This sample Pneumonia Research Paper is published for educational and informational purposes only. If you need help writing your assignment, please use our research paper writing service and buy a paper on any topic at affordable price. Also check our tips on how to write a research paper, see the lists of health research paper topics, and browse research paper examples.
Introduction
The lung is constantly exposed to microorganisms. A complex system of host defenses is required to prevent these organisms from gaining access to the lung and for removing them from the lung. This process, however, may be breached and pathogenic microorganisms may reach the alveoli. The combined effects of microorganism multiplication and host response determine the clinical condition known as pneumonia.
The most useful classification of pneumonia is based on the origin of the infection because this factor implies a different etiology, prognosis and treatment. The term ‘community-acquired pneumonia’ (CAP) refers to the appearance of infection in a nonhospitalized population with no risk factors for multi-drug-resistant pathogens whereas the term ‘hospital acquired pneumonia’ (HAP) or ‘nosocomial pneumonia’ is used when there is no evidence that the infection was present or incubating at the time of hospital admission. The latter type of pneumonia is most frequently found in patients receiving mechanical ventilation, hence the term ‘ventilator-associated pneumonia.’
Community-Acquired Pneumonia
This common condition is generally more prevalent in nonindustrialized and less developed countries. In adults the incidence differs among countries, from 1.6 to 11 per 1000 adults. Incidence is higher in the elderly and more common in men than in women with this difference increasing with age. In the United States CAP affects more than 4 million adults and accounts for more than 1 million hospital admissions per year; moreover, in the United States pneumonia and influenza are the sixth leading cause of death and the age-adjusted mortality attributed to this disease is on the rise. In children pneumonia is the leading cause of mortality worldwide.
Several populations are at risk of pneumonia especially by pneumococcal invasive disease: people 65 years and older, immunocompromising conditions/medications, patients with anatomic or functional asplenia, congestive heart failure, COPD, diabetes mellitus, liver disease, and alcoholism, and long-term care facility residents. Smoking is the strongest risk factor for invasive pneumococcal disease in immunocompetent nonelderly adults.
Clinical Features
Patients with pneumonia usually present a constellation of symptoms and signs that include cough, dyspnea, sputum production, and pleuritic chest pain, although nonrespiratory symptoms (mainly in elderly patients who may report fewer symptoms) such as consciousness status changes or falls may also predominate. Unfortunately, information obtained from patient history and physical examination is not sufficient to achieve adequate diagnostic accuracy, and thus diagnosis requires the presence of an infiltrate on chest X-ray (Metlay et al., 1997; Fine et al., 1999). The presence of an infiltrate confirms the diagnosis but cannot predict a specific etiologic agent.
Etiology
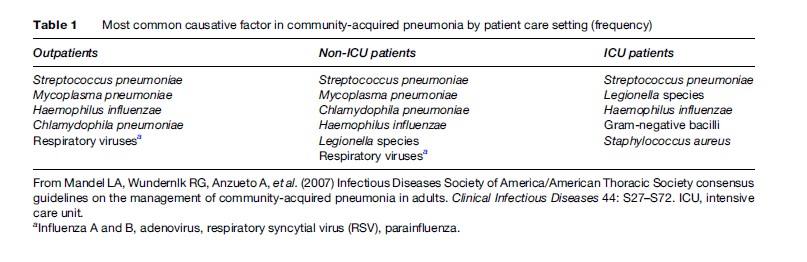
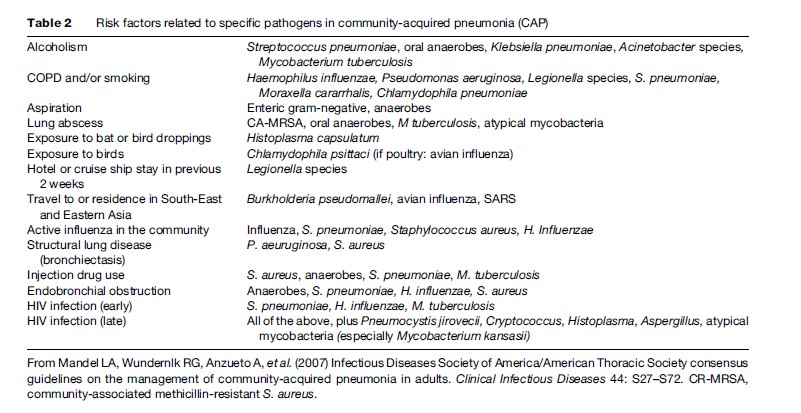
Although many pathogens have been associated with pneumonia, only a small range of key pathogens cause most cases. The most predominant pathogen observed is Streptococcus pneumoniae (pneumococcus), which accounts for about two thirds of all cases of bacteremic pneumonia. Other agents vary according to the severity of the disease (Table1) and the epidemiological condition or risk factors (Table 2). Concurrent infection by multiple microorganisms may lead to CAP; for example, influenza A may be followed by a secondary infection with S. pneumoniae or Staphylococcus aureus. Chlamydophila pneumoniae may also be followed by S. pneumoniae (File, 2003).
Recent human infections caused by avian influenza A (H5N1) in Vietnam, Thailand, Cambodia, China, Indonesia, Egypt, and Turkey have raised the possibility of a pandemic in the near future. The severity of H5N1 in humans distinguishes it from that caused by routine seasonal influenza. Patients with an illness compatible with influenza and with known exposure to poultry in areas with previous H5N1 infection should be tested for this infection. More specific guidance can be found on the World Health Organization (WHO), Infectious Diseases Society of America (IDSA), and American Thoracic Society (ATS) websites.
