, Boffetta P, Oreggia F, Fiero L, and Mendilaharsu M (1998) Hard liquor drinking is associated with higher risk of cancer of the oral cavity and pharynx in Uruguay. Oral Oncology 34: 99–104.
- De Stefani E, Fiero L, Barrios E, et al. (1994a) Cancer mortality trends in Uruguay. International Journal of Cancer 56: 634–639.
- De Stefani E, Oreggia F, Ronco AL, et al. (1994b) Salted meat consumption as a risk factor for cancer of the oral cavity and pharynx; a case control study from Uruguay. Cancer Epidemiology Biomarkers and Prevention 3: 381–385.
- Derk CT, Rasheed M, Spiegel JR, and Jimenez SA (2005) Increased incidence of carcinoma of the tongue in patients with systemic sclerosis. Journal of Rheumatology 32(4): 637–641.
- Downer MC, Moles DR, Palmer S, and Speight PM (2004) A systematic review of test performance in screening for oral cancer, precancer. European Journal of Cancer. Part B: Oral Oncology 40(3): 264–273.
- Edwards DM and Jones J (1999) Incidence of and survival from upper aerodigestive tract cancers in the UK; the influence of deprivation. European Journal of Cancer 35: 968–972.
- Ehlinger P, Fossion E, and Vrielinck L (1993) Carcinoma of the oral cavity in patients over 75 years of age. International Journal of Oral and Maxillofacial Surgery 22: 218–220.
- Elmore JG and Horwitz RI (1995) Oral cancer and mouthwash use: evaluation of the epidemiologic evidence. Otolaryngology, Head and Neck Surgery 113: 253–261.
- Ferlay J, Bray F, Pisani P, and Parking DM (eds.) (2001) Gobocan 2000 Cancer incidence, Mortality and Prevalence Worldwide. Lyon, France: International Agency for Research on Cancer.
- Firth NA (1997) Marijuana use and oral cancer: a review. Oral Oncology 33: 398–401.
- Foulkes WD, Brunet JS, Kowalski LP, et al. (1995) Family history of cancer is a risk factor for squamous cell carcinoma of the head and neck in Brazil; a case control study. International Journal of Cancer 63: 769–773.
- Franceschi S, Talamini R, Barra S, et al. (1990) Smoking and drinking in relation to cancers of the oral cavity, pharynx, larynx, and esophagus in northern Italy. Cancer Research 50: 6502–6507.
- Franceschi S, Levi F, Dal Maso L, et al. (2000) Cessation of alcohol drinking and risk of cancer of the oral cavity and pharynx. International Journal of Cancer 85: 787–790.
- Franco EL, Kowalski LP, Oliveira BV, et al. (1989) Risk factors for oral cancer in Brazil: a case-control study. International Journal of Cancer 43: 992–1000.
- Franco EL, Kowalski LP, and Kanda JL (1991) Risk factors for second cancers of the upper respiratory and digestive systems; a case control study. Journal of Clinical Epidemiology 44: 615–625.
- Fung M, Gallagher C, and Machtay M (1999) Lung and aero-digestive cancers in young marijuana smokers. Tumori 85: 140–142.
- Galeone C, Pelucchi C, Talamini R, et al. (2005) Role of fried foods and oral/pharyngeal and oesophageal cancers. British Journal of Cancer 92(11): 2065–2069.
- Gillison ML, Koch WM, and Shah KV (1999) ‘‘Human papillomavirus in head and neck squamous cell carcinoma: are some head and neck cancers a sexually transmitted disease?’’ Current Opinions in Oncology 11: 191–199.
- Gloeckler Ries LA, Miller BA, Hankey BF, Kosary CL, Harras A, and Edwards BK (eds.) (1994) SEER Cancer Statistics Review, 1973–1991. Bethesda, MD: US Department of Health and Human Services, Public Health Service, National Cancer Institute Report no. NIH-94–2789.
- Goldberg H, Lockwood S, Wyatt S, and Crossett L (1994) Trends and differentials in mortality from cancers of the oral cavity and pharynx in the United States, 1973–1987. Cancer 74: 565–572.
- Greenwood M, Thomson PJ, Lowry RJ, and Steen IN (2003) Oral cancer: material deprivation, unemployment and risk factor behaviour—an initial study. International Journal of Oral and Maxillofacial Surgery 32(1): 74–77.
