This sample Pharmaceuticals as Environmental Pollutants Research Paper is published for educational and informational purposes only. If you need help writing your assignment, please use our research paper writing service and buy a paper on any topic at affordable price. Also check our tips on how to write a research paper, see the lists of health research paper topics, and browse research paper examples.
Introduction
Human exposure to pharmaceuticals other than through actual therapeutic, lifestyle, or recreational usage can occur by two primary but very distinct routes. These two exposure routes have totally different consequences and pose completely different challenges with regard to their control or reduction. The first route is unintended, unexpected exposure through the consumption of water and foods contaminated by pharmaceutical residues that have entered the environment as a result of their intended use, such as by excretion and bathing or upon disposal, and that then become ‘recycled.’ This route can theoretically lead to chronic, inadvertent exposure to extremely low levels of complex mixtures of pharmaceuticals (as well as other chemical stressors that are part of the ambient environment). The second route involves both the unintended and purposeful human exposure to leftover, unused drugs being stored as wastes but that then become diverted from eventual disposal. This route is known to result in acute, high-level exposures, generally to single drug entities at a time, and is responsible for significant human morbidity and mortality.
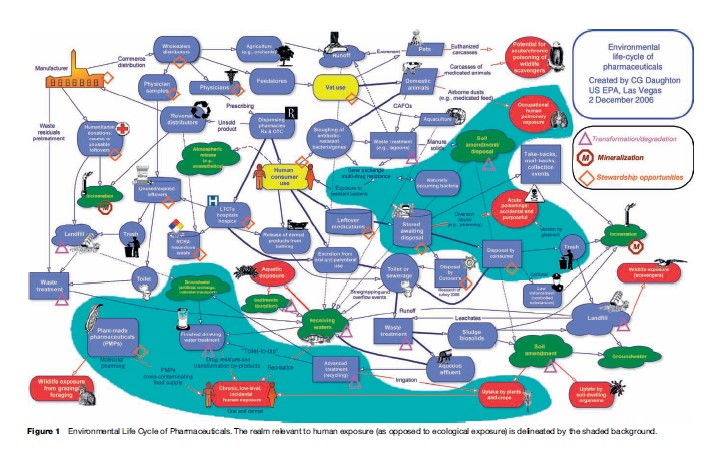
While the risks associated with exposure to environmental pharmaceutical residues are probably most significant with regard to ecological exposure (especially in the aquatic environment – see Figure 1), the public’s concern is understandably more focused on human exposure. Most of the published literature, however, addresses the occurrence of pharmaceuticals in sewage effluent and natural waters, information not of immediate use for assessing human exposure, where consumption of tap water (or bottled water) is the key concern. Other exposures can result, however, from consumption of foods contaminated with residues whose origins are from their discharge to the environment. For a number of reasons, the concentrations of pharmaceuticals when they persist in tap water can be orders of magnitude lower than in the aquatic environment. Such low concentrations (parts per trillion/billion (ppt/ppb) and lower) pose major challenges even for advanced chemical analysis. For this reason, sparse data are available on the occurrence of pharmaceuticals in point-of-use (POU) drinking waters – that is, tap water dispensed for end use from plumbing fixtures.
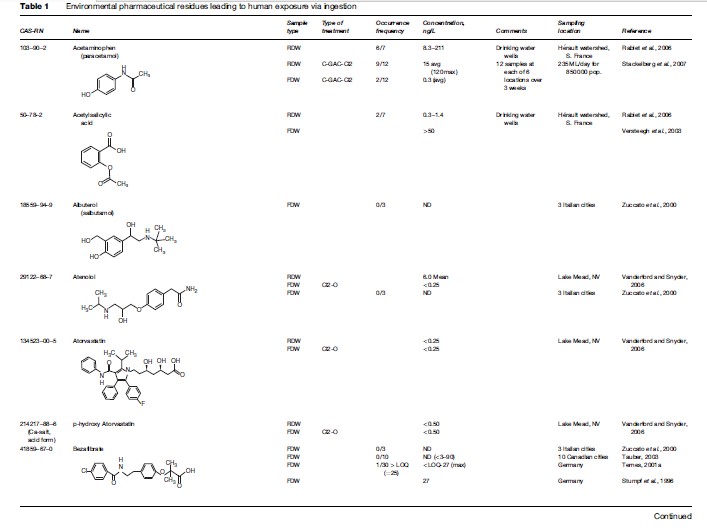
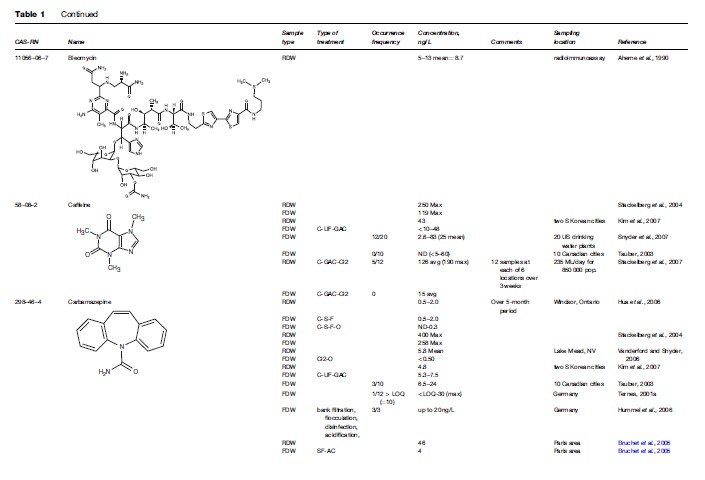
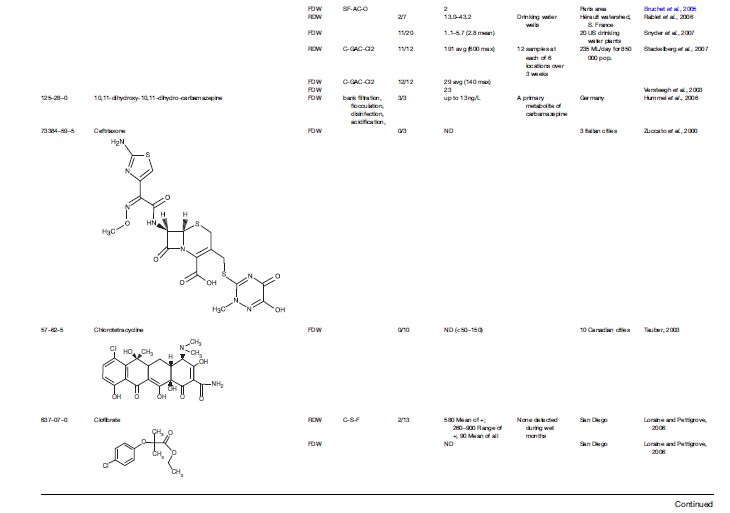
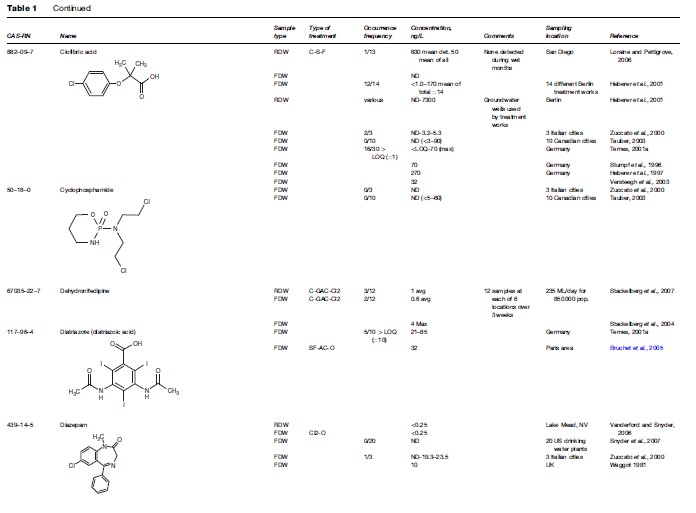
Although advances in analytical chemistry have made possible the detection of many drug residues in the environment at individual concentrations of roughly 1 ng/L, these analytical tools are still insufficient for comprehensively identifying and rigorously quantifying the entire universe of pharmaceuticals that might exist in drinking waters or as unintended residues in foods – residues that the consumer should have no reason to expect. Even more difficult is trace-level determination of the unknown numbers of bioactive transformation or degradation products pre-existing from drug usage (e.g., bioactive metabolites) or newly created in drinking water; disinfection byproducts from drugs themselves are one example of the latter. The trace residues of pharmaceuticals that remain after treatment of sewage, for example, are usually further removed during later treatment to create finished potable water. The concentrations in drinking water, therefore, can be one or more orders of magnitude lower yet than in source waters. These concentrations challenge the most advanced detection methods currently used by environmental chemists. The analytical wherewithal to implement routine monitoring of drinking waters simply does not yet exist. The little that is known of the occurrence of pharmaceuticals in drinking water represents an unknown portion of the overall exposure picture. Knowledge of unintentional food residues that are derived from the ambient environment is practically nonexistent. A databank on drug occurrence in drinking waters simply does not exist. A first step toward establishing such a database is shown in Table 1 (‘Environmental pharmaceutical residues leading to human exposure via ingestion’).