Risk Stratification
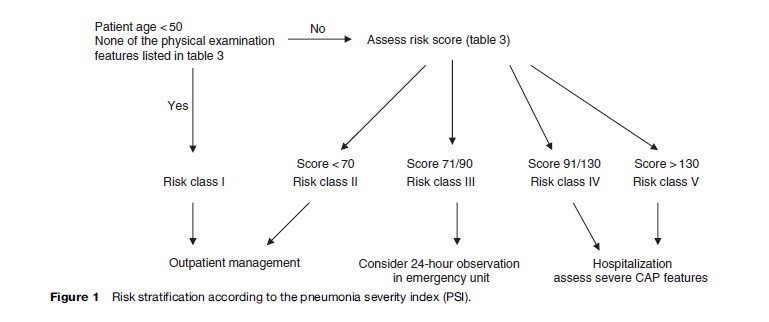
The most important issue in the management of a patient with CAP is the decision concerning the most appropriate care setting: outpatient, hospitalization on a medical ward, or admission to an intensive care unit (ICU). Between 30% and 50% of patients who are hospitalized are at low risk of death and may potentially be managed at home. The decision regarding hospitalization should be based on the stability of the clinical condition, the risk of death and complications, and the presence or absence of other medical problems and social characteristics. Severity or prognostic scores should be used to identify patients who may be candidates for outpatient treatment.
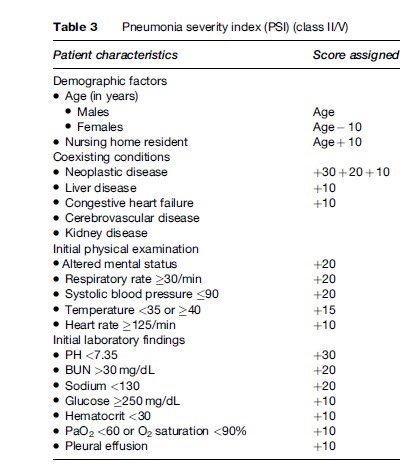
The most widely used prediction score is the ‘pneumonia severity index’ (PSI), which stratifies the patients to five risk categories (Figure 1 and Table 3); the higher the score, the higher the risk of death (Fine et al., 1997). Patients in classes I and II have a 30-day mortality risk of lower than 1% and can be safely treated as outpatients, while patients in class III have a risk of death of 0.9% to 2.9%. The risk of clinical worsening (although very low) is highest during the first 24 hours after presentation and decreases thereafter. It is therefore prudent to place these patients for a short period in an observation unit in the emergency department. Lastly, patients in classes IV and V have a risk of mortality of between 8.9% and 29.2% and should be hospitalized, considering the presence of severe CAP markers. An easy-to-use version of the PSI is available on Internet.
An alternative and simpler score that may be used at bedside is the ‘CURB 65’ score which assesses five variables each of which is assigned one point: Confusion; Urea greater than 7mmol/L; Respiratory rate greater than 30 per minute; low Blood pressure (systolic <90 mm Hg or diastolic ≤60 mm Hg); and age over 65 (Lim et al., 2003). A patient with 1 point has a risk of death of 1.5% and may be treated as an outpatient. Patients with a CURB-65 score of 2 or greater are not only at increased risk of death but also likely to have clinically important physiologic derangements requiring active intervention and should usually be considered for hospitalization. A simplified version (CRB-65, which does not require BUN levels) has demonstrated a similar ability of prediction and may be appropriate for decision making in primary care (Espana et al., 2003).
Other considerations such as the presence of hypotension (systolic blood pressure lower than 90), hypoxemia, exacerbation or active comorbidities, inability to reliably take oral medications, or social characteristics that compromise treatment compliance should all be taken into account when evaluating the need for hospital admission (Metlay et al., 2003).
On hospitalization of the patient, the next step is to evaluate the severity of the disease and to consider the need for admission to the ICU. The presence of septic shock (vasopressor requirement despite appropriate fluid resuscitation) or the need for mechanical ventilation defines severe CAP and these patients should be admitted directly to the ICU. However, not all patients eventually admitted to an ICU present such findings at presentation and delay in the transfer due to delayed respiratory failure or delayed onset of septic shock is associated with increased mortality. Thus, other criteria have been established to predict a worse prognosis with intensive care requirements. The presence of three or more of these criteria is accepted as criteria for ICU admission: respiratory rate 30/min; arterial oxygen pressure to oxygen inspired fraction rate (PaO2/FiO2) 250; multilobar infiltrates; confusion; uremia (BUN 20 mg/dL); leukopenia (<4000 white blood cells/mm3); thrombocytopenia (<100 000 platelets/mm3); hypothermia; and hypotension requiring aggressive fluid resuscitation (Mandel et al., 2007).
Diagnostic Testing
The length of diagnostic testing for specific pathogens should be based on the following factors: the probability that such a finding would significantly alter standard (empirical) management decisions, the severity and care setting of patients, and suspicion of a specific pathogen on an epidemiological basis.
Extensive tests for outpatients with suspected bacterial CAP are optional because of the low probability of treatment failure and good prognosis with recommended empirical antibiotic treatment. The presence of epidemiological variables that point to a specific pathogen requiring different treatment clearly justifies extensive testing (Table 2).
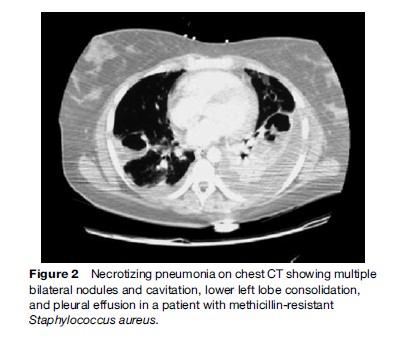
Blood cultures have a poor sensitivity (5%–14%) but should be done in hospitalized patients, especially in cases of severe CAP, ICU patients, asplenia, liver disease or alcohol abuse or other causes of immunusuppression, and in the presence of cavitary infiltrates with the suspicion of methicillin-resistant S. aureus (Figure 2).