- Gronbaek M, Becker U, Johansen D, Tonnesen H, Jensen G, and Sorensen TI (1998) A population based cohort study of the association between alcohol intake and cancer of the upper digestive tract. British Medical Journal 317: 844–847.
- Gupta PC (1984) A study of dose-response relationship between tobacco habits and oral leukoplakia. British Journal of Cancer 50: 527–531.
- Gupta PC (1999) Mouth cancer in India; a new epidemic? Journal of the Indian Medical Association 97: 370–373.
- Gupta PC, Mehta FS, Daftary DK, et al. (1980) Incidence of oral cancer and natural history of precancerous lesions in a 10-yr follow-up study of Indian villagers. Community Dentistry and Oral Epidemiology 8: 287–333.
- Hashibe M, Jacob BJ, Thomas G, et al. (2003) Socioeconomic status, lifestyle factors and oral premalignant lesions. Oral Oncology 39: 664–671.
- Hashibe M, Ritz B, Le AD, Li G, Sankaranarayanan R, and Zhang ZF (2005a) Radiotherapy for oral cancer as a risk factor for second primary cancers. Cancer Letters 220(2): 185–195.
- Hashibe M, Straif K, Tashkin DP, Morgenstern H, Greenland S, and Zhang ZF (2005b) Epidemiologic review of marijuana use and cancer risk. Alcohol 35(3): 265–275.
- Hindle I, Downer MC, and Speight PM (1996) The epidemiology of oral cancer. British Journal of Oral and Maxillofacial Surgery 34: 471–476.
- Hindle I, Downer MC, Moles DR, and Speight PM (2000) Is alcohol responsible for more intra-oral cancer? Oral Oncology 36(4): 328–333.
- Hindle I and Nally F (1991) Oral cancer; a comparative study between 1962–67 and 1980–84 in England and Wales. British Dentistry Journal 170: 15–20.
- Hirsch JM (2002) Snuff induced cancer in Sweden. 3rd International Conference on Smokeless Tobacco. Stockholm, Sweden Sept 22–25.
- Huang WY, Winn DM, Brown LM, et al. (2003) Alcohol concentration and risk of oral cancer in Puerto Rico. American Journal of Epidemiology 157: 881–887.
- Huebner WW, Schoenberg JB, Kelsey JL, et al. (1992) Oral and pharyngeal cancer and occupation; a case control study. Epidemiology 3: 300–309.
- International Agency for Research on Cancer (1986a) IARC monographs on the evaluation of carcinogenic risks of chemicals to humans. Tobacco Habits other than Smoking: Betel Quid, Areca Nut Chewing, Some Related Nitrosamines Vol. 37. Lyon, France: International Agency for Research on Cancer (IARC).
- International Agency for Research on Cancer (1986b) IARC Monographs on the evaluation of carcinogenic risks to humans. Tobacco smoking Vol 38. Lyon, France: International Agency for Research on Cancer (IARC).
- International Agency for Research on Cancer (1988) IARC monographs on the evaluation of carcinogenic risks to humans. Alcohol drinking Vol. 44. Lyon, France: International Agency for Research on Cancer (IARC).
- Jaber MA, Porter SR, Gilthorpe MS, Bedi R, and Scully C (1999) Risk factors for oral epithelial dysplasia – the role of smoking and alcohol. Oral Oncology 35: 151–156.
- Jacob BJ, Straif K, Thomas G, et al. (2004) Betel quid without tobacco as a risk factor for oral precancers. Oral Oncology 40: 697–704.
- Jeng JH, Chang MC, and Hahn LJ (2001) Role of areca nut in betel quid-associated chemical carcinogenesis; current awareness and future perspectives. Oral Oncology 37: 477–492.
- Kari S, Alho OP, Jokinen K, et al. (1997) Carcinoma of the oral tongue in Northern Finland; trends in overall incidence and patient and tumour characteristics. Journal of Oral Pathology and Medicine 26: 480–483.
- Kujan O, Glenny AM, Duxbury AJ, Thakker N, and Sloan P (2003) Screening programmes for the early detection, prevention of oral cancer. The Cochrane Database of Systematic Reviews. Issue 4. Art. No.: CD004150. DOI: 10.1002/14651858.CD004150.
