This sample Peripheral Arterial Disease Research Paper is published for educational and informational purposes only. If you need help writing your assignment, please use our research paper writing service and buy a paper on any topic at affordable price. Also check our tips on how to write a research paper, see the lists of health research paper topics, and browse research paper examples.
Definition Of Peripheral Arterial Disease (PAD)
Peripheral arterial disease (PAD) is defined ranging from the broad to the specific. For many, PAD defines a wide variety of disorders that obstruct the blood supply to the arteries of the noncoronary circulation. However, PAD is also used solely to describe obstructive disease in the arteries to the legs. Here, we define PAD by the latter definition. Other terms have been used to describe this disorder including peripheral vascular disease, peripheral arterial occlusive disease, arteriosclerosis obliterans, and lower extremity arterial disease.
Clinical Presentation And Natural History Of The Disease
Peripheral arterial disease remains asymptomatic for long periods of time and only presents clinically when it is relatively advanced. The cardinal symptom of PAD is intermittent claudication (IC), a cramping leg pain, which is brought on by ambulation/exercise and relieved by rest. Some patients develop ‘atypical’ leg pain, which presents with lower extremity discomfort on exertion but does not consistently resolve with rest. More severe forms of the disease include critical limb ischemia (CLI), characterized by persistent rest pain becoming worse when the legs are elevated, for example, in bed at night. In more severe cases, patients develop gangrene and ulceration and may undergo leg amputation or other surgical intervention. Acute limb ischemia denotes a sudden decrease or worsening of limb perfusion causing a potential threat to limb viability.
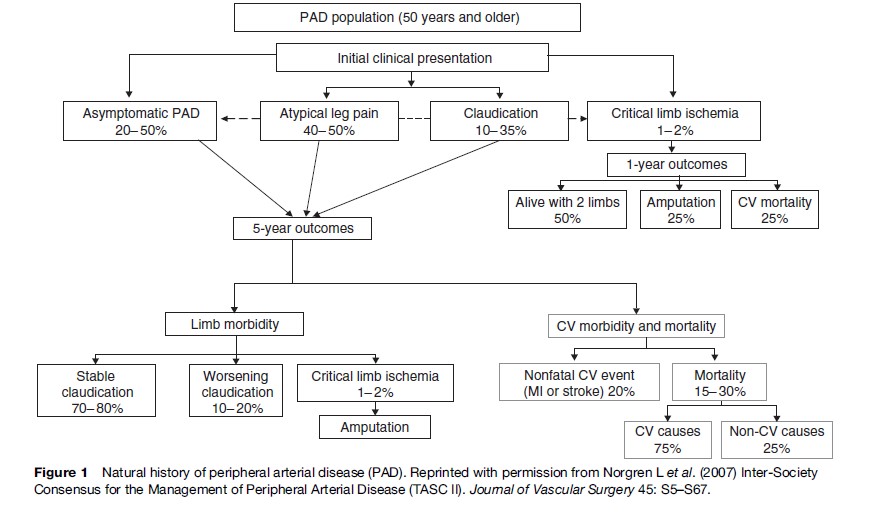
The prognosis of patients with PAD is characterized by an increased risk of cardiovascular disease affecting other arterial beds such as coronary heart disease and stroke. Progression of local disease in the legs is less common (Figure 1). In fact, patients with IC in most cases either improve or stabilize. Less frequently, worsening of claudication may require surgical intervention and finally amputation. For example, after 5 years’ follow-up of the Edinburgh Artery Study, out of 116 identified claudicants at baseline, only 29% still had symptoms of claudication, 8% had surgical revascularization or amputation, and 1% had developed leg ulcers (Leng et al., 2006). In contrast, amputation rates are much higher (10–40%) once symptoms of CLI (rest pain, tissue loss) commence. Asymptomatic disease has also been shown to progress slowly over time with approximately 11% of asymptomatic individuals showing significant progression of disease over 5 years (Aboyans et al., 2006).
Prevalence And Incidence Of PAD
PAD is a relatively common disease that affects many adults worldwide. For example, approximately 8 million out of 300 million people suffer from PAD in the United States (Hirsch, 2001). Overall, the estimated prevalence of the disease is highly dependent on the age of the population and the criteria used to define the disease. For example, it was estimated that 12–20% of Americans age 65 and older have PAD yet only about 25% of PAD patients undergo treatment (Becker et al., 2002).