A valid expectorated sputum sample for microbiologic processing requires more than 25 polymorphonuclear cells and less than 10 epithelial squamous cells per field and should be obtained whenever possible, especially in the group of patients mentioned earlier. However, the collection of pretreatment expectorated sputum should not delay the initiation of antibiotic administration. A respiratory sample (endotracheal aspiration or bronchoscopically obtained) is always recommended for patients intubated for severe CAP.
The urinary antigen test for S. pneumoniae and Legionella pneumophila is a rapid and very useful tool whose main advantages are simplicity and ability to detect a specific pathogen with reasonable accuracy even after antibiotic therapy has been started (File, 2003). For S. pneumoniae, studies in adults have shown a sensitivity of 50% to 80% and a specificity of over 90%. For Legionella, all the assays available detect only L. pneumophila serogroup 1, which accounts for 80% to 95% of community-acquired cases of Legionnaire’s disease and has a sensitivity ranging from 70% to 90% and a specificity of nearly 99%.
Treatment
In previously healthy outpatients a macrolide (azitromycin, clarithromycin, or erythromycin) is recommended.
However, the prevalence of drug-resistant S. pneumoniae worldwide has increased and the risk factors for b-lactam resistance have been defined: age over 65 or under 5, b-lactam treatment within the previous 3 months, alcoholism, medical comorbidities, and immunosuppressive disease or treatment; despite such findings its clinical relevance remains controversial because the outcomes of patients with ‘beta-lactam resistance’ (S. pneumoniae) usually do not result in treatment failures. Additionally, the rates of S. pneumoniae macrolide resistance have risen substantially in several parts of the world, contributing to an increased risk of macrolide treatment failure (Daneman et al., 2006). It is therefore prudent to treat these patients with a respiratory fluoroquinolone (levofloxacin, gatifloxacin) or b-lactam (amoxicillin 1 g tid or amoxicillin/clavulanate 2g bid or ceftriaxone) plus a macrolide in regions with recognized high-resistance rates. In nonICU inpatients the following treatment options are recommended: a respiratory fluoroquinolone or b-lactam plus macrolide. In ICU inpatients the following regimen is the minimal treatment recommended: b-lactam (ceftriaxone, cefotaxime, ampicillin-sulbactam) plus fluoroquinolone (Mandel et al., 2007).
Prevention
Vaccines against pneumococci and influenza remain the mainstay for preventing CAP. All persons 50 years and older, contact with high-risk persons and health-care workers should receive the influenza vaccine. The pneumococcal polysaccharide vaccine is recommended for people 65 years and older, the immunocompromised, and patients with anatomic or functional asplenia and chronic disease with high-risk morbi-mortality associated with pneumococcal disease (congestive heart failure, COPD, diabetes mellitus, liver disease, alcoholism). Smoking cessation should be a goal for all persons, particularly those with pneumonia because of the increased risk of invasive pneumococcal disease in immunocompetent nonelderly adults who smoke.
Hospital-Acquired Pneumonia
Hospital-acquired pneumonia (HAP) is defined as pneumonia that occurs 48 hours or more after admission, which was not incubating at the time of admission. Pneumonia is the second most common nosocomial infection and remains the in-hospital acquired infection with the highest associated mortality. The overall incidence of HAP varies from 6 to 8.6 per 1000 admissions with the highest incidence reported in the ICU: from 12% to 29%, 90% of which occurs during mechanical ventilation and is known as ventilator-associated pneumonia (VAP). The mortality related to this disease or ‘attributable mortality’ is reportedly between 33% and 50%. Host characteristics (advanced age and sex female), underlying diseases (higher-severity disease scores), and etiology (nonfermenting gram-negative bacilli) are associated with a poor prognosis. It is important to note that inappropriate or delayed antibiotic treatment is associated with a higher mortality. The presence of HAP increases hospital stay by an average of 7 to 9 days per patient and accounts for a large proportion of antibiotics prescribed.
In addition to the definitions established earlier, a diverse population of patients have been found to have a spectrum of pathogens causing pneumonia that more closely resembles HAP, that is, multi-drug-resistant pathogens (Table 4). Pneumonia in this group of patients is known as ‘health-care-associated pneumonia’ (HCAP) and the diagnostic and therapeutic considerations should be the same as the previously mentioned groups (HAP and VAP) (Bonten et al., 2005).

Etiology
The time of onset of pneumonia is an important epidemiologic variable and risk factor for specific pathogens and outcomes in patients with HAP and VAP. Early-onset pneumonia is defined as that occurring within the first 4 days of hospitalization. It usually carries the best prognosis and is more likely to be caused by microorganisms that are already carried by the host (‘community flora’): S. pneumoniae, Haemophilus influenzae, S. aureus, and anaerobes. ‘Late-onset’ pneumonia (5 days or more) is more likely to be caused by multi-drug-resistant pathogens and is associated with a poor prognosis: Pseudomona aeruginosa (30%), S. aureus including methicillin-resistant (25%), Gramnegative enteric bacilli (25%), L. pneumophila (5%), S. pneumoniae (5%), H. influenzae (5%), Aspergillus and Candida species (5%), and polymicrobial (30%).