It is not currently possible to directly assess human exposure to environmental residues of pharmaceuticals by measuring biomarkers of exposure (as done in biomonitoring programs; see the excellent general discussion on biomonitoring provided by Paustenbach and Galbraith, 2006) because such markers would be present at concentrations far below those that are detectable and impossible to distinguish from natural background and variation. For the time being, gross estimates of exposure can only be derived indirectly from empirical environmental occurrence data.
Defining ‘Drinking Water’
For this discussion, it is important to develop a common understanding of what is meant by ‘drinking water.’ The published research literature is often confusing because it uses this term interchangeably when referring to ‘source waters’ (an example being ‘terminal reservoirs’ that serve as drinking water sources), finished or polished distributed water (as it leaves water treatment facilities), and POU water. With regard to human exposure, however, it is really only the latter that is pertinent. Most monitoring data purported to apply to ‘drinking water’ actually derive from source waters, which commonly undergo further treatment prior to potable use, rather than from POU drinking water. These important distinctions are often not made clear in the literature. POU concentration data are extremely limited worldwide, with even fewer data available for the United States. Note, however, that special cases must be acknowledged, in which drinking water can be contaminated with abnormally high levels, such as with well water that might be subjected to only nominal treatment before drinking (for example, depth filtration) and that has been contaminated by leaching from septic systems or abandoned landfills.
Assessing Risk Resulting From Exposure
The knowledge required for assessing whatever risks might exist for ecological exposure to trace levels of pharmaceuticals is more extensive than for assessing risks posed to humans. Although the first paper to address the issue of human exposure was published nearly a decade ago (Christensen, 1998), only a few risk assessments have been published with regard to drinking water exposure, and these rather rudimentary studies have been based on extrapolations from the comparatively high doses required for therapeutic effects or ‘safe’ therapeutic doses such as acceptable daily intakes (ADIs), which are orders of magnitude higher than the levels that could possibly impart less obvious, perhaps subtle, nontherapeutic effects (e.g., diminution in learning or behavioral alterations); these studies also could not cover all ADIs or even representative ADIs that span all mechanisms of action (since many of these mechanisms are unknown). These initial studies include those of Jones et al. (2004, 2005), Schulman et al. (2002), Schwab et al. (2005), and Webb et al. (2003). These approaches use predicted no effect concentrations determined for each pharmaceutical under consideration. These values are then compared with measured (or predicted) environmental concentrations. Using this extrapolative approach, risks for the time being have generally been ruled out for human exposure via drinking water.
Scope Of Human Exposure
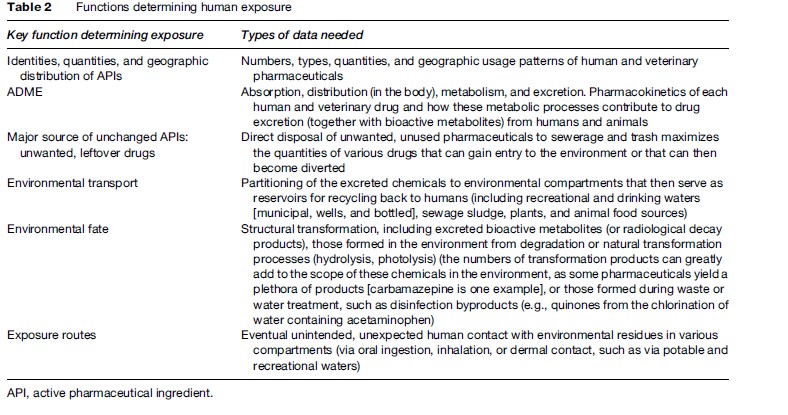
Human exposure to pharmaceuticals from the environment is a complex function of several variables, all of which require a better understanding; see Table 2.
Human exposure is but one part of the much larger issue of pharmaceuticals as environmental contaminants (see Figure 1 for a detailed perspective on the complex routes by which pharmaceuticals travel through the environment). Exposure is an extremely complex process, one that yields an effect only when a wide spectrum of processes happen to be suitably coordinated spatially and temporally so as to perturb the homeostasis mechanisms of an organism; this is embodied in the concept of the 4Ts: toxicant, totality, tolerance, and trajectory (see Daughton (2005) for further discussion). With respect to ‘totality,’ it is important to remember that environmental pharmaceutical residues are but one of many classes of anthropogenic and naturally occurring stressors. But isolating one stressor class from all others in order to assess risk has long been a necessary step in attempting to simplify an otherwise overwhelmingly complex task.
Human Exposure: The Major Unanswered Questions And Major Outcomes
Of all the aspects of assessing the significance of pharmaceutical residues in the environment, one aspect has received far less attention than the others – human exposure. Little research is currently underway in assessing human risk, perhaps because it is perceived as minimal at best. The data gaps and uncertainties that are apparent from the very limited data regarding human exposure to the recycling of pharmaceuticals from the environment, and from exposure via leftover drug waste awaiting disposal, involve some key questions and uncertainties (Table 3).

The needs outlined under 2 and 5 in Table 3, especially those related to low-dose effects and effects from exposure to multiple stressors, are particularly amenable to addressing through toxicogenomics; see Oberemm et al. (2005) for an overview.
Successfully addressing the unknowns surrounding these key, unanswered questions could eventually lead to some major outcomes, such as those in Table 4.

Human Exposure Routes
There are two primary routes leading to human exposure of nontherapeutic or recreational drugs: (1) ingestion of ambient residues by way of their recycling from the environment (via drinking water and foods), (2) and ingestion of leftover drug waste (accidental and purposeful). When considering these routes, the following factors become important to understand.
Critical Aspects Of Human Exposure
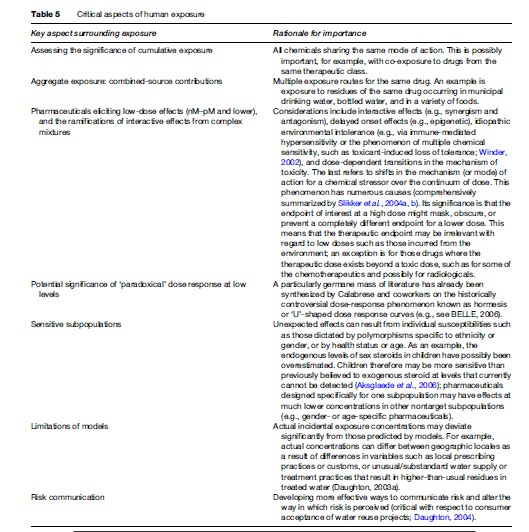
Exposure to trace residues of drugs from the environment highlights some of the major challenges facing both environmental and human toxicology today. There are many questions but few answers. These consumer chemicals epitomize the importance of obtaining a better understanding of the significance of a number of aspects surrounding exposure (Table 5).
An overlooked aspect of prioritizing pharmaceuticals for assessing risk is the toxicological significance of those pharmaceuticals that have no inherent toxicity of their own but that can potentiate the toxicity of other stressors. Such indirect toxicants include inhibitors of efflux pumps or multidrug transporters, and modulators or disruptors of P450 and the cellular stress response (Daughton, 2005), or immunosuppressants that lead to increased rates of infection.
Ingestion Of Trace Residues Present As Trace Pollutants In The Environment
The first major route of exposure is ingestion of trace residues that have entered the environment and persist as contaminants in drinking water or that have been assimilated into foods. While this route holds the potential for chronic, simultaneous exposure (cumulative and aggregate) to very low levels of drugs, its full scope, extent, and significance is poorly understood, mainly because of (1) the analytical difficulties in analyzing polished drinking waters and foods for such low concentrations (ng/L and lower) of a wide spectrum of possible analytes (most of which are relatively polar), and (2) the toxicology of chronic human exposure to low-level mixtures being poorly understood. It is important to note that part of the scope of this exposure route also includes the environmental degradates and transformation products, as well as excreted human and domestic animal metabolites. Some of these chemicals possess significant biological activity themselves; some are even the actual therapeutic entity or are responsible for adverse effects when used therapeutically. This exacerbates the overall complexity of the problem, especially since transformation products are generally not the current targets of environmental monitoring.
Drinking Water As A Source Of Exposure
Table 1 compiles the majority of data published (as of January 2007) for the occurrence of pharmaceuticals in actual drinking water – both ‘raw’ and polished (finished). ‘Raw’ drinking water pertains to the water as it enters a treatment regime (if any) at a drinking water facility; note that exposure via drinking water includes not only municipal drinking water but also private well water, as well as commercial bottled water (e.g., see Perret et al., 2006). Necessarily excluded from Table 1 are occurrence data for ambient-environment waters (for example, the source waters as they exist in drinking water reservoirs) that have not yet been drawn into a drinking water treatment system. The rationale is to include only those data that are most closely relevant to actual point-of-use human consumption. It is important to recognize that extremely few monitoring studies or surveys have ever been attempted for actual point-of-use waters – that is, those waters as they are drawn from a household plumbing fixture. Included in Table 1 are data (when appropriate) from other countries because the data specific for the United States are so limited. A substantial amount of negative occurrence data exist in the literature. While these ‘data of absence’ are valuable for drawing conclusions concerning those drugs that are less likely to occur in drinking water, only a few have been captured in this compilation. Purposefully omitted from this compilation are studies resulting from special instances, for example, where drinking water sources are unusually contaminated by drug residues. An example is groundwaters highly contaminated by leachate from defective or abandoned landfills or manufacturing wastes; these types of data do not represent the norm with respect to drinking waters.