- Kujan O, Duxbury AJ, Glenny AM, Thakker NS, and Sloan P (2006) Opinions and attitudes of the UK’s GDPs and specialists in oral surgery, oral medicine and surgical dentistry on oral cancer screening. Oral Diseases 12(2): 194–199.
- Landis SH, Murray T, Bolden S, and Wingo PA (1999) ‘‘Cancer statistics, 1999.’’ CA: A Cancer Journal for Clinicians 49(1): 8–31.
- La Vecchia C, Bidoli F, Barra S, et al. (1990) Type of cigarettes and cancers of the upper digestive and respiratory tract. Cancer Causes and Control 1: 69–74.
- La Vecchia C, Tavani A, Franceschi S, Levi F, Corrao G, and Negri E (1997) Epiedmiology and prevention of oral cancer. Oral Oncology 33: 302–312.
- La Vecchia C, Franceschi S, Favero A, et al. (1999a) Alcohol intake and cancer of the upper aerodigestive tract. British Medical Journal 318: 1289–1290.
- La Vecchia C, Franceschi S, Bosetti C, Levi F, Talamini R, and Negri E (1999b) Time since stopping smoking and the risk of oral and pharyngeal cancers. Journal of the National Cancer Institute 91: 726–728.
- La Vecchia C, Lucchini F, Negri E, and Levi F (2004) Trends in oral cancer mortality in Europe. Oral Oncology 40(4): 433–439.
- Levi F, Lucchini F, Boyle P, Negri E, and La Vecchia C (1998) Cancer incidence and mortality in Europe, 1988–92. Journal of Epidemiological Biostatistics 3: 295–361.
- Levi F, Lucchini F, Negri E, Boyle P, and La Vecchia C (1999) Cancer mortality in Europe, 1990–1994, and an overview of trends from 1955 to 1994. European Journal of Cancer 35: 1477–1516.
- Levi F, Lucchini F, Negri E, and La Vecchia C (2003) The end of the tobacco-related lung cancer epidemic in Europe. Journal of the National Cancer Institute 95: 631–632.
- Lissowska J, Pilarska A, Pilarski P, et al. (2003) Smoking, alcohol, diet, dentition and sexual practices in the epidemiology of oral cancer in Poland. European Journal of Cancer Prevention 12(1): 25–33.
- Llewellyn CD, Linklater K, Bell J, Johnson NW, and Warnakulasuriya KA (2003) Squamous cell carcinoma of the oral cavity in patients aged 45 years and under: a descriptive analysis of 116 cases diagnosed in the South East of England from 1990 to 1997. Oral Oncology 39(2): 106–114.
- Llewellyn CD, Johnson NW, and Warnakulasuriya S (2004) Factors associated with delay in presentation among younger patients with oral cancer. Oral Surgery Oral Medicine Oral Pathology Oral Radiology & Endodontics 97(6): 707–713.
- Lopez LAM, Gomez GCE, Navarro AG, Lapiedra RC, Hernandez MJG, and Rojas VD (2000) Risk of oral cancer associated with tobacco smoking, alcohol consumption and oral hygiene: a case-control study in Madrid, Spain. Oral Oncology 36: 170–174.
- Luna-Ortiz K, Guemes-Meza A, Villavicencio-Valencia V, and Mosqueda-Taylor A (2004) Lip cancer experience in Mexico. An 11-year retrospective study. Oral Oncology 40(10): 992–999.
- MacFarlane GJ, Boyle P, and Scully C (1987) Rising mortality from cancer of the tongue in young Scottish males. Lancet 2: 912.
- MacFarlane GJ, Boyle P, and Scully C (1992) Oral cancer in Scotland: Changing incidence and mortality. British Medical Journal 305: 1121–1123.
- Macfarlane GJ, Boyle P, Evstifeeva TV, et al. (1994a) Rising trends of oral cancer mortality among males worldwide: the return of an old public health problem. Cancer Causes Control 5: 259–265.
- Macfarlane GJ, Evstifeeva TV, Robertson C, et al. (1994b) Trends of oral cancer mortality among females worldwide. Cancer Causes Control 5: 255–258.