Diagnosis
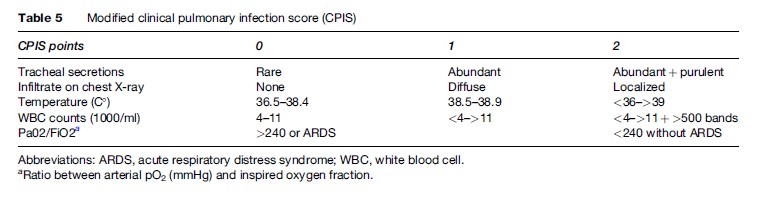
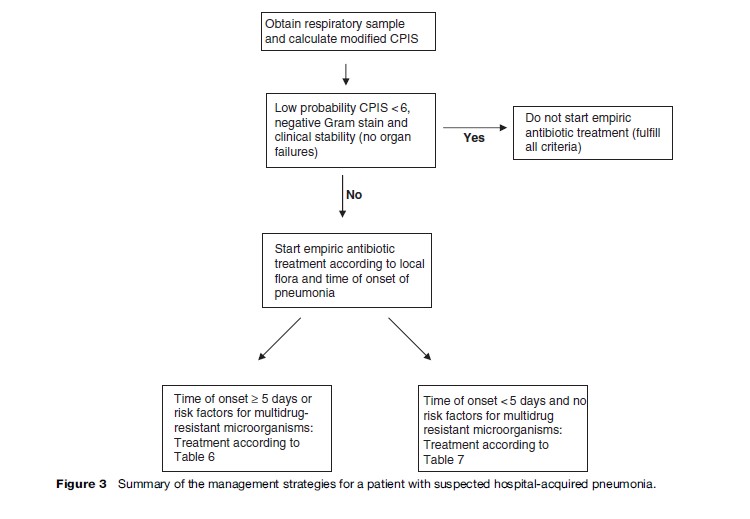
The clinical features that point to nosocomial pneumonia include the presence of new and persistent pulmonary infiltrates, temperature greater than 38.3 C or less than 36 C, white blood cell count greater than 12 000/mm3 or less than 4000/mm3, and purulent secretions. Unfortunately, many hospitalized patients present such findings for other reasons, especially intubated patients, thus making diagnosis difficult. The diagnostic criteria of a radiographic infiltrate and at least one of the additional criteria previously mentioned have a high sensitivity but low specificity (especially in VAP), while the presence of a pulmonary infiltrate plus two additional clinical findings result in a sensitivity of 69% and a specificity of 75% (Fabregas et al., 1999). Given the poor accuracy of an isolated clinical finding, a score of clinical findings (a ‘modified clinical pulmonary infection score’ – CPIS) can be used for initial decision making. This score is based on previously mentioned findings and an oxygenation index (Table 5). If the sum is 6 or greater than 6, a respiratory sample is justified and empirical antibiotic treatment is begun (Torres et al., 2006).
Blood cultures are obligatory when considering a diagnosis of nosocomial pneumonia. Unfortunately, the sensitivity is low (10% to 20%) and the specificity is reduced in critically ill patients who are at risk from multiple infectious foci.
The most important challenges in the management of suspected HAP are to avoid inappropriate or delayed antibiotic treatment with the higher mortality that this entails, and, on the other hand, to prevent the emergence of multi-drug-resistant microorganisms by avoiding indiscriminate antibiotic prescription. To achieve this double purpose it is imperative to obtain a lower respiratory tract sample for Gram stain and culture (to confirm the diagnosis) and antibiogram (adjusting the antibiotic to the sensitivity pattern when available) (Bonten et al., 2005).
In patients breathing spontaneously, respiratory secretions can be obtained by expectoration (with the same considerations as CAP for ‘valid’ sputum). The diagnostic accuracy of sputum in HAP has yet to be established. In intubated patients, lower respiratory tract cultures can be obtained bronchoscopically or nonbronchoscopically, and can be cultured semiquantitatively or quantitatively with different cut-off points for discriminating ‘colonization’ from ‘infection’ according to the method. Quantitative cultures and more invasive methods increase the specificity of the diagnosis without deleterious consequences. However, each institution should choose the technique based on local expertise, experience, and availability, because, when the diagnostic and therapeutic approaches are protocol driven and the initial treatment is adequate, the clinically relevant outcomes and use of antibiotics are the same with both the invasive and noninvasive (and quantitative and semiquantitative) methods. In order to facilitate the diagnostic approach, a tracheobronchial aspirate with Gram stain and semiquantitative or quantitative culture (cut-off point to differentiate colonization from infection: 105 colony forming units/mL for the quantitative method) (Canadian Critical Care Trials Group, 2007) should be obtained. Figure 3 presents a summary of the management of these patients.
Therapy
Inappropriate therapy (microorganism not sensitive to the antibiotic) is a risk factor for excess mortality, and multidrug-resistant microorganisms are frequent pathogens commonly associated with inappropriate therapy. On the other hand, delay in initiating antibiotic therapy may add to excess mortality, thus the importance of treating the most probable etiologic pathogens promptly.
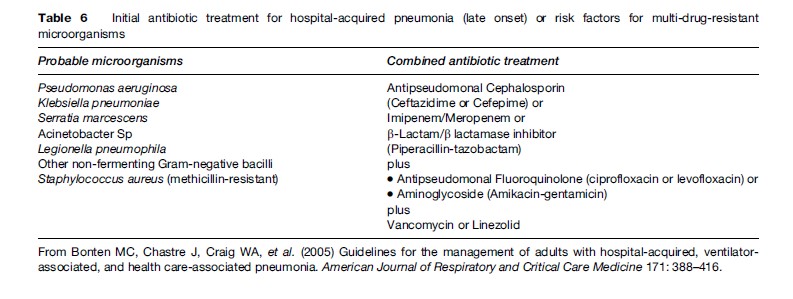
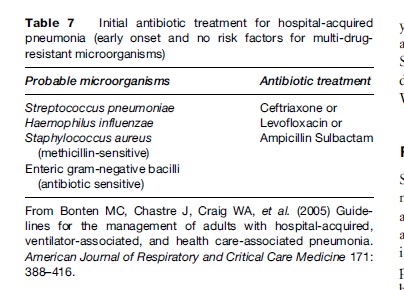
The choice of specific agents should be dictated by local flora and the pattern of sensitivity. Additionally, initial empiric therapy is more likely to be appropriate if the antibiotic selection is based on protocols designed according to the local patterns of antibiotic resistance. Table 6 and Table 7 describe general recommendations according to the time of onset of HAP, although they may be adapted, as mentioned earlier.