The available data (as of early 2007) show that the number of APIs and bioactive metabolites that had been detected above their quantitation limits in finished drinking waters was roughly 45 (see Table 1). Generally, their concentrations tend to be less than 1–10 ng/L, some in the 10–100 ng/L range, and a few in the range of hundreds of ppt (sub-ppb), such as: carbamazepine (258 ng/L max; Stackelberg et al., 2004), ibuprofen (930 ng/L: 120 ng/L mean; Loraine and Pettigrove, 2006), ibuprofen methyl ester (4 950 ng/L: 330 mean; Loraine and Pettigrove, 2006), and triclosan (734 ng/L: 49 ng/L mean; Loraine and Pettigrove, 2006). Note that in order to get a complete perspective regarding the relative concentrations of drugs in the environment, mass concentrations also need to be converted to molar concentrations, especially for drugs of wildly disparate molecular weights. Environmental scientists tend to report concentrations on the basis of mass, whereas pharmacologists and those in the life sciences often focus in molarity. Probably an even larger number of instances have been reported where certain drugs could not be detected above their quantitation limits – data of absence; only a select few of these data are also shown in Table 1. Of significance, however, is that even the combined numbers of those originally targeted by analysis (those above and those below their limits of quantitation) represent but a small fraction of those hundreds of drugs that are used most routinely and an even smaller fraction of the thousands in use commercially. The diversity and magnitude of the commercial market for human pharmaceuticals is provided in a discussion by Daughton (2007); these figures do not include the very large amounts of certain drugs (such as antibiotics) used in agriculture.
Worth noting is that of the chemicals listed in Table 1, the endogenous steroids (e.g., estradiol, estrone, progesterone, testosterone) are included because they are also used in certain pharmaceutical formulations, for example, hormone replacement therapies. The concentrations of these steroids in the environment originate from both natural, endogenous synthesis and from therapeutic administration (oral and dermal). Furthermore, with the steroid biosynthesis pathway (with all of its branches and interconversions), the administration of a therapeutic steroid drug leads to the synthesis of its pathway metabolites – metabolites that would not have been produced (or would not have reached their new concentrations) had the drug not been administered. This further complicates the issue as to whether sex steroids in the environment result from ‘natural’ processes or from pharmaceutical therapy.
With respect to these empirical occurrence data, it is critical to recognize that published data on drug occurrence can be inherently biased. Bias occurs because new target-based monitoring studies often use the positive occurrence data from prior studies as the basis for selecting their targets (partly in order to increase the chances of collecting positive occurrence data). The data for a given pharmaceutical may also not be representative because of the probability of very high spatial and temporal fluctuations in sewage effluents. There is a distinct probability that samples are taken during transiently high or low concentrations. Discrete samples (e.g., ‘grab’ samples) will not necessarily represent average concentrations. But the greater mixing achieved during transit from the time of uptake until drinking water treatment would possibly serve to smooth these fluctuations, reducing their impact on concentration variability.
A particular group of pharmaceuticals with little monitoring data or assessment of potential risks comprises the low levels of radionuclides used in a growing number of diagnostic procedures, and the higher levels of certain radionuclides used in medical treatments. One example is metastable technetium (Tc-99m). While Tc-99m has a short half-life (6 h), its progeny, Tc-99, is much longer lived and can accumulate in receiving waters after excretion. Other radionuclides, used in much larger amounts, are iodine 131 and samarium 153.
Alternative approaches for assessing drinking water contamination are possible by using the occurrence of certain drugs as indicators of the likely presence of other drugs. One example is the use of occurrence data for iodinated X-ray contrast media. These diagnostic pharmaceuticals are used in very high quantities and are excreted unchanged. They are also among those that are most refractory to removal by drinking water treatment technologies (especially processes including oxidation and sorption by activated carbon). Therefore, if X-ray contrast media are present in the feed for drinking water but absent from the treated (polished) water, this could be an excellent indication that most other pharmaceuticals should also be absent (e.g., Bruchet et al., 2005).
Highly Conservative Assessments Show Extremely Low Risks From Incidental Mixture Exposures
In the inadvertent, incidental exposure of humans to APIs also known as pharmacons via drinking water and food, the risks ordinarily deemed as acceptable for doses needed to achieve therapeutic outcomes do not apply, because the doses resulting from these surreptitious exposures from the environment are orders of magnitude lower. Furthermore, those experiencing incidental exposure (beyond their control or knowledge) are not necessarily presuming that they are receiving any benefit. For these people, the so-called ‘nocebo’ effect might even apply (Daughton, 2004); see later discussion in section titled ‘Perception of exposure as a confounding factor in assessing risk.’ Consequently, the use of benchmark therapeutic doses of APIs for assessing the risks of incidental exposure are not applicable. A more conservative approach is therefore required.
One possible conservative approach for roughly estimating the risk associated with incidental ingestion of environmental residues of APIs recycled back via water and food would be to calculate the numbers of APIs that could be ingested on an ongoing lifetime daily basis without exceeding a predetermined nontherapeutic acceptable daily intake. Note that the complicating issue of bioconcentration is not a factor with most pharmaceuticals, as this is a design property purposefully avoided for most human medications; also note that dermal exposure to environmentally recycled APIs is probably not an issue because most APIs have poor dermal transport. The problem is in defining sufficiently conservative ADIs (especially if they must be individually determined for each API). An approach that could be broadly applied across nearly all APIs would be most useful for providing a rough but highly conservative assessment of the potential for human risk due to ongoing exposure to multiple APIs.
Because of a lack of data, it is not possible to assume the worst-case but highly improbable scenario – namely, synergistic interactions among the APIs. But another conservative assumption – namely that of dose additivity – can be easily considered for all of the APIs as representing a very adverse scenario. By selecting an adverse endpoint such as genotoxicity, one can estimate the number of APIs to which continual exposure can be sustained and for which the lifetime excess cancer risk is limited to the upper bound of 1 in 105 to 106; this is the approach used for assessing risk from impurities introduced in drug manufacturing or from the degradates resulting after manufacturing. The threshold of toxicological concern (TTC) for carcinogens, based on lifetime exposure, is accepted to be roughly 1 mg/day (except for very highly potent carcinogens, which have TTCs of about 0.15 mg/day) (see Dolan et al., 2005; McGovern and Jacobson-Kram, 2006; Mu¨ller et al., 2006); it must be noted, however, that this approach assumes a body weight of 60 kg, and therefore the TTC could be lower for those who are lighter, such as children. These levels of exposure are considered ‘virtually safe’ (or to pose ‘reasonable certainty of no harm’) for carcinogens; background on the history behind the TTC is provided by Barlow et al. (2001) and Kroes et al. (2005).
For some perspective on the risk associated with the 1-mg/day TTC, consider one of the best-known naturally occurring toxicants. Aflatoxin B1, a ubiquitous mycotoxin naturally occurring especially in grains, is a potent human carcinogen. The FDA action level in human food is 20 ppb for total aflatoxins, with the exception of milk, where the action level is 0.5 ppb 0.5 mg/L) for aflatoxin M1 (an aflatoxin metabolite); a dosage of 1 mg/day for total aflatoxins would be reached upon consuming 50 g of grain, which just meets the FDA action level. Although the possibility exists that the thresholds could be lower for other endpoints such as allergenicity or neurologic, immunologic, endocrine, or developmental toxicity, data show that these TTCs are probably at least one order of magnitude higher (Barlow et al., 2001) than for genotoxicity. If it were assumed that all APIs act as genotoxicants, and that the nominal concentration for any given API present in POU drinking water in the United States were assumed to be upwards of 10 ng/L (this number would need to be refined after expansion of the limited occurrence data; see Table 1), and the water intake per day were assumed to be 2 L (Webb et al., 2003), then the number of APIs that could be simultaneously present on a perpetual basis but remain below the TTC would be 50 (TTC 1000 ng/day ×L/10 ng×day/2 L).