- Mackenzie J, Ah-See K, Thakker N, et al. (2000) Increasing incidence of oral cancer amongst young persons; what is the aetiology? Oral Oncology 36: 387–389.
- Mathew B, Sankaranarayanan R, and Nair PP (1995) Evaluation of chemoprevention of oral cancer with Spirulina fusiformis. Nutrition and Cancer 24(2): 197–202.
- McLeod NM, Saeed NR, and Ali EA (2005) Oral cancer: delays in referral and diagnosis persist. British Dental Journal 198(11): 681–684.
- Merletti F, Boffetta P, Ferro G, et al. (1991) Occupation and cancer of the oral cavity or oropharynx in Turin, Italy. Scandinavian Journal of Work, Environment & Health 17: 248–254.
- Moles DM, Downer MC, and Speight PM (2002) Meta-analysis of measures of performance reported in oral cancer and precancer screening studies. British Dentistry Journal 192: 340–344.
- Moller H (1989) Changing incidence of cancer of the tongue, oral cavity and pharynx in Denmark. Journal of Oral Pathology and Medicine 18: 224–229.
- Moore SR, Johnson NW, Pierce AM, and Wilson DF (1999) The epidemiology of lip cancer; a review of global incidence and aetiology. Oral Diseases 5: 185–195.
- Moore SR, Johnson NW, Pierce AM, and Wilson DF (2000) The epidemiology of mouth cancer; a review of global incidence. Oral Diseases 6: 65–74.
- Mork J and Glattre E (1998) Squamous cell carcinomas of the head and neck in Norway, 1953–92: an epidemiologic study of a low-risk population. Cancer Causes and Control 9: 37–48.
- Morse DE, Katz RV, Pendrys DG, et al. (1997) Mouthwash use and dentures in relation to oral epithelial dysplasia. Oral Oncology 33: 338–343.
- Myers J, Elkins T, Roberts D, and Byers R (2000) Squamous cell carcinoma of the tongue in young adults; increasing incidence and factors that predict treatment outcomes. Otolaryngology, Head and Neck Surgery 122: 44–51.
- Nair U, Bartsch H, and Nair J (2004) Alert for an epidemic of oral cancer due to the use of betel quid substitutes gutkha and pan masala; a review of agents and causative mechanisms. Mutagenesis 19: 251–262.
- Nandakumar A, Thimmasetty KT, Sreeramareddy NM, et al. (1990) A population-based case-control investigation on cancers of the oral cavity in Bangalore, India. British Journal of Cancer 62: 847–851.
- Nordby KC, Andersen A, and Kristensen P (2004) Incidence of lip cancer in the male Norwegian agricultural population. Cancer Causes Control 15(6): 619–626.
- Ogden GR (2005 Apr) Alcohol and oral cancer. Alcohol 35(3): 169–173.
- Onizawa K, Nishihara K, Yamagata K, Yusa H, Yanagawa T, and Yoshida H (2003) Factors associated with diagnostic delay of oral squamous cell carcinoma. Oral Oncology 39(8): 781–788.
- Orregia F, De Stefani E, Correa P, et al. (1989) Occupational exposure in cancer of the mouth, pharynx and larynx. Anales Otorrinolaringolo´gicos Ibero-Americanas 16: 365–376.
- Papas RK, Logan HL, and Tomar SL (2004) Effectiveness of a community-based oral cancer awareness campaign (United States). Cancer Causes & Control 15(2): 121–131.
- Parkin DM, Pisani P, and Ferlay J (1999) Estimates of the worldwide incidence of 25 major cancers in 1990. International Journal of Cancer 80: 827–841.
- Petti S and Scully C (2005 Sep) Oral cancer: the association between nation-based alcohol-drinking profiles and oral cancer mortality. Oral Oncology 41(8): 828–834.
- Potter JD, Chavez A, Chen J, et al. (1997) Food, nutrition and the prevention of cancer: a global perspective. Washington DC: World Cancer Research Fund/American Institute of Cancer Research.
- Rahman M, Sakamoto J, and Fukui T (2005) Calculation of population attributable risk for bidi smoking and oral cancer in south Asia. Preventative Medicine 40(5): 510–514.
- Reed SG, Duffy NG, Walters KC, and Day TA (2005) Oral cancer knowledge and experience: a survey of South Carolina medical students in 2002. Journal of Cancer Education 20(3): 136–142.