Community-Acquired Pneumonia In Childhood
Pneumonia is the leading cause of death in children. More than 2 million children under 5 years of age die from pneumonia each year, accounting for almost one in five under-5 deaths worldwide. That is, pneumonia kills more children than any other illness – more than AIDS, malaria, and measles combined (UNICEF/WHO, 2007). This figure does not include deaths due to pneumonia during the first 4 weeks of life. If these deaths were included in the overall estimate, pneumonia would account for up to one third of under-5 deaths each year. In terms of lost healthy life years (measured as disability-adjusted life years), acute respiratory infections are the chief cause of global ill health because of their great impact in young children.
More than 150 million episodes of childhood pneumonia occur every year in the developing world, accounting for more than 95% of all new cases worldwide. Between 11 and 20 million children with pneumonia are hospitalized, and more than 2 million die every year. South Asia and sub-Saharan Africa together bear the burden of more than half of all childhood pneumonia cases worldwide, and three quarters of all childhood pneumonia cases occur in just 15 countries. Only about one half of children with pneumonia receive appropriate medical care and less than 20% of children with pneumonia receive antibiotics.
The cost of reducing pneumonia deaths is relatively low. The lives of approximately 600 000 could be saved yearly through universal treatment with antibiotics alone, at a cost of $US600 million. South Asia and sub- Saharan Africa, where 85% of childhood pneumonia deaths occur, have the lowest treatment cost (UNICEF/ WHO, 2007).
Risk Factors
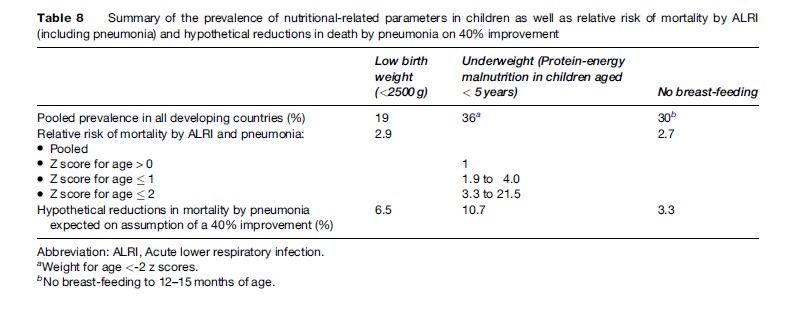
Several studies have suggested that low birth weight, malnutrition (underweight), and lack of breastfeeding are important risk factors for pneumonia morbidity and mortality (Victoria et al., 1999). In low-birth-weight infants (weight <2500 grams) a clear pattern of decreased pneumonia mortality with increased birth weight has been proved. The risk of mortality by pneumonia is highest in the first 24 months of life and the pooled relative risk of mortality has been estimated at 2.9%.
Protein-energy malnutrition is a risk factor for pneumonia mortality and a clear dose-response pattern has been observed with the highest mortality in the populations with the lowest weight according to weight-for-age Z score references. Additionally, several studies have pointed to an association between anthropometric status and different morbidity outcomes: in underweight children the relative risk for pneumonia requiring hospitalization ranges from 1.2% to 3.9% and seems to have a dose response trend. As a whole it has been estimated that child undernutrition contributes to more than half of child deaths each year, and more than 1 million deaths in children aged 0 to 4 years. Some micronutrient deficiency, particularly zinc, seems to predispose to pneumonia. Zinc intake helps reduce the incidence of pneumonia, as well as the severity of the disease and, potentially, death due to pneumonia. During the acute phase of severe pneumonia, zinc intake reduces the duration of the disease, the severity of the disease, and the treatment failure rate.
Breastfeeding protects against acute lower respiratory infections (ARLI) because of the unique anti-infective properties of breast milk. Children who are not breast-fed are 3.6-fold more likely to die from ARLI than are those who received breast milk, and the risk does not appear to vary by the age of the infants. Table 8 summarizes the prevalence of nutritional risk factors, the relative risk of different outcomes, and the hypothetical effect of their reduction in developing countries.
The lack of regular hand washing with soap has been clearly established as a risk factor for respiratory tract infections in several settings. In developing countries where pneumonia and diarrhea are leading causes of death, a community-based program with intervention to promote regular hand-washing habits in participants younger than 5 years decreased the incidence of pneumonia by 50% and that of diarrhea by 53% (Luby et al., 2005).
Indoor and outdoor environments are widely contaminated by complex mixtures of gases and particles that are produced by combustion and these pollutant mixtures increase the incidence of respiratory infections (Smith et al., 2000). Nearly half of the households worldwide are thought to cook daily with unprocessed solid fuels, such as biomass or coal that can release 50 times more pollution during cooking compared with gas stoves. Indoor air pollution in developing countries has been found to be dramatically high. In several studies it has been found that exposure to different pollutants increases the odds of clinically relevant acute lower respiratory infection (ALRI) (including pneumonia) from 2.2% to 6.0%. The attributable mortality fraction of ALRI /pneumonia due to air pollution probably represents the largest class of health impact from air pollution exposure worldwide.