With this relatively worst-case scenario, we see that upwards of 50 APIs could be perpetually present in drinking water at 10 ng/L while maintaining the lifetime excess cancer risk at less than 10-6. Since only a few APIs have ever been identified as occurring simultaneously in given POU drinking waters, and if the assumption is made that exposure to a multitude of APIs occurs only infrequently (because of their episodic release to the environment), then the TTC increases to 120 mg/day (for exposures less than 1 month; Mu¨ller et al., 2006). The number of APIs that could be safely present at individual concentrations of 10 ng/L increases to 6000, a number that clearly would never be reached under any condition. Furthermore, the TTC of 1 mg/L is a rough estimate for compounds that are ‘likely to be carcinogenic.’ Since this does not broadly apply to APIs (with the exception of some chemotherapeutics), the more relevant TTC would be 10 mg/day for compounds that are ‘likely to be potent or highly toxic’ or 100 mg/day for those that are ‘not likely to be potent, highly toxic, or carcinogenic’ (Dolan et al., 2005). This would allow the acceptable exposure concentrations of APIs or their total numbers to increase another one to two orders of magnitude. Numbers this high would not even be very sensitive to highly conservative uncertainty factors.
Another factor to consider regarding human exposure to APIs is that the ADI in a therapeutic setting is necessarily a direct function of the therapeutic dose, which in turn is a function of potency. Assuming pharmacokinetics not wildly different (similar excretion of the parent API) and overall usage rates that were similar, if the amount of an API entering the environment exceeds that of others, it also means that its ADI is very possibly higher. Predicted environmental concentrations (PECs) would therefore tend to be inversely related to potency. Risk due to environmental residues would then be considered ‘self-leveling’ to a certain extent. This contrasts sharply with industrial chemicals, for example, whose usage rates have much less connection with potencies.
In general, the finding of low risk to humans posed by pharmaceutical residues in drinking water is corroborated by the published literature (Jones et al., 2004, 2005; Schulman et al., 2002; Schwab et al., 2005; Webb et al., 2003), albeit limited. This is also the official position of the Pharmaceutical Research and Manufacturers of America (PhRMA).
The Other Side Of The Exposure Debate
With these points in mind, however, we cannot conclude that the absence of any evidence for adverse human effects from extremely low-level exposure to pharmaceuticals eliminates cause for concern. There are simply too many unknowns facing toxicology today. This is articulated by Grandjean (2005):
Toxicology has failed the purpose of science in society by striving to reach only the limited goals of solving simplified riddles and in recognizing, rather than exploring uncertainty. By ignoring the larger perspectives of chemical causation of disease, it has failed its responsibility to contribute to the foundation of disease prevention.
Moreover, the possibility for adverse human health effects at the extremely low levels of ambient drug residues hinges in part on the meaning of the so-called ‘noobserved-effect level’ (NOEL), which is the maximum concentration of a chemical stressor known to produce an ‘observed’ adverse effect. The key word is ‘observed.’ The endpoint measure for the effect is one that is either anticipated or subsequently observed during testing. Necessarily, the NOEL can change with the revelation of new effects never previously observed at concentrations below the established NOEL. This is especially germane to effects that are sufficiently subtle that they cannot currently be anticipated or revealed using current testing protocols; this is a concern first proposed by Daughton and Ternes (1999). One example of a phenomenon that can confound the interpretation of low-level effects is hormesis. The possibility that hormesis (nonrandom biological activity below the NOEL) is a widespread phenomenon is becoming clearer with closer re-examination of classic dose-response studies, where it is seen that a threshold-like model for dose response may not hold (Calabrese et al., 2006).
Knowledge Gained From Epidemiology
Another source of potential information for indirectly assessing human exposure derives from certain previously conducted epidemiological studies. These studies have compared populations consuming water derived at least in part from sewage after varying degrees of treatment. Those studies designed around water reuse projects are particularly germane. The studies on water reused for drinking are few (less than a dozen) and largely conclude that neither microbiological nor chemical contaminants have led to any overt, adverse health effects, although the incidence of certain health problems has been elevated in some of these studies (DHH\PD, 2001; NRC, 1998). While no adverse health effects in communities using reclaimed water have been documented to result from nonmicrobiological contaminants, it is important to note that these studies had to use as controls populations relying on conventional sources of water. This approach is therefore obviously insensitive to detecting potential effects resulting from contaminants shared by both types of water supplies. A recent study, among a number of others preceding, found no evidence for an association between partially wastewater-derived drinking water and breast cancer in a locale with a higher-than-predicted incidence of breast cancer (Brody et al., 2006).
Complexities Of Low-Level Multiple Exposure: The Potential For Mixture Effects
On the other side of the equation, the evidence pointing to the possible absence of human health effects during chronic or repeated exposure to multiple pharmaceuticals at very low levels is tempered by studies just beginning to emerge that focus on more subtle endpoints.
Studies examining the potential for effects from simultaneous or sequential exposure to low individual concentrations of pharmaceuticals are rare, even for nonhuman targets. Studies designed for revealing human risks have only just begun, the first being published in 2006 (Pomati et al., 2006). This study is the first to examine (using an effects endpoint relevant to humans) the important issue of mixture effects with regard to pharmaceuticals. The study used a mixture comprising ‘near’-real-world concentrations of drugs from disparate therapeutic classes: atenolol, bezafibrate, carbamazepine, cyclophosphamide, ciprofloxacin, furosemide, hydrochlorothiazide, ibuprofen, lincomycin, ofloxacin, ranitidine, albuterol, and sulfamethoxazole. The concentration of each drug by itself was insufficient to impart measurable effects for the endpoint – several aspects of the growth of human embryonic cells – but the mixture could. Although the concentration of each component was probably somewhat higher than what exists in the environment (but still in the ng/L range, sub-ppb), the actual number of drugs in the mixture (roughly a dozen) may better represent reality. Note, however, that it is not known whether these concentrations in cell culture, while reflective of drinking water concentrations, would mimic the actual concentrations that would be available to cells in a human.
Although exposure to multiple drugs sharing the same specific target of action is one potential mode by which simultaneous exposure to ‘ultra-low’ pharmaceutical doses could potentially impart effects, another that has received comparatively little attention is via a biochemical target with extremely broad capacity to interact with a wide spectrum of chemical stressors. A common biochemical pathway by which large numbers of stressors could act would provide the means by which additive effects could easily reinforce each other – a mechanism by which a receptor pathway could integrate the ordinarily small individual responses elicited by each of a wide array of chemicals having disparate mechanisms of therapeutic action. Such a pathway was recently reported by Li et al. (2007), who studied oligodendrocyte precursor cells, from which the myelin-forming oligodendrocytes of the central nervous system are formed. The oligodendrocytes respond to small changes in redox conditions, and determine whether these cells divide or differentiate. The latter is caused by oxidative stressors. While the authors used nonpharmaceutical stressors, the work was conducted at low, environmentally relevant concentrations and has broad implications. Since many drugs are known to elicit oxidative stress (a few examples being indomethacin, tamoxifen, thioridazine, chloroquine, cyclosporine A, diazepam, fibrates (and clofibric acid), and acetaminophen), a plausible mechanism exists by which adverse effects could theoretically arise – in this case, a developing central nervous system could be affected.
Ingestion Of Waste Medications
The second route to human exposure is intimately related to the accumulation and storage of unused, unwanted medications by consumers and other end users, as a prelude to disposal. Leftover drugs constitute household waste. Many other sources of stockpiled unwanted drugs also exist and serve as potential sources of acute exposure for humans; these are presented in a comprehensive flow chart prepared by Ruhoy and Daughton (2008). Once medications accumulate unused and unwanted by the consumer, they become chemical waste and therefore represent an exposure route outside of their intended use in medical therapy. That portion of the leftover drugs that is immediately disposed to sewerage constitutes one of the principal sources for environmental residues – excretion being the other major source. That portion of leftover drugs not immediately disposed of, but rather stored in the household or other locations, as well as that portion discarded to domestic trash is subject to diversion (use by those for whom the medications were not prescribed). Drugs discarded to curbside trash or municipal landfills can be reclaimed and reused by those who scavenge for them (e.g., human ‘gleaners’); some landfills also practice manual sorting, where the presence of discarded drugs could be readily revealed. Diversion of controlled substances is a major concern of the U.S. Drug Enforcement Administration (DEA) and the White House Office of National Drug Control Policy (White House, 2007).
Waste medications serve as a principal source of acute human exposure, leading to substantial morbidity and mortality as a result of their purposeful ingestion by those for whom they were not intended (e.g., recreational drug users, drug abusers) or as a result of unintentional or accidental ingestion, for example by infants, toddlers, children, and the elderly (AAPCC, 2005). The relatively large problem of acute drug poisonings, some of which results directly by inappropriate storage of waste pharmaceuticals, represents an intersection of the domain of human health and safety with that of environmental protection. Measures to protect humans from drug poisonings will also serve to protect the environment. Storage of waste drugs in domiciles (as well as a broad spectrum of other places; see Ruhoy and Daughton, 2008) is a major source of drug diversion. The growing prevalence of teen ‘pharming’ is one example of diversion, and scavenging domestic refuse is another.