Intermittent Claudication
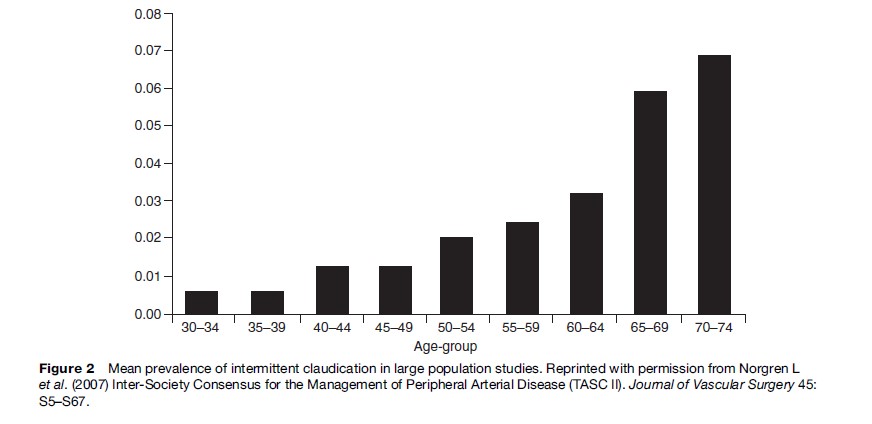
Most epidemiological studies use IC as a symptomatic marker of PAD, which is usually assessed via the World Health Organization (WHO) IC questionnaire. The estimated prevalence is small in men and women under 55 years but increases rapidly in those aged 55 and above. Overall the prevalence of claudication ranges between 0.4–14.4% (Dormandy and Rutherford, 2000). Figure 2 shows a calculated mean prevalence by age weighted by study sample size as reported by the Trans-Atlantic Inter-Society Consensus (TASC) Working Group on the management of peripheral arterial disease. The incidence of PAD follows a similar trend. In the Framingham Heart Study, the age-specific annual incidence of intermittent claudication for ages 30 to 44 years was 6 per 10 000 men and 3 per 10 000 women; this incidence increased to 61 per 10 000 men and 54 per 10 000 women ages 65 to 74 years (Kannel et al., 1970). Sex differences are most apparent in middle-aged populations with men showing a higher prevalence or incidence of disease whereas in older populations sex differences are minimal in keeping with an equilibration in the frequency of cardiovascular disease between men and women at older ages. Ethnic differences in the prevalence of PAD also exist, with a higher prevalence/ incidence in the United States of PAD in subjects with black ethnicity. Some studies suggest that the prevalence of PAD in persons of Hispanic origin is similar to or slightly higher than that in whites (Criqui et al., 2005).
Asymptomatic Disease
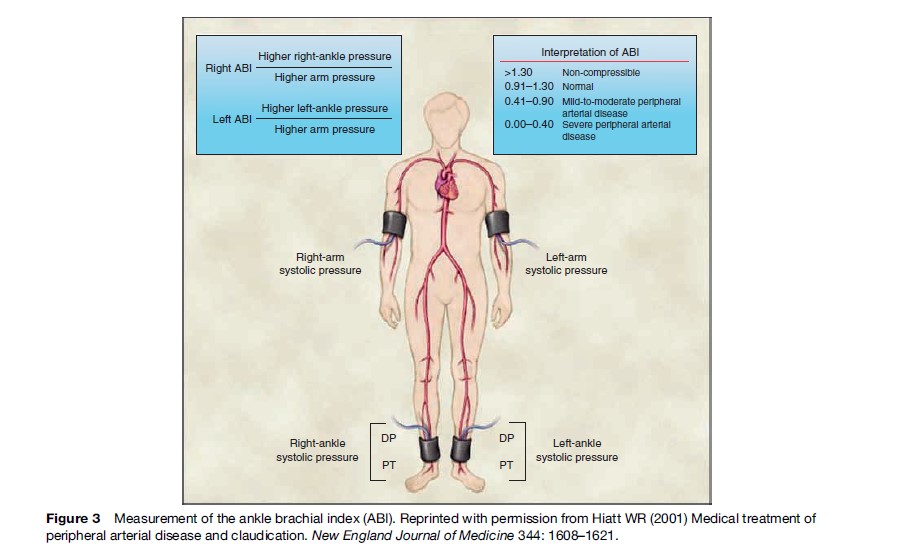
For every individual with IC there are another 3 with asymptomatic disease causing a 50% or greater stenosis of the arteries supplying the legs (Hiatt et al., 1995). Therefore, the prevalence of asymptomatic disease is higher than that estimated on the basis of IC symptoms alone and ranges between 3–10%, increasing to 15–20% in populations older than 70 years of age. Asymptomatic disease may be assessed by noninvasive modalities. The most commonly used test is the ankle brachial index (ABI), which is the ratio of the ankle to the brachial systolic blood pressure (Figure 3). An ABI less than unity (or less than 0.9 in practice) at rest is highly suggestive of significant arterial obstruction in the legs. Other noninvasive tests include pulse palpation, flow velocity determination, and duplex ultrasound. In the San Diego Study, Criqui et al. (1985) used a battery of three different diagnostic tests (ABI, pulse wave analysis, and pulse examination) along with the WHO questionnaire in 613 men and women. The estimated prevalence of PAD was 12%, which was considerably greater than that estimated by the claudication questionnaire alone (2.2%). In a high-risk population (aged >70 years or 50–69 years with a cardiovascular risk factor) the prevalence of asymptomatic disease was found to be 29% (Hirch et al., 2001).