HIV-positivity is a strong risk factor for pneumonia. Pneumocystis jiroveci (PCP) is the leading cause of pneumonia in this population. In some developing countries PCP causes at least one out of every four deaths among HIVpositive infants under the age of 1 and about 5% of pneumonia deaths among children worldwide.
Etiology
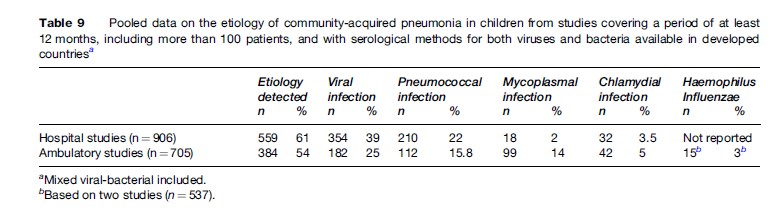
The most extensive knowledge on the etiology of CAP in children is mainly based on serologic studies in developed countries. Serologic evidence of mixed etiology, either viral-bacterial or bacterial-bacterial, is common. In inpatient studies, young children and those with severe disease are over-represented, emphasizing the role of S. pneumoniae and invasive viruses, such as respiratory syncytial virus (RSV), parainfluenza 3 virus, and adenovirus. In outpatient studies, school-aged children and those with mild diseases are included, emphasizing the role of Mycoplasma pneumoniae.
Viruses have been shown to be the causative agents of pediatric CAP in 26% to 62% of cases. The proportion of viral etiology alone, as well as viral-pneumococcal etiology, is highest in young children, decreasing with age. RSV causes about one third of CAP cases in young children treated in-hospital. In addition, the influenza A and B viruses, parainfluenza 1, 2, and 3, adenoviruses, and rhinoviruses may be causative agents in pediatric CAP. S. pneumoniae was reported to be the causative agent in 11% to 37% and 8% to 28% in hospital and ambulatory settings, respectively. The figures for other microorganisms are: M. pneumoniae in <5% and 7%–30% (inpatients and outpatients, respectively), and Chlamydophila species in >10% and 1%–9% (hospital and ambulatory settings, respectively). Table 9 summarizes the etiology of CAP based on studies covering a period of at least 12 months (given the high dependence on epidemiological conditions) with more than 100 patients included and with serological methods for both viruses and bacteria available.
In developing countries data on pathogen-specific causes are limited. It is known that S. pneumoniae is the leading cause of severe pneumonia. In Africa this microorganism is responsible for over 50% of severe pneumonia cases, a proportion that may vary in different parts of the world. H. influenzae type b (Hib) is a major cause of pneumonia and vaccine studies in different countries have suggested that it may cause around 20% of severe cases (UNICEF/WHO, 2007). Bacteria also contribute to nonsevere cases, but to a lesser extent, and more cases are probably of viral origin. Data from etiology studies in different countries that include inpatients with ARLI have reported pneumonia in 40% to 50% of cases. In these studies viruses were detected in 32% to 68% of the cases with the most frequent being RSV (28%–78% of viral etiology) and adenovirus, parainfluenza and influenza A and B having variable frequencies. Mixed (bacterial/ viral) etiology has been found in 9% to 40% of cases. Although the severity and case-fatality in ARLI of viral origin is classically described as low, in some regions, such as sub-Saharan Africa and perhaps other tropical subregions, concomitant multiple viruses and bacteria infection observed in malnourished children may explain the greater clinical severity of ARLI in this group. PCP is a particularly important cause in young children with AIDS.
Despite these data there is an urgent need to better understand the cause of pneumonia in developing countries where most cases occur. Knowing the pathogens that lead to pneumonia is critical for guiding treatment policies.
Clinical Manifestations
Fever, cough, increased respiratory rate, chest retractions on inspection, and crackles (crepitations) on auscultation are clinical signs of pneumonia in children. If breathing sounds are not audible, chest radiography is mandatory to diagnose or rule out pleural fluid, atelectasis, or other complications of pneumonia (Korppi, 2006).
Fever and cough are common in most respiratory syndromes. Tachypnea is an essential feature of bronchiolitis and is common in both wheezing bronchitis and asthma exacerbation. Chest indrawings may be present in all respiratory distress syndromes, correlating more with age than with clinical syndromes.
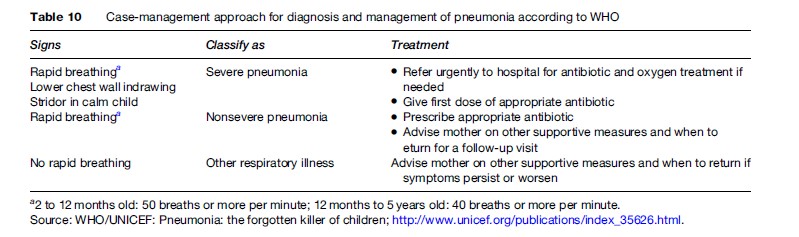
In developed countries it may be important to differentiate bronchiolitis and wheezing bronchitis from pneumonia. Nonetheless, in developing countries (accounting for more than 95% of all new cases of pneumonia worldwide) with limited access to health services, prompt treatment might require trained community health workers to diagnose pneumonia. The WHO has established a simple algorithm based on counting respiratory rates to diagnose and treat pneumonia (Table 10). The respiratory rates set are 50/min for infants <12 months of age and 40/min for children of 1–5 years of age. This strategy – named the ‘case-management approach’ – has shown a low specificity when compared with radiographic evidence in nonsevere pneumonia. However, it is simple and inexpensive to implement, having been evaluated in nine studies with a marked reduction in mortality (Sazawal et al., 2003).