Another means that consumers sometimes employ for dealing with leftover drugs that would otherwise be destined for waste disposal is the practice of reusing drugs by providing them to another end user. While not always legal (e.g., for prescription medications) or medically prudent, reuse (also called drug ‘sharing’) is usually accomplished by donation to charitable organizations or by sharing with family and friends. Drug ‘sharing’ probably does not play a significant widespread role as an added source of environmental residues, but it does pose substantial acute risks with regard to human health and safety. Drug sharing is a practice that continues to grow as a result of the public’s frustration with not being able to make use of drugs that one person no longer wants or needs but that another person does. Both consumers and physicians sometimes practice it as an alternative to disposal. The practice of self-medication is hazardous itself and is responsible for a large percentage of hospitalizations from adverse drug responses (e.g., wrong drug, wrong dosage). With respect to charitable drug donations, it is worth noting that the World Health Organization and other international organizations involved with humanitarian relief now discourage the donation of drugs during relief efforts because of the high prevalence of inappropriate, unusable, or expired drugs (Daughton, 2007). Extremely large quantities of donated drugs accumulate on site and create large financial burdens (for warehousing and disposal) as well as opportunities for diversion.
Currently, the only aspect of pharmaceuticals (outside their intended use) known to directly impact human morbidity and mortality is their substantial contribution to accidental and purposeful poisonings (AAPCC, 2005).
One of the factors determining or encouraging inappropriate or undesired access to drugs is the prevalence of improper storage or misguided attempts at disposal, which are in turn caused by the accumulation of leftover drugs. Many factors lead to the unnecessary storage of unwanted pharmaceuticals in domestic residences. Poor levels of adherence (compliance) by patients to prescribed medication regimes is one of the major factors leading to the accumulation and eventual expiration of unused drugs in the household. Adherence to prescription regimes is an issue of great importance to health care. Its causes are many and complex (e.g., see Kenreigh and Wagner, 2005). A variety of ways to improve compliance, ranging from simple to technologically sophisticated, currently exist or are under development.
Once the consumer has accumulated a certain number of unwanted or unusable medications in the home, the quandary of disposal is confronted. Conflicting needs and motivations make disposal of pharmaceuticals a confusing issue in the United States, where national take-back programs do not exist. Water treatment facilities increasingly no longer want drugs unnecessarily discharged via sewers, while at the same time, poison control centers have long advised against discarding them to trash and have always recommended discarding to sewerage (since this is historically the easiest means available for protecting humans and pets from accidental and purposeful poisonings). Drugs discarded to municipal trash/landfills pose not just potential future environmental exposure risks but also ongoing risks with regard to reuse by those who scavenge for them (e.g., human ‘gleaners’ or animal scavengers). Discarding to sewerage, in contrast, is the surest simple means (currently available) for preventing drug diversion. With these conflicting issues aside, the U.S. government, as a first step, issued its first public guidance for drug disposal in February 2007 (ONDCP, 2007; White House, 2007):
Unless stated otherwise on the label, proper disposal methods include intermingling drugs with undesirable substances … and depositing them in the garbage or bringing the drugs to a community pharmaceutical take-back or solid waste program. Unless otherwise directed, prescription drugs should not be flushed down the toilet due to the risk of contaminating water sources.
Ingestion Of Ambient Environmental Residues Incorporated Into Foods
Drinking water is not the only source of human exposure to ambient environmental residues of drugs. Another source is food contaminated by uptake of ambient residues from the environment. These residues are not expected by the consumer to be present, in contrast to residues from those drugs approved for use during the commercial production of food. Ambient residues can be concentrated in the tissues of aquatic organisms and taken up by food crops that have been irrigated with reused water or amended with sewage biosolids. A screening study targeting 24 pharmaceuticals in the tissues of fish and clams from an effluent-dominated stream revealed residues of 13 at levels up to 80 ppb in muscle and liver (Chambliss et al., 2006). Residues resulting from aquaculture would not be part of this consideration because they result from the purposeful, approved use of drugs. The occurrence of nontherapeutic biocides not intended for ingestion (e.g., triclosan and triclocarban) in human breast milk (Adolfsson-Erici et al., 2002) points to the possibility of this route occurring for lactating women exposed to environmental residues of drugs that they would ordinarily not be consuming.
With regard to human exposure, of potential significance is that veterinary drugs and human drugs comprise sets that have little overlap. Human exposure to residues from veterinary drugs from any inadvertent, unintended consumption (e.g., via foods) may have less predictable consequences than exposure to human drugs.
Potential sources for human exposure to ambient residues include not just drinking water and the well-known but less publicized routes such as domestic livestock and fish treated with veterinary drugs, but also the lessknown route of edible plants. Foods can potentially become contaminated with pharmaceuticals not just by the recycling of environmental residues originating from therapeutic use in confined animal feeding operations (CAFOs) (veterinary drugs such as antibiotics and estrogenic and androgenic steroids, for both therapeutic treatment and growth promotion), agriculture (e.g., use of antibiotics for plant disease control), and aquaculture (antibiotics for disease prevention and treatment), but also from uptake of drug residues by food crops grown on land where sewage biosolids have been applied. When biosolids from municipal sewage treatment facilities or the excrement from domestic animals treated with veterinary medicines are used on arable lands (e.g., as soil amendment or fertilizer), vegetation has the potential to remove the drug residues that partition to the soil pore water. These residues can accumulate in shoots and roots. For a limited number of targeted drugs evaluated under controlled conditions, the residues found to accumulate in certain plants hold the potential for yielding human intakes that approach 10% of the ADIs (Boxall et al., 2006).
Aquaculture can release drugs directly to open waters (from excess medicated feed and from excreta). Aquaculture also experiences off-label and illegal usage of certain drugs, especially highly toxic antibiotics such as chloramphenicol, furazolidone, and nitrofurazone, all of whose use is banned in many countries but continues nonetheless. These released residues can be taken up by wild fin and shell fish, which can then serve as a direct human exposure route via recreational or subsistence fishing; exposure could be enhanced for those drugs known to bioconcentrate, for example in fish. For thorough background on the environmental aspects of concentrated aquatic animal production (CAAP) and the role of pharmaceuticals, refer to the materials available from EPA (2006).
Another exposure route related to foods that could emerge in the future is inadvertent exposure to foods genetically engineered to produce proteinaceous pharmaceuticals – ‘plant-made pharmaceuticals’ (PMPs) produced by ‘molecular farming’ or ‘biopharming’; see Elbehri (2005) for an overview. Current transgenic biotechnology has the potential for using food crop species (primarily corn, soybeans, rice) for producing hundreds of distinct proteinaceous therapeutics (especially enzymes, hormones, vaccines, monoclonal antibodies). PMPs raise a host of questions regarding risk, primarily centered around allergenicity and toxicity in the form of direct endocrine disruption or other mechanisms, should these crops or genes enter the food supply (such as by crosspollination of food crops) or their PMPs be released directly to the environment. Although drugs based on peptides and proteins would ordinarily not be expected to persist in the environment because they can be easily degraded or denatured, a possible exception is the cyclic peptides and circular proteins. Natural products of the former include cyclosporin (an immunosuppressant) and gramicidin S (an antibiotic); these are distinguished from the circular proteins in being synthesized by enzymatic pathways as opposed to being synthesized ribosomally. Synthetic versions of these chemicals (which cross over into the domain of self-assembling nanostructures) can be designed with broad-ranging biological activities, especially antimicrobial. The significant aspects of this class of drugs is that they resist chemical, thermal, and enzymatic alteration and therefore have the potential to persist in the environment and participate in later exposure events.
Other Routes For Exposure
There are several less common or unique routes by which humans can be inadvertently exposed to pharmaceuticals. These routes hold the potential for acute exposures. More than 150 drugs are formally recognized as hazardous in the occupational health-care setting (NIOSH, 2004); NIOSH plans to add to this list in 2007. In contrast to human therapeutic use, however, agricultural use poses concerns with regard to occupational and bystander exposure, especially by way of handling medicated feeds. One example is the inhalation of pharmaceuticals sorbed to dust particles generated by the handling of medicated feeds (Hamscher et al., 2003). Another exposure concern resulting from the use of medications with domestic animals is airborne antibiotic-resistant bacteria (Gibbs et al., 2006); indeed, the genes from antibiotic-resistant bacteria are becoming to be recognized as pollutants themselves (Pruden et al., 2006).
Another example involves various ill-conceived proposals to prevent the reuse of drugs destined for disposal to trash. These proposals all increase the risk of unusual exposures for the consumer. For example, an often recommended but ill-advised step prior to discard to trash is to render unwanted medications unsuitable for consumption by adding reactive chemicals, by heating, or by disassembly of capsules or crushing tablets and mixing with unpalatable ‘inerts.’ Such procedures could prove hazardous themselves because they promote the unnecessary handling of active ingredients and could lead to dermal or pulmonary exposure (e.g., by hand contact or inhalation of dusts) or the generation of highly hazardous vapors (e.g., if denaturing chemicals, such as bleach, or heat are used). Furthermore, unnecessary handling of individual medications also increases their risk of falling unnoticed onto floors or counters from where infants and children could consume them.