Critical Limb Ischemia
CLI is rare, affecting between 0.05–0.24% of the general population (Dormandy and Rutherford, 2000). In 1995 there were approximately 20 000 people with CLI in the UK, thus an annual incidence of approximately 400 per million per year (Vascular Surgical Society of Great Britain and Ireland, 1995). In other Western countries, the incidence of CLI has been estimated to be between 500–1000 per million per year. These estimates have been based on the number of amputations performed and the assumption that 25% of people with CLI require amputation.
Risk Factors For PAD
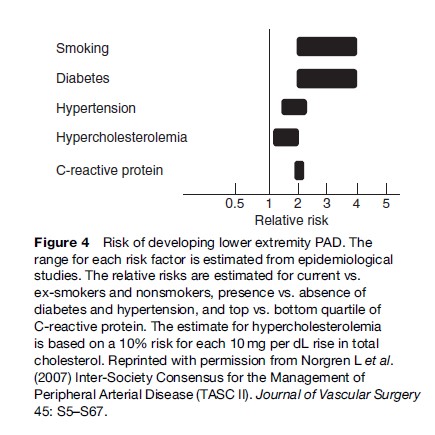
The underlying cause of PAD is, in the vast majority of cases, atherosclerotic disease. Thus, risk factors for PAD are those associated with atherosclerotic disease and are similar to those of coronary heart disease and stroke. Figure 4 shows the range of relative risks for PAD as estimated by epidemiological studies for some important factors including cigarette smoking, diabetes, dyslipidemia, and hypertension.
Smoking
Most risk factors for PAD are almost identical to those associated with coronary heart disease. However, some, such as smoking, would appear to be particularly important in the development of peripheral atherosclerosis. Overall, the prevalence of symptomatic PAD is increased 2.6-fold in current smokers (Willigendael et al., 2005). In addition, the risk ratio in smokers compared to nonsmokers for PAD has been shown to be approximately double that for coronary heart disease. Smoking has also been associated with progression of PAD. Patients with IC who continued to smoke have been shown to more often develop CLI and to have higher amputation rates than those patients with IC who quit smoking.
Diabetes
People with diabetes have an approximately twoto fourfold increase in prevalence of PAD compared to nondiabetics (Luscher et al., 2003). The duration and severity of diabetes would appear to correlate with the incidence and extent of PAD. Increased rates of absent pedal pulses, femoral bruits, and decreased ABI in people with diabetes have also been shown. Moreover, patients with diabetes are more likely to develop severe symptomatic forms of the disease, such as rest pain or ulceration as well as IC, and have more aggressive progression of PAD. Patients with IC and diabetes have been shown to have a 35% risk of sudden ischemia and a 21% risk of major amputation compared to claudicants without diabetes who had 19% risk of sudden ischemia and 3% risk of major amputation. In addition, amputation or gangrene was 10 times more frequent in PAD subjects with diabetes than in those without diabetes (Dormandy and Rutherford, 2000).
Hypercholesterolemia
The effect of blood lipids on PAD is less well established. In fact, elevated cholesterol levels seem to be associated somewhat less with PAD than with coronary heart disease. A protective effect of increased high-density lipoprotein (HDL) cholesterol has been observed in several studies. An association between increased triglycerides and PAD has been reported by many but the strength of this association is reduced by multivariate analysis and the effect of triglycerides on PAD remains unclear. In the Physicians Health Study, low-density lipoprotein (LDL) cholesterol, HDL cholesterol, triglycerides, and ratio of total HDL cholesterol were compared as predictors of PAD; the ratio of total HDL cholesterol was found to have the greatest independent effect (Ridker et al., 2001).