Chest radiography is essential in all febrile children with a decreased general condition, in CAP treated in-hospital as well as children treated at home, if there is no significant improvement within 48 hours after starting antibiotics. In primary care, the diagnosis of CAP must be achieved (and the antibiotic treatment started) on the basis of clinical symptoms and signs alone since radiography does not improve the outcome in ambulatory children with acute respiratory infections. On the other hand, the infiltrate pattern does not predict the causative agent or even the main etiologic classification (bacterial-viral).
Management
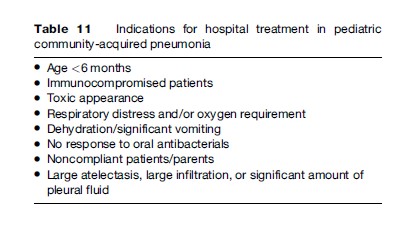
The treatment of CAP in children is empirical: the selection of antimicrobials is based on clinical experience and knowledge of the etiology of CAP. In clinical practice, a pathogen-specific treatment is rarely possible; even rapid tests for respiratory viruses do not rule out bacterial coinfection. Most children with CAP can be treated at home. The indications for hospital admission are presented in Table 11. Oral therapy is sufficient for most patients (Addo et al., 2004).
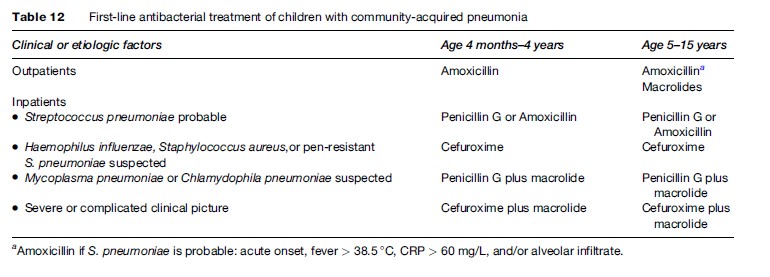
The choice of antibiotics should be based on the age of the patient, clinical presentation, and local resistance patterns of predominant bacterial pathogens. In all cases the first antibacterial choice should cover S. pneumoniae. Amoxicillin is the drug of choice for oral antibacterial therapy in children up to and including 5 years of age at a dose of 40 to 50 mg/kg per day. In areas with high risk of pneumococcal penicillin resistance a higher dose of 90 to 100 mg/kg per day is recommended. Table 12 presents the firstline antibiotics in CAP children.
From a global perspective, because pneumonia kills more children than any other illness, mostly in developing countries where health human resources are scarce, an affordable intervention strategy is necessary to diminish mortality. The most feasible intervention at this point is the case-management approach proposed by the WHO (Table 10). This approach is based on the assumptions that (1) a high proportion of fatal pneumonia is of bacterial origin, (2) timely antibiotic therapy of these infections can considerably reduce mortality by pneumonia, (3) a simple algorithm is sensitive and adequately specific to identify children with pneumonia requiring antibiotic therapy (Table 10), and (4) community health workers can use this algorithm and provide antibiotics to children. A meta-analysis of nine studies that investigated the impact of community-based case management of pneumonia showed a consistent reduction in mortality: total mortality was reduced by 27%, 20%, and 24% among neonates, infants, and children of 0–4 years, respectively. The mortality by pneumonia among these same three groups was found to be reduced by 42%, 36%, and 36%, respectively (Sazawal et al., 2003). Community-based interventions have, therefore, a significant impact on under-5 mortality and should urgently be incorporated in primary health care in developing countries.
Prevention
Three vaccines have the potential to significantly reduce child death by pneumonia. Measles is an acute viral infection that often causes only a self-limiting illness in children, but complications that can lead to disability or death are relatively common, especially in undernourished children. Pneumonia is a serious complication of measles, and the most common cause of death associated with this disease. A safe and effective measles vaccine has been available for use in developed countries for the past 40 years. In 2002 a global effort to expand the use of the measles vaccine, following a strategic plan of the WHO and UNICEF, has resulted in the greatest measurable reduction in under-5 years child mortality from measles, with the annual death rate reduced by 48%, from 871 000 deaths in 1999 to 454 000 in 2004.
A new vaccine suitable for infants and toddlers, called the pneumococcal conjugate vaccine, has been developed to protect against S. pneumoniae. The 7-valent vaccine (PCV7) has been approved for use in routine immunization of all infants in the United States. Recent results in a trial including 17 000 children in the Gambia showed that for children immunized with a 9-valent vaccine there were 37% fewer cases of pneumonia, 15% fewer hospitalizations, and a 16% reduction in overall mortality (Cuttus et al., 2005).
Hib is an important cause of pneumonia and meningitis in developed countries. A trial of Hib vaccination in the Gambia showed an efficacy of 100% for Hib pneumonia and 95% for prevention of invasive Hib disease; the global incidence of radiologically confirmed pneumonia was reduced by 21% (Mulholland et al., 1997).
As mentioned earlier, adequate nutrition and exclusive breast-feeding at least up to 6 months of age reduce the risk of pneumonia and mortality. Zinc intake helps reduce the incidence of pneumonia and the severity of the disease. Additional measures include hand washing with soap and a reduction in indoor pollution.
For prevention of pneumonia in children with HIV infection the WHO and UNICEF recommend prophylaxis with cotrimoxazole for all HIV-positive children, as well as infants born to HIV-infected mothers.
Bibliography:
- Addo-Yobo E, Chisaka N, Hassan M, et al. (2004) Oral amoxicillin versus injectable penicillin for severe pneumonia in children aged 3–59 mo. The Lancet 364: 1141–1148.