An undocumented exposure route might involve inhalation of the combustion gases from incinerators employed for the ultimate destruction of pharmaceuticals (both licit and illicit) that have been collected or confiscated in large quantities (e.g., by local take-back projects or by law enforcement). Except for incinerators designed and approved for hazardous wastes, the efficiencies of other incinerators are not fully understood. Improper or insufficient incinerator conditions (especially temperature) or design could lead to the airborne emission of unaltered parent drug entities or of toxic pyrolysis/oxidation byproducts. A scenario in which airborne exposure could be substantial is with small incinerators used in humanitarian relief efforts and for on-site disposal of confiscated drugs by law enforcement.
A little-recognized potential route of exposure is the possibility of bodily exchange of drugs between people. A potential route by which this might occur is from the secretion of systemic drugs through the skin, from where they could possibly be transferred directly to another person by direct contact. As an example that systemic drugs can be excreted through the skin, consider the appearance of loratadine on the skin surface 40 min after ingesting a 10-mg oral dose of the antihistamine (Taka´ts, 2004).
Finally, there are two routes by which acute dermal exposure can occur. First, certain medications that can be absorbed by the dermis are applied to the skin as gels or creams by use of the fingers or hand; examples are testosterone and progesterone, which are dermally applied in very high concentrations (parts per hundred). The residuals remaining on the hand are not fully removed by casual washing and can then be transferred from the hand to any surface subsequently contacted (telephones, doors, handles, etc.). These residues can then be transferred to others. The second route is by contact with dermal patches that have been disposed after usage or expiry. This is especially dangerous because many of these medications tend to be very potent (e.g., fentanyl and testosterone).
Perception Of Exposure As A Confounding Factor In Assessing Risk
While no evidence yet exists that trace levels of drugs in drinking water pose any risk for humans, their mere presence can create a refractory obstacle to public acceptance of, and trust in, recycled wastewater. This important outcome results from the way risk is perceived, which in turn is often little influenced by factual weight of evidence. Traces of drugs in drinking water supplies, regardless of how low their concentrations might be, are essentially considered ‘out-of-place’ chemicals, and as such are sometimes looked upon as ‘chemical weeds’ by the consumer (Daughton, 2005). The problem with low-level exposure has more to do with the repercussions from the way in which risk is perceived rather than overt toxicological hazard.
At the least, the occurrence of minute quantities of drugs in drinking water could cause what is known as the nocebo response (Daughton, 2005) – nocebo deriving from Latin ‘I will harm.’ The nocebo effect (the opposite of the placebo effect) is a real, physiologically adverse outcome caused simply by the suggestion or belief that something (such as a chemical) is harmful, despite the absence of any inherent toxicity. The nocebo effect could play a key role in the manifestation of adverse health consequences from exposure even to nontoxic trace levels of contaminants – simply by the power of suggestion and belief. Public education and a better understanding of how risk is perceived and how it is best communicated are particularly important for minimizing the incidence of the nocebo response (Daughton, 2004).
Particularly germane to the concept of ‘chemical weeds’ is the way in which the public perceives the technical concept of a contaminant’s ‘concentration.’ Whereas scientists readily distinguish the enormous, orders-of-magnitude differences between ppm, ppb, ppt, and other measures of concentration, the public often does not perceive these as measures of quantity, but rather as variant shorthands for the fact that a chemical is merely present – all measures of trace contaminants perceived as being essentially the same with regard to risk. For the general public, the dose is often not correlated with the potential for an effect – all doses are essentially equal. Another confusing aspect of concentration terminology can derive from the use of the ‘parts-per’ notation, where the quantity descriptors denote relative amounts that have opposite relative sizes when used by themselves. For example, although ‘trillion’ is 1000 times larger than ‘billion,’ it is only one-thousandth the size of a billion when used with ‘parts-per.’ This would represent a million fold discrepancy from reality for the public. So the lower the concentration of a toxicant reported using ‘parts-per’ notation, the higher the perceived risk might be, because the focus is on the quantifiers, and the ‘parts-per’ are simply ignored. Making things even more complicated is the fact that numbers larger than 109 do not have the same meanings worldwide; for example, a trillion in the United States is 1012 (a thousand billion) but in many other countries (such as Britain) a trillion is called a billion, and a trillion for them is a quintillion in the United States. Even worse, a trillion in Britain can also mean 1018, a billion. This is why the National Institute of Standards and Technology (NIST) does not condone the parts-per notation.
One of the ways in which risk is subconsciously framed or valued during its perception derives from a form of ‘logic’ or valuation explained by what are known as the ‘common laws of magic’. One of these laws is the law of association, which in turn comprises the sublaws of similarity and of contact or contagion. The ‘magical law of contagion’ constitutes one of the sympathetic laws of magic as introduced over a century ago by anthropologists (see Nemeroff and Rozin, 1994). Of particular relevance to drinking water as a source of pharmaceuticals, the law of contagion holds that once contaminated, always contaminated: ‘Things that have once been in contact with each other continue to act on each other at a distance even after physical contact has been severed.’ Once objects come into contact with each other, they will continue to influence each other, even after separation. The presence of pharmaceuticals essentially serves as a reminder that the drinking water or food was at one time in ‘contact’ with human waste. This can lead to rejection by the consumer of recycled water for drinking (Daughton, 2004).
As an example of the difficulty in communicating risk, consider the following hypothetical scenario. A city’s drinking water generally contained about 10 ppt of each of nine different pharmaceuticals from various therapeutic classes. For those consumers drinking 2 L per day, the potential for exposure (assuming 100% absorption from the gut) resulted in doses that were roughly the equivalent of 0.0002% of the safe therapeutic dose for each drug (20-ng residue versus 100 mg of a normal dose). Even though such an exposure would be considered safe, the extenuating factor is that the mere occurrence of the drug residues verifies that the water was derived from human waste and therefore is still ‘tainted.’ Communication of facts does not necessarily influence perception based on beliefs and fears.
Antimicrobial Resistance
Of the numerous classes of pharmaceuticals, the two that have attracted the most attention as environmental pollutants are the sex steroids and antibiotics. Attention has been focused on the first because of their endocrine modulating effects on wildlife at extraordinarily low concentrations (e.g., 1 ng/L); ethynylestradiol is one example. The second has attracted concern because of the issue of selection for antibiotic resistance in human pathogens. In fact, selection for antibiotic-resistant pathogens is often highlighted as one of the major drivers for concern over pharmaceutical residues in the environment. Despite this concern, there is little evidence that the trace concentrations of antibiotic residues in most environmental settings is sufficiently high to serve as a selecting force for development of resistance; but this is an unresolved issue. The levels monitored in the dissolved aqueous environment (ppt to ppb) are orders of magnitude below those believed necessary to select for antibiotic resistance. These ambient, trace concentrations of antibiotics are insufficiently high to select for resistance whether this were to occur in the environment or in those who ingest ambient, trace residues from water. The limited data on the concentrations of certain antibiotics in biosolids, however, reveal concentrations that can be orders of magnitude higher than in the aqueous environment (100s of ppb versus ppt; e.g., see Jones-Lepp and Stevens, 2007; Kinney et al., 2006). At these greatly enhanced concentrations, it might be possible to select for resistance among the bacteria occurring in the biosolids.
The co-occurrence of antibiotic residues with antibiotic-resistant bacteria in environmental settings is often interpreted as causal. The tendency has been to simply note correlations between the occurrence of trace concentrations of antibiotics and the co-occurrence of resistant bacteria, leading to erroneous conclusions that the former leads to the latter. In reality, these resistant bacteria are partly introduced to the environment by their shedding from humans and animals (together with excreted antibiotics) that have been treated with therapeutic levels of antibiotics. These bacteria probably developed resistance during the therapeutic exposures, not from exposure to trace levels in the environment. Any correlation of occurrence of trace levels of antibiotics with the co-occurrence of resistant bacteria most likely results from the sloughing of resistant bacteria from people and animals receiving antibiotic therapy and from horizontal gene transfer after these enteric bacteria are shed to the environment.
Important to recognize at the same time, however, is that antibiotic resistance is also a widespread natural phenomenon due to the ubiquitous production of natural antibiotics by autochthonous soil bacteria. The phenomenon of multiple antibiotic resistance (spanning all significant therapeutic antibiotics, whether naturally or artificially synthesized) and multiple means of resistance may be widespread in the environment (D’Costa et al., 2006). It leads to questions of (1) the origin(s) of widespread resistance (whether naturally evolved as a result of allelopathic defenses or by gene transfer, or both), and (2) whether the soil domain might serve as a reservoir for the transfer of naturally acquired resistance to pathogens. Exposure to an antibiotic of one class can also select for resistance to those from other classes (multidrug resistance, or MDR) if the mechanism of resistance depends on processes with little ligand specificity (such as overexpression of cellular efflux pumps).
It is also important to recognize that development of transient antibiotic resistance can stem not just from exposure to antibiotics but also from a wide array of socalled ‘non-antibiotic’ antibiotics, some of which have no inherent toxicity of their own (indirect toxicants). Examples include those pharmaceuticals that induce overexpression of the efflux pumps responsible for MDR (these include many widely used drugs, such as certain selective serotonin reuptake inhibitors, or SSRIs).