- Reichart PA, Dietrich T, Khongkhunthian P, and Srisuwan S (2003) Decline of oropharyngeal cancer in Chiangmai province, Thailand, between 1988 and 1999. Oral Oncology 39: 569–573.
- Ries LAG, Kosary CL, Hankey BF, et al. (1999) SEER Cancer Statistics Review, 1973–1996. Washington, DC: National Cancer Institute.
- Robinson KL and MacFarlane GJ (2003) Oropharyngeal cancer incidence and mortality in Scotland; are rates still rising? Oral Oncology 39(1): 31–36.
- Rodriguez T, Altieri A, Chatenoud L, et al. (2004) Risk factors for oral and pharyngeal cancer in young adults. Oral Oncology 40: 207–213.
- Sankaranarayanan R (1990) Oral cancer in India; an epidemiologic and clinical review. Oral Surgery, Oral Medicine, Oral Pathology 69: 325–330.
- Sankaranarayanan R, Duffy SW, Padmakumary G, Day NE, and Nair MK (1990) Risk factors for cancer of the buccal and labial mucosa in Kerala, Southern India. Journal of Epidemiology and Community Health 44: 286–292.
- Sankaranarayanan R, Ramadas K, Thomas G, et al. (2005) Effect of screening on oral cancer mortality in Kerala, India: a clusterrandomised controlled trial. Lancet 365(9475): 1905–1906.
- Schantz S and Yu GP (2002) Head and neck cancer incidence trends in young Americans 1973–1997, with special analysis for tongue cancer. Archives of Otolaryngology–Head and Neck Surgery 128: 268–274.
- Scheifele C, Reichart PA, Hippler-Benscheidt M, Neuhaus P, and Neuhaus R (2005) Incidence of oral, pharyngeal, and laryngeal squamous cell carcinomas among 1515 patients after liver transplantation. Oral Oncology 41(7): 670–676.
- Scott SE, Grunfeld EA, and McGurk M (2005) The idiosyncratic relationship between diagnostic delay and stage of oral squamous cell carcinoma. Oral Oncology 41(4): 396–403.
- Scully C and Bedi R (2000) Ethnicity and oral cancer. Lancet Oncology 1: 37–42.
- Scully C, Field JK, and Tanzawa H (2000) Genetic aberrations in oral or head and neck squamous cell carcinoma 2: chromosomal aberrations. Oral Oncology 36: 311–327.
- Scully C, Newman L, and Bagan JV (2005) The role of the dental team in preventing and diagnosing cancer: 2. Oral cancer risk factors. Dental Update 32(5): 261–262, 264–266, 269–270.
- Seedat HA and van Wyk CW (1988) Betel chewing and dietary habits of chewers without and with submucous fibrosis and with concomitant oral cancer. South African Medical Journal 74: 572–575.
- Shah SM, Mercant AT, Luby SP, and Choitani RA (2002) Addicted schoolchildren; prevalence and characteristics of areca nut chewers among primary school children in Karachi, Pakistan. Journal of Pediatrics and Child Health 38: 507–510.
- Shanks TG and Burns DM (1998) Disease consequences of cigar smoking. Smoking and Tobacco Control Monograph 9. Cigars Health effects and Trends, pp. 105–158. Bethesda MD: National Cancer Institute
- Sheiham A and Watt RG (2000) The common risk factor approach: a rational basis for promoting oral health. Community Dentistry Oral Epidemiology 28(6): 399–406.
- Shiboski C, Shiboski S, and Silverman SJ (2000) Trends in oral cancer rates in the United States, 1973–1996. Community Dentistry and Oral Epidemiology 28: 249–256.
- Shiboski CH, Schmidt BL, and Jordan RC (2005) Tongue and tonsil carcinoma: increasing trends in the U.S. population ages 20–44 years. Cancer 103(9): 1843–1849.
- Sohn W, Ismail AI, and Kolker JL (2005) Knowledge of oral cancer and screening practices of primary care providers at Federally Qualified Health Centers. Journal of Public Health Dentistry 65(3): 160–165.
- Sorahan T, Kinlen LJ, and Doll R (2005) Cancer risks in a historical UK cohort of benzene exposed workers. Journal of Occupational and Environmental Medicine 62(4): 231–236.