Hypertension
High blood pressure is likely to be a risk factor for PAD but may not be as strong as others such as smoking. Approximately 50–92% of claudicants have hypertension and subjects with elevated blood pressure have a 2.5to 4-fold increased risk of developing IC prospectively (Makin et al., 2001). However, the relation between high blood pressure and PAD is not consistent between studies.
Other Risk Factors
Other major cardiovascular risk factors such as obesity and physical inactivity are less well established in peripheral atherosclerosis. Few studies have examined the relationship between obesity, mainly measured by the body mass index (BMI); incident PAD and the overall evidence for an association was weak. The role of physical activity as an etiological factor is particularly difficult to evaluate in PAD because symptomatic disease often results in reduced physical activity. A relatively weak association between a physically active lifestyle and a reduced risk of developing PAD has been observed.
A series of novel risk factors have been proposed over the past few years as potential risk factors for cardiovascular disease. Although most studies have focused on coronary heart disease some evidence exists for PAD. Among these newly proposed markers of atherosclerosis, C-reactive protein (CRP), a marker of activated inflammation, has received considerable attention. However, the clinical utility of CRP for cardiovascular disease prediction is currently open to question. For example, the Edinburgh Artery Study reported a 40% increased risk of PAD for people with CRP levels in the top third of the distribution but a small added value of CRP over and above that of traditional cardiovascular risk factors (Tzoulaki et al., 2007). Other factors such as fibrinogen and D-dimer have been associated with the presence and development of the disease but again the evidence to date would suggest that measuring these plasma markers in patients in primary care is not justified.
PAD And Presence Of Other Cardiovascular Diseases
Patients suffering from PAD face an increased risk of other cardiovascular events, which is equal to or greater than that of patients with clinical coronary arterial disease. This may be at least partly due to concomitant coronary and cerebrovascular disease in these patients. Among patients with IC, 40–60% have coexisting coronary disease and 26–50% have coexisting cerebrovascular disease. The reported coprevalence is highly dependent on the sensitivity of the diagnostic tools used to define atherosclerotic disease, whether PAD, coronary heart disease, or stroke. More sensitive diagnostic techniques correlate with higher coprevalence of diseases. Also, among subjects with asymptomatic PAD (ABI < 0.9), the percentage of coexisting cardiovascular disease is higher than those without PAD and ranges between 56–71%. Finally, ABI has been shown to correlate well with measures of arterial disease in other vascular beds such as carotid intima media thickness and coronary artery calcium levels.
PAD And Prediction Of Cardiovascular Morbidity And Mortality
Patients with PAD have been shown to have an increased risk of angina, heart failure, fatal and nonfatal myocardial infarction (MI), fatal and nonfatal stroke, and cardiovascular disease; all-cause mortality as reported by largescale epidemiological studies (Figure 1). Patients with IC have an approximately threefold increase in cardiovascular mortality risk (Leng et al., 2006). Also, claudicants have 2.5 times higher all-cause mortality rates than nonclaudicants. Asymptomatic PAD defined by an ABI < 0.9 is associated with future MI and stroke with risk ratios ranging between 1.1–2.7 and 1.1–2.0, respectively. Also a twofold risk for cardiovascular mortality has been observed in those with ABI < 0.9 (Heald et al., 2006). Lower cut-off points for the ABI (ABI < 0.8) have been associated with greater cardiovascular risks. PAD is also a powerful predictor of all-cause mortality: subjects with asymptomatic PAD have a 1.6 relative risk of death compared to healthy individuals. Mortality and morbidity risks are incrementally higher in patients with asymptomatic, symptomatic, and severe PAD. Patients with CLI have a 20% mortality rate within one year (Figure 1). The 30-day mortality of those experiencing acute limb ischemia is approximately 15%.
Conclusions
PAD is a common problem in the community causing considerable morbidity and is an indication of an increased risk of major cardiovascular events and mortality. The increased aging of the population and improved survival of atherosclerotic patients following myocardial infarction and stroke would suggest that the burden of PAD will persist for the foreseeable future and will be a major consideration for the public health challenge of reducing cardiovascular disease.
Bibliography:
- Aboyans V, Criqui MH, Denenberg JO, Knoke JD, Ridker PM, and Fronek A (2006) Risk factors for progression of peripheral arterial disease in large and small vessels. Circulation 113: 2623–2629.
- Becker GJ, McClenny TE, Kovacs ME, Raabe RD, and Katzen BT (2002) The importance of increasing public and physician awareness of peripheral arterial disease. Journal of Vascular and Interventional Radiology 13: 7–11.