The prevalence of ubiquitous ‘nonculturable’ bacteria (often resulting from antibiotic therapy) also impedes determining the prevalence of antibiotic resistance. The many unknowns presented by the fact that most microbes are not culturable (and therefore resistance cannot be determined) is a major complicating factor. These bacteria may serve as a significant but unidentifiable reservoir of resistance genes for horizontal transfer to human pathogens. Indeed, recent studies have shown that healthy people could be the source of many of the antibiotic resistance genes that have led to the rise of ‘superbugs’ in hospitals. One study has found that more than 90% of the harmless E. coli in the guts of healthy people are multidrug resistant (Hall, 2006). Overexpression of broad-spectrum efflux pumps, plasmid-addiction systems, and exposure to naturally produced antibiotics may conserve resistance in the absence of continued ‘anthropogenic’ selective pressure.
Although antibiotic resistance is a major and growing human health concern, it is probably little connected with the issue of pharmaceutical residues being released to the environment as pollutants, but rather related to the imprudent use of these drugs in medical therapy. As opposed to the purported development of resistance among microorganisms exposed to trace levels of antibiotics in the environment, the more salient concern with respect to human exposure is probably the horizontal transfer of resistance genes to human pathogens. The problem is probably more related to environmental ‘pollution’ with resistance genes rather than by antibiotics themselves.
In contrast to therapeutic antibiotics, the high production volume, broad-spectrum biocides might pose a separate concern. Two of the major high-volume, broad-spectrum biocides are triclosan and triclocarban. Whether these two chemicals select for widespread resistance to themselves (or for cross-resistance to narrow-spectrum antibiotics in the environment) is very controversial. Another concern is the possible widespread employment of antivirals, such as oseltamivir carboxylate, during epidemics (Singer et al., 2007). Excretion of antivirals resistant to sewage treatment might lead to significantly higher ambient water concentrations than for antibiotics, which are used by a much smaller percentage of the populace for everyday health care. These concentrations could be sufficiently high as to select for resistant viruses carried by wildlife and later subject to crossing over to humans. This is an example of indirect hazard, where the initial exposure (to a chemical) could lead to future exposure to an infectious agent. So the significance of an exposure cannot be considered outside the context of the larger picture.
Proactive Measures To Reduce Exposure Or Hazard Perception
Certain measures can be implemented to begin reducing or limiting human exposure or to improve public acceptance of the fact that because of the ever-improving ability of chemists to detect lower and lower concentrations, ‘zero’ cannot be economically achieved with respect to the concentrations of contaminants in water. These measures include the following.
Multiple Barriers
Given the uncertainties regarding the true magnitude of potential risk, coupled with the difficulty in communicating risk, a reasonable strategy may be to implement the multiple-barrier approach, whose historic usage in the water treatment industry began with the need to maximize the removal of microbial pathogens. The multiple barrier approach partly involves the use of a series of approaches to maximize the spatial and temporal separation between wastewater and drinking water sources – essentially, to make the loop in water recycling as long and circuitous as possible.
Reducing Real Or Perceived Exposure Via Drinking Water
The consumer can be proactive to further ensure that drinking water contaminants are minimized, including ultra-trace levels of whatever pharmaceuticals might still be present, by use of ‘point-of-use’ treatment systems that install on individual water fixtures, or with ‘point-of-entry’ systems that treat all the potable water entering a building regardless of whether it is used as drinking water; systems using reverse osmosis and activated carbon sorption are particularly effective. The consumer should also be aware of alternatives to the disposal of drugs via the toilet.
Scope Of Occurrence
Compared with environmental monitoring, significantly less effort has been invested in determining the extent, frequency, and distribution of pharmaceutical residues in finished (POU) drinking waters. More extensive monitoring surveys could be designed and implemented.
Status And Trends Monitoring
Trends data for drugs in drinking water may be the key for determining monitoring priorities relevant to human exposure. Once routine methods can be established, risk assessments might focus on those target analytes found to be trending upward in occurrence frequencies or in concentrations, or the emergence of newly approved APIs. Likewise, methods for nontarget analysis could spot newly emerging drugs, triggering risk assessments. If trends data revealed the occurrence of previously unseen drugs or increasing concentrations of existing pharmaceuticals in the aquatic environment or in biosolids, then we would be positioned to take faster action – before these chemicals and their quantities might prove significant. Occurrence data could also be used simply to establish baseline exposure status (‘reference’ ranges) instead of linking it with risk. Negative concentration trends could be useful in assessing the effectiveness of waste treatment or pollution prevention controls. Drinking water occurrence data that is representative and reproducible could also be used by the public to compare the effectiveness of water treatment among municipalities.
Bibliography:
- Adolfsson-Erici M, Pettersson M, Parkkonen J, and Sturve J (2002) Triclosan, a commonly used bactericide found in human milk and in the aquatic environment in Sweden. Chemosphere 46(9–10): 1485–1489.
- Aksglaede L, Juul A, Leffers H, Skakkebæk NE, and Andersson A-M (2006) The sensitivity of the child to sex steroids: Possible impact of exogenous estrogens. Human Reproduction Update 12: 341–349.
- Barlow SM, Kozianowski G, Wu¨ rtzen G, and Schlatter J (2001) Threshold of toxicological concern for chemical substances present in the diet. Food and Chemical Toxicology 39(9): 893–905.
- Boxall ABA, Johnson P, Smith EJ, Sinclair CJ, Stutt E, and Levy LS (2006) Uptake of veterinary medicines from soils into plants. Journal of Agriculture and Food Chemistry 54(6): 2288–2297.
- Brody JG, Aschengrau A, McKelvey W, Swartz CH, Kennedy T, and Rudel RA (2006) Breast cancer risk and drinking water contaminated by wastewater: A case control study. Environmental Health 5(1): 28 [online].
- Bruchet A, Hochereau C, Picard C, Decottignies V, Rodrigues JM, and Janex-Habibi ML (2005) Analysis of drugs and personal care products in French source and drinking waters: The analytical challenge and examples of application. Water Science and Technology 52(8): 53–61.
- Calabrese EJ, Staudenmayer JW, Stanek EJ III, and Hoffmann GR (2006) Hormesis out performs threshold model in national cancer institute antitumor drug screening database. Toxicological Sciences 94(2): 368–378.
- Chambliss C, Ramirez AJ, Mottaleb MA, and Brooks BW (2006) Liquid Chromatography-Tandem Mass Spectrometry Screening Analysis of Select Pharmaceuticals in Aquatic Organisms Collected from an Effluent-Dominated Stream, poster 82 presented at SETAC, Montreal, 6 November.
- Christensen FM (1998) Pharmaceuticals in the Environment—A Human Risk? Regulatory Toxicology and Pharmacology. 28(3): 212–221.
- D’Costa VM, McGrann KM, Hughes DW, and Wright GD (2006) Sampling the Antibiotic Resistome. Science 311(5759): 374–377.
- Daughton CG (2004) Groundwater Recharge and Chemical Contaminants: Challenges in Communicating the Connections and Collisions of Two Disparate Worlds, Ground Water Monitor. Remed 24(2): 127–138; available: http://www.epa.gov/nerlesd1/bios/daughton/waterreuse.pdf.
- Daughton CG (2005) ‘Emerging’ Chemicals as Pollutants in the Environment: a 21st Century Perspective. Renewable Resources J. 23(4): 6–23.
- Daughton CG (2007) Pharmaceuticals in the Environment: Sources and Their Management, Chapter 1, 1–58, In: Analysis, Fate and Removal of Pharmaceuticals in the Water Cycle (M. Petrovic and D. Barcelo, Eds.) Wilson & Wilson’s comprehensive Analytical Chemistry Series (D. Barcelo, Ed.), volume 50, Elsevier Science, p. 564. http://www. epa.gov/nerlesd1/bios/daughton/Chap1_Petrovic8 Barcelo.pdf
- Daughton CG and Ternes TA (1999) Pharmaceuticals and Personal Care Products in the Environment: Agents of Subtle Change? Environmental Health Perspectives 107(suppl 6): 907–938.
- DHH\PD Review of health issues associated with potable reuse of wastewater, report No. RFT200/00, Department of Health and Aged Care, Department of Health and Aged Care, Canberra, Commonwealth of Australia. 324 pp, available: http://www.health. gov.au/internet/wcms/publishing.nsf/Content/health-pubhlthpublicat-document-metadata-env_water.htm/$FILE/env_water.pdf.
- Dolan DG, Naumann BD, Sargent EV, Maier A, and Dourson M (2005) Application of the threshold of toxicological concern concept to pharmaceutical manufacturing operations. Regulatory Toxicology and Pharmacology 43(1): 1–9.
- Elbehri A (2005) Biopharming and the food system: Examining the potential benefits and risks. AgBioForum 8(1): 18–25 available: http://www.agbioforum.missouri.edu/v8n1/v8n1a03-elbehri.htm.