- Speight PM, Downer MC, and Zakrzewska J (1993) Screening for oral cancer and precancer: Report of the UK Working Group on Screening for Oral Cancer and Precancer. Community Dental Health 10(supplement 1): 87–89.
- Speight PM, Palmer S, Moles DR, et al. (2006) The cost-effectiveness of screening for oral cancer in primary care. Health Technology Assessment (Winchester, England) 10(14): 1–144, iii-iv.
- Stahl S, Meskin LH, and Brown LJ (2004) The American Dental Association’s oral cancer campaign: the impact on consumers and dentists. Journal of the American Dental Association 135(9): 1261–1267.
- Sunny L, Yeole BB, Hakama M, et al. (2004) Oral cancers in Mumbai, India: a fifteen years perspective with respect to incidence trend and cumulative risk. Asian Pacific Journal of Cancer Research 5(3): 294–300.
- Swango PA (1996) Cancer of the oral cavity and pharynx in the United States: an epidemiologic overview. Journal of Public Health Dentistry 56(6): 309–318.
- Swerdlow AJ, Marmot MG, Grulich AE, and Head J (1995) Cancer mortality in Indian and British ethnic immigrants from the Indian subcontinent to England and Wales. British Journal of Cancer 72: 1312–1319.
- Tarvainen L, Suuronen R, Lindqvist C, and Malila N (2004) Is the incidence of oral and pharyngeal cancer increasing in Finland? An epidemiological study of 17,383 cases in 1953–1999. Oral Diseases 10(3): 167–172.
- Tumino R and Vicario G (2004) Head and neck cancers: oral cavity, pharynx, and larynx. Epidemiologia E Prevenzione 28(2 Suppl): 28–33.
- Van Wyk CW, Stander I, Padayachee A, et al. (1993) The areca nut chewing habit and oral squamous cell carcinoma in South African Indians. A retrospective study. South African Medical Journal 83: 425–429.
- Warnakulasuriya KA and Nanayakkara BG (1991) Reproducibility of an oral cancer and precancer detection program using a primary health care model in Sri Lanka. Cancer Detection & Prevention 15(5): 331–334.
- Warnakulasuriya KAAS, Johnson NW, Linklater KM, and Bell J (1999) Cancer of the mouth, pharynx and nasopharynx in Asian and Chinese immigrants resident in Thames regions. Oral Oncology 35: 471–475.
- Warnakulasuriya KAAS (2004) Smokeless tobacco and oral cancer. Oral Diseases 10: 1–4.
- Wennerberg J, Anderson H, Bjorklund A, and Moller T (1994) Carcinoma of the oral tongue in young adults. Head Neck 16: 480.
- Wilson JMG and Junger G (1968) Principles, practice of screening for disease. (Public health papers No 34) Geneva, Switzerland: World Health Organization.
- Winn DM, Blot WJ, McLaughlin JK, et al. (1991) Mouthwash use and oral conditions in the risk of oral and pharyngeal cancer. Cancer Research 51: 3044–3047.
- World Cancer Research Fund (WCRF), American Institute for Cancer Research (AICR) (1997) Food, Nutrition and the Prevention of Cancer: a Global Perspective. Washington DC: AICR.
- World Health Organisation (WHO) (2002) Food and Agriculture Organisation (FAO). Diet, Nutrition and the Prevention of Chronic Diseases: Report of a Joint WHO/FAO Expert Consultation. Geneva, Switzerland: WHO.
- Zaridze D, Evstifeeva T, and Boyle P (1993) Chemoprevention of oral leukoplakia and chronic esophagitis in an area of high incidence of oral and esophageal cancer. Annals of Epidemiology 3(3): 225–234.
- Zatonski W (1998) Alcohol and health: what is good for the French may not be for Russians. Journal of Epidemiology and Community Health 52: 766–767.
- Zavras AI, Laskaris C, Kittas C, and Laskaris G (2003) Leukoplakia and intraoral malignancies: female cases increase in Greece. Journal of the European Academic of Dermatology and Venereology 17(1): 25–27.
See also:
Free research papers are not written to satisfy your specific instructions. You can use our professional writing services to buy a custom research paper on any topic and get your high quality paper at affordable price.