- Criqui MH, Fronek A, Barrett-Connor E, Klauber MR, Gabriel S, and Goodman D (1985) The prevalence of peripheral arterial disease in a defined population. Circulation 71: 510–515.
- Criqui MH, Vargas V, Denenberg JO, et al. (2005) Ethnicity and peripheral arterial disease: The San Diego Population Study. Circulation 112: 2703–2707.
- Dormandy JA and Rutherford RB (2000) Management of peripheral arterial disease (PAD). TASC Working Group. Trans-Atlantic Inter-Society Consensus (TASC). Journal of Vascular Surgery 31: S1–S296.
- Heald CL, Fowkes FGR, Murray GD, and Price JF (2006) Risk of mortality and cardiovascular disease associated with the ankle-brachial index: Systematic review. Atherosclerosis 189: 61–69.
- Hiatt WR, Hoag S, and Hammen RF (1995) Effect of diagnostic criteria on the prevalence of peripheral arterial disease. Circulation 92: 1472–1479.
- Hirsch AT, Criqui MH, Treat-Jacobson D, et al. (2001) Peripheral arterial disease detection, awareness, and treatment in primary care. Journal of the American Medical Association 286: 1317–1324.
- Kannel WB, Skinner JJ Jr., Schwartz MJ, and Shurtleff D (1970) Intermittent claudication. Incidence in the Framingham Study. Circulation 41: 875–883.
- Leng GC, Lee AJ, Fowkes FG, Whiteman M, Dunbar J, Housley E, and Ruckley CV (1996) Incidence, natural history and cardiovascular events in symptomatic and asymptomatic peripheral arterial disease in the general population. International Journal Epidemiology 25: 1172–1181.
- Luscher TF, Creager MA, Beckman JA, and Cosentino F (2003) Diabetes and vascular disease: Pathophysiology, clinical consequences, and medical therapy: Part II. Circulation 108: 1655–1661.
- Makin A, Lip GY, Silverman S, and Beevers G (2001) Peripheral vascular disease and hypertension: A forgotten association? Journal of Human Hypertension 15: 447–454.
- Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, and Fowkes FG on behalf of the TASC II Working Group (2007) Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). Journal of Vascular Surgery 45: S5–S67.
- Ridker PM, Stampfer MJ, and Rifai N (2001) Novel risk factors for systemic atherosclerosis: A comparison of creactive protein, fibrinogen, homocysteine, lipoprotein(a), and standard cholesterol screening as predictors of peripheral arterial disease. Journal of the American Medical Association 285: 2481–2485.
- The Vascular Surgery Society of Great Britain and Ireland (1995) Critical limb ischaemia anagement and outcome. Report of a national survey. European Journal of Vascular and Endovascular Surgery 10: 108–113.
- Tzoulaki I, Murray GD, Lee AJ, Rumley A, Lowe GDO, and Fowkes FG (2007) Inflammatory, haemostatic, and rheological markers for incident peripheral arterial disease: Edinburgh Artery Study. European Heart Journal 28: 354–362.
- Willigendael EM, Teijink JAW, Bartelink ML, Peters RJG, Buller HR, and Prins MH (2005) Smoking and the patency of lower extremity bypass grafts: A meta-analysis. Journal of Vascular Surgery 42: 67–74.
- Cassar K (2006) Intermittent claudication. British Medical Journal 11: 1002–1005.
- Golomb BA, Dang TT, and Criqui MH (2006) Peripheral arterial disease: Morbidity and mortality implications. Circulation 114: 688–699.
- Meijer WT, Hoes AW, Rutgers D, Bots ML, Hofman A, and Grobbee DE (1998) Peripheral arterial disease in the elderly: The Rotterdam Study. Arteriosclerosis Thrombosis and Vascular Biology 18: 185–192.
- Meru AV, Mittra S, Thyagarajan B, and Chugh A (2006) Intermittent claudication: An overview. Atherosclerosis 187: 221–237.
- Ouriel K (2001) Peripheral arterial disease. The Lancet 358: 1257–1264.
- Selvin E and Erlinger TP (2004) Prevalence of and risk factors for peripheral arterial disease in the United States: Results from the National Health and Nutrition Examination Survey, 1999–2000. Circulation 110: 738–743.
See also:
Free research papers are not written to satisfy your specific instructions. You can use our professional writing services to buy a custom research paper on any topic and get your high quality paper at affordable price.