- EPA Aquatic Animal Production Industry Effluent Guidelines (2006) web page, U.S. Environmental Protection Agency, Washington, D.C. available: http://epa.gov/guide/aquaculture/#rule
- Gibbs SG, Green CF, Tarwater PM, Mota LC, Mena KD, and Scarpino PV (2006) Isolation of Antibiotic-Resistant Bacteria from the Air Plume Downwind of a Swine Confined or Concentrated Animal Feeding Operation. Environmental Health Perspectives 114(7): 1032–1037.
- Grandjean P (2005) Non-precautionary aspects of toxicology. Regulatory Toxicology and Pharmacology 207(2 suppl. 1): 652–657.
- Hall R (2006) Antibiotic Resistance Genes on the Move, presented at the Australian Society for Microbiology Annual Conference, 2–6 July, Gold Coast, QLD Australia.
- Hamscher G, Pawelzick HT, Sczensky S, Nau H, and Hartung J (2003) Antibiotics in dust originating from a pig-fattening farm: A new source of health hazard for farmers? Environmental Health Perspectives 111 (13): 1590–1594.
- Jones OA, Lester JN, and Voulvoulis N (2005) Pharmaceuticals: a threat to drinking water? Trends in Biotechnology 23(4): 163–167.
- Jones OA, Voulvoulis N, and Lester JN (2004) Potential ecological and human health risks associated with the presence of pharmaceutically active compounds in the aquatic environment. Crit. Rev. Toxicol. 34(4): 335–350.
- Jones-Lepp TL and Stevens R (2007) Pharmaceuticals and personal care products in biosolids/sewage sludge: the interface between analytical chemistry and regulation. Anal. Bioanal. Chem. 387: 1173–1183.
- Kenreigh CA and Wagner LT (2005) Medication Adherence: A Literature Review. Medscape Pharmacists 6(2) available: http://www. medscape.com/viewarticle/514164_print.
- Kinney CA, Furlong ET, Zaugg SD, et al. (2006) Survey of Organic Wastewater Contaminants in Biosolids Destined for Land Application. Environ. Sci. Technol. 40(23): 7207–7215.
- Kroes R, Kleiner J, and Renwick A (2005) ‘‘The Threshold of Toxicological Concern Concept in Risk Assessment,’’ Toxicol. Sci. 86(2): 226–230.
- Li Z, Dong T, Pro¨ schel C, and Noble M (2007) Chemically Diverse Toxicants Converge on Fyn and c-Cbl to Disrupt Precursor Cell Function. PLoS Biology 5(2): e35.
- McGovern T and Jacobson-Kram D (2006) Regulation of Genotoxic and Carcinogenic Impurities in Drug Substances and Products. TrAC Trends Anal. Chem. 25(8): 790–795.
- Mu¨ ller L, Mauthe RJ, Riley CM, Andino MM, De Antonis D, Beels C, et al. (2006) A rationale for determining, testing, and controlling specific impurities in pharmaceuticals that possess potential for genotoxicity. Reg. Toxicol. Pharmacol. 44(3): 198–211.
- Nemeroff C and Rozin P (1994) The Contagion Concept in Adult Thinking in the United States: Transmission of Germs and of Interpersonal Influence. Ethos 22(2): 158–186.
- Preventing Occupational Exposure to Antineoplastic and Other Hazardous Drugs in Health Care Settings, September 2004, Publication No. 2004–165, National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention, Atlanta GA. available: http://www.cdc.gov/niosh/docs/2004-165/#r.
- NRC (1998) Issues in potable reuse: The viability of augmenting drinking water supplies with reclaimed water. Committee to Evaluate the Viability of Augmenting Potable Water Supplies with Reclaimed Water, Water Science and Technology Board, Commission on Geosciences, Environment, and Resources, National Research Council, National Academy Press report. available: http://www.nap. edu/books/ 0309064163/html.
- Oberemm A, Onyon L, and Gundert-Remy U (2005) How can toxicogenomics inform risk assessment? Toxicol. Appl. Pharmacol. 207(2, suppl 1): 592–598.
- Proper Disposal of Prescription Drugs: Federal Guidelines. Office of national Drug Control Policy, Washington, DC. February 2007, available: http://www.whitehousedrugpolicy.gov/publications/pdf/prescrip_disposal.pdf.
- Paustenbach D and Galbraith D (2006) Biomonitoring: Is body burden relevant to public health? Reg. Toxicol. Pharmacol. 44(3): 249–261.
- Pomati F, Castiglioni S, Zuccato E, Fanelli R, Vigetti D, Rossetti C, and Calamari D (2006) Effects of a Complex Mixture of Therapeutic Drugs at Environmental Levels on Human Embryonic Cells. Environ. Sci. Technol. 40(7): 2442–2447.
- Pruden A, Pei R, Storteboom H, and Carlson KH (2006) Antibiotic Resistance Genes as Emerging Contaminants: Studies in Northern Colorado. Environ. Sci. Technol. web release date 15 August 2006.
- Schulman LJ, Sargent EV, Naumann BD, Faria EC, Dolan DG, and Wargo JP (2002) A human health risk assessment of pharmaceuticals in the aquatic environment. Hum. Ecol. Risk Assess. 8(4): 657–680.
- Schwab BW, Hayes EP, Fiori JM, et al. (2005) Human pharmaceuticals in US surfaces waters: a human health risk assessment. Reg. Toxicol. Pharmacol. 42: 296–312.
- Singer AC, Nunn MA, Gould EA, and Johnson AC (2007) Potential risks associated with the proposed widespread use of Tamiflu. Environ. Health. Perspect. 115: 102–106.
- Slikker W Jr, Andersen ME, Bogdanffy MS, et al. (2004a) Dosedependent transitions in mechanisms of toxicity. Toxicol. Appl. Pharmacol. 201(3): 203–225.
- Slikker W Jr, Andersen ME, Bogdanffy MS, et al. (2004b) Dosedependent transitions in mechanisms of toxicity: case studies. Toxicol. Appl. Pharmacol. 201(3): 226–294.
- Taka´ ts Z, Wiseman JM, Gologan B, and Cooks RG (2004) Mass Spectrometry Sampling Under Ambient Conditions with Desorption Electrospray Ionization. Science 306(5695): 471–473.
- Webb S, Ternes T, Gibert M, and Olejniczak K (2003) Indirect human exposure to pharmaceuticals via drinking water. Toxicol. Lett. 142(3): 157–167.
- White House. The President’s National Drug Control Strategy, Office of National Drug Control Policy. The White House, Washington DC. February 2007, (see page 31); avaialble: http://www. whitehousedrugpolicy.gov/publications/policy/ndcs07/ndcs07.pdf.
- Winder C (2002) Mechanisms of multiple chemical sensitivity. Toxicol. Lett 128(1–3): 85–97.
- Anderson PD, D’Aco VJ, Shanahan P, et al. (2004) Screening analysis of human pharmaceutical compounds in US surface waters. Environmental Science and Technology 38(3): 838–849.
- Boxall ABA (2004) The environmental side effects of medication: How are human and veterinary medicines in soils and water bodies affecting human and environmental health? EMBO Reports 5(12): 1110–1116.
- Daughton CG (2003a) Cradle-to-cradle stewardship of drugs for minimizing their environmental disposition while promoting human health. I. Rationale for and avenues toward a green pharmacy. Environmental Health Perspectives 111: 757–774.
- Daughton CG (2003b) Cradle-to-cradle stewardship of drugs for minimizing their environmental disposition while promoting human health. II. Drug disposal, waste reduction, and future direction. Environmental Health Perspectives 111: 775–785.
- Daughton CG and Jones-Lepp TL (eds.) (2001) Pharmaceuticals and Personal Care Products in the Environment: Scientific and Regulatory Issues. Washington, DC: American Chemical Society.
- Halling-Sorensen B, NorsNielsen SN, Lanzky PF, Ingerslev F, Lutzhoft HCH, and Jorgensen SE (1998) Occurrence, fate and effects of pharmaceutical substances in the environment: A review. Chemosphere 36: 357–394.
- Kolpin DW, Furlong ET, Meyer MT, Thurman EM, Zaugg SD, Barber LB, and Buxton HT (2002) Pharmaceuticals, hormones, and other organic wastewater contaminants in US streams, 1999–2000: A national reconnaissance. Environmental Science and Technology 36: 1202–1211.
- Ku¨ mmerer K (ed.) (2004) Pharmaceuticals in the Environment: Sources, Fate, Effects, and Risk. 2nd ed. Berlin: Springer.
- Ruhoy IS and Daughton CG (2007) Types and quantities of leftover drugs entering the environment via disposal to sewage: Revealed by coroner records. Science of the Total Environment 388(1): 137–148.
- Weber S, Khan S, and Hollender J (2006) Human risk assessment of organic contaminants in reclaimed wastewater used for irrigation. Desalination 187: 53–64.
- Williams RT (ed.) Human Pharmaceuticals: Assessing the Impacts on Aquatic Ecosystems. Pensacola, FL: SETAC Press.
See also:
Free research papers are not written to satisfy your specific instructions. You can use our professional writing services to buy a custom research paper on any topic and get your high quality paper at affordable price.