- Bonten MC, Chastre J, Craig WA, et al. (2005) Guidelines for the management of adults with hospital-acquired, ventilator-associated, and health care-associated pneumonia. American Journal of Respiratory and Critical Care Medicine 171: 388–416.
- Canadian Critical Care Trials Group (2006) A randomized trial of diagnostic techniques for ventilator-associated pneumonia. New England Journal of Medicine 355: 2619.
- Cuttus F, Enwere G, Jaffar S, et al. (2005) Efficacy of nine-valent pneumococcal conjugate vaccine against pneumonia and invasive pneumococcal disease in The Gambia: Randomised, double-blind, placebo-controlled trial. The Lancet 365: 1139–1146.
- Daneman N, McGeer A, Green K, et al. (2006) Macrolide resistance in bacteremic pneumococcal disease: Implications for patient management. Clinical Infectious Diseases 43: 432.
- Espana PP, Capelastegui A, Quintana JM, et al. (2003) A prediction rule to identify allocation of inpatient care in community-acquired pneumonia. European Respiratory Journal 21: 695.
- Fabregas N, Ewing S, Torres A, et al. (1999) Clinical diagnosis of ventilator-associated pneumonia revisited: Comparative validation using immediate post-mortem lung biopsies. Thorax 54: 867.
- File TM (2003) Community-acquired pneumonia. The Lancet 362: 1991–2001.
- Fine MJ, Auble TE, Yealy DM, et al. (1997) A prediction rule to identify low-risk patients with community-acquired pneumonia. New England Journal of Medicine 366: 243–250.
- Fine MJ, Stone RA, Singer DE, et al. (1999) Process and outcome of care for patients with community-acquired pneumonia. Archives of Internal Medicine 159: 970–980.
- Lim WS, van der Eerden MM, Laing R, et al. (2003) Acquired pneumonia severity on presentation to hospital: An international derivation and validation study. Thorax 58: 377.
- Luby S, Agboatwalla M, Feikin D, et al. (2005) Effect of hand-washing on child health: A randomised controlled trial. The Lancet 366: 225–233.
- Mandel LA, Wundernik RG, Anzueto A, et al. (2007) Infectious Diseases Society of America / American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clinical Infectious Diseases 44(supplement): S27–S72.
- Metlay J and Fine M (2003) Testing strategies in the initial management of patients with community-acquired pneumonia. Annals of Internal Medicine 138: 109–118.
- Metlay J, Kapoor W, and Fine M (1997) Does this patient have community-acquired pneumonia? Diagnosing pneumonia by history and physical examination. Journal of the American Medical Association 278: 1440.
- Mulholland K, Hilton S, and Adegbola R (1997) Randomised trial of Haemophilus influenzae type b tetanus protein conjugate for prevention of pneumonia and meningitis in Gambian infants. The Lancet 349: 1191–1197.
- Sazawal S and Black R (2003) Effect of pneumonia case management on mortality in neonates, infants, and preschool children: A metaanalysis of community-based trials. The Lancet Infectious Diseases 3: 547–556.
- Smith K, Smet J, Romieu I, et al. (2000) Indoor air pollution in developing countries and acute lower respiratory infections in children. Thorax 55: 518–532.
- Torres A, Alcon A, and Fabregas N (2006) Ventilator-associated pneumonia. In: Albert RK, Sluutsky A, and Ranieri M (eds.) Clincal Critical Care Medicine, pp. 175–186. Oxford, UK: Elsevier.
- UNICEF/WHO (2007) Pneumonia: The Forgotten Killer of Children. http://www.unicef.org/publications/index_35626.html (accessed October 2007).
- Victora C, Kirkwood B, Ashworth A, et al. (1999) Potential interventions for the prevention of childhood pneumonia in developing countries: Improving nutrition. American Journal of Clinical Nutrition 70: 309–320.
- Woodhead M, Blasi F, and Ewing S (2005) Guidelines for the management of adult lower respiratory tract infections. European Respiratory Journal 26: 1138–1180.
- Bonten MC, Chastre J, Craig WA, et al. (2005) Guidelines for the management of adults with hospital-acquired, ventilator-associated, and health care-associated pneumonia. American Journal of Respiratory and Critical Care Medicine 171: 388–416.
- Halm EA and Teirstein AS (2002) Management of community-acquired pneumonia. New England Journal of Medicine 347: 2039–2045.
- Korppi M (2006) Community-acquired pneumonia and bronchiolitis in childhood. In: Torres A, Ewig S, Mandel L, and Woodhead M (eds.) Respiratory Infections, pp. 371–383. New York: Oxford University Press: A Hodder Arnold Publication.
- Mandel L, Wundernik RG, Anzueto A, et al. (2007) Infectious Diseases Society of America / American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clinical Infectious Diseases 44(supplement): S27–S72.
- Torres A (2005) Hospital-acquired pneumonia. In: Albert SK, Spiro SG, and Jett J (eds.) Clinical Respiratory Medicine, pp. 315–320. St. Louis, MO: Mosby.
- Valencia M and Torres A (2006) Emergency treatment of community- acquired pneumonia. In: Nava S and Welte T (eds.) Respiratory Emergencies, pp. 183–199. Sheffield, UK: European Respiratory SocietyEuropean Respiratory Monograph.
- Woodhead M, Blasi F, and Ewing S (2005) Guidelines for the management of adult lower respiratory tract infections. European Respiratory Journal 26: 1138–1180.
See also:
Free research papers are not written to satisfy your specific instructions. You can use our professional writing services to buy a custom research paper on any topic and get your high quality paper at affordable price.








