This sample Radiation Therapy Research Paper is published for educational and informational purposes only. If you need help writing your assignment, please use our research paper writing service and buy a paper on any topic at affordable price. Also check our tips on how to write a research paper, see the lists of health research paper topics, and browse research paper examples.
Radiation oncology is the discipline of human medicine focused on generation, conservation, and dissemination of knowledge concerning etiology, prevention, and treatment of cancer and some benign diseases involving special expertise in the therapeutic applications of ionizing irradiation (Smith and McKenna, 2004). It addresses the therapeutic uses of ionizing irradiation given either alone or in combination with other treatment modalities, such as surgery or chemotherapy. Radiation oncology also includes investigation of basic principles of tumor biology, the biologic interaction of irradiation with tissues, normal or malignant, as well as the physical principles of therapeutic irradiation. As a medical profession, radiation oncology involves patient care, scientific research, and education of professionals within the discipline.
Radiation therapy is a clinical modality dealing with the use of ionizing irradiation in the treatment of patients with cancer and occasionally benign disease (Smith and McKenna, 2004). Its aim is to deliver a precisely measured irradiation dose to a defined tumor volume with as minimal damage as possible to surrounding healthy tissue, thus resulting in tumor eradication, improved quality of life, and prolonged survival. Indications for radiation therapy include cases in which it can improve either local control, hence overall survival, or ameliorate symptoms of the disease.
The aim of therapeutic irradiation can be either curative or palliative (Price and Sikora, 2005). The aim of curative radiation therapy is to eradicate the tumor and cure the patient, in cases in which the patient is willing to accept a small risk of significant adverse events in return for the possibility of cure. Palliative radiation therapy has the aim of either ameliorating the symptoms of the disease or preventing it to increase the quality of life without a significant risk of serious adverse events.
Specific issues of radiation oncology are the volume, techniques, and the dose (Halperin et al., 2004). Although the appropriate volume to be irradiated needs to be specified for achieving the desired goal, the appropriate technique to irradiate a specified volume of tissue must also be taken into account. There are two techniques: teletherapy, using either distant-positioned focal source of cobalt-60 machines or linear accelerators as external beam treatment machines, and brachytherapy, with various radioactive isotopes that could be used interstitially, intracavitary, or intraluminally, or as molds. Although teletherapy remains the standard technique for the majority of patients, there is an increasing interest in the use of novel brachytherapy applications in tumors such as prostate cancer. Finally, the irradiation dose must be chosen by taking into account total dose, total number of fractions, number of fractions per day, irradiation dose per fraction, and the overall treatment time. Choice of irradiation dose is therefore a complex issue encompassing treatment goals, volumes, and techniques, as well as knowledge of radiobiology, such as the dose–response relationship for a particular tumor type and normal tissue tolerance.
This research paper provides an introduction into the science of radiation oncology from the standpoint of clinical exercise, radiobiology, and medical physics, all representing specific aspects merged into an important treatment method that nowadays represents an indispensable part of treatment of cancer.
Radiobiology
Radiobiology is a branch of science that deals with the action of ionizing radiation on biological tissues and their cellular and molecular components (Hall and Giacca, 2006). The biological effects of radiation result mainly from damage to the DNA, which is the most critical target within the cell. Damage to DNA occurs by both direct and indirect action. In direct action the radiation interacts directly with the DNA, but about two-thirds of the biological damage by low-LET radiations (sparsely ionizing radiations, called low linear energy transfer), such as X-rays or electrons, is due to indirect action. In indirect action the radiation interacts with other molecules and atoms (mainly water, since 80% of a cell is composed of water) within the cell to produce free radicals that can, through diffusion in the cell, damage the DNA. The indirect action can be modified by chemical sensitizers or radiation protectors. Direct action is the dominant process in interaction of high-LET particles, such as neutrons and charged heavy particles, with DNA.
Irradiation of a cell can result in division delay (the cell is delayed from going through division), apoptosis (the cell dies before it can divide or after division by fragmentation into smaller bodies that are taken up by neighboring cells), or reproductive failure (the cell dies when attempting the first or subsequent mitosis). Radiation damage to cells can be lethal, sublethal (damage can be repaired in hours unless additional sublethal damage is inflicted and eventually leads to lethal damage), or potentially lethal (can be manipulated by repair processes when cells are allowed to remain in a nondividing state).
Much of our understanding of the mechanisms of the response of tissues and organs to dose fractionation and dose rate has come from studies of colony-forming cells in culture. A cell survival curve describes the relationship between the surviving fraction of cells, that is, the fraction of irradiated cells that maintain their reproductive integrity (clonogenic cells), and the absorbed dose. Cell survival as a function of radiation dose is conventionally represented graphically by plotting the surviving fraction on a logarithmic scale on the y-axis against dose on a linear scale on the x-axis.
The type of radiation influences the shape of the cell survival curves (Steel, 2002). On the one hand, densely ionizing radiations exhibit a cell survival curve that is almost an exponential function of dose, shown by almost a straight line on the log-linear plot. For sparsely ionizing radiation, on the other hand, the curves show an initial slope followed by a curving shoulder region and then become nearly straight at higher doses. Factors that make cells less radiosensitive are removal of oxygen to an hypoxic state, the addition of chemical radical-scavengers, the use of low dose-rates or multifractionated irradiation, and cells synchronized in the late-S phase of the cell cycle. The linear-quadratic model is now most often used to describe the cell survival curve assuming that there are two components to cell killing by radiation. A constant a describes the linear component of cell sensitivity to killing on a semi-log plot of (log) survival versus (linear) dose, and b describes the increasing sensitivity of cells to higher radiation doses. The ratio a/b is a measure of the curvature of the survival curve, and is the dose at which the linear and quadratic components of cell killing are equal. The a/b ratio is lower and the curve on a semilog plot is more pronounced for homogeneous, slowly proliferating cell populations, such as in slow-renewing organ systems like kidney and spinal cord. The a/b ratio is higher and the survival curve is straighter for heterogeneous, rapidly proliferating cell populations, such as the regenerative target cell populations in oral mucosa and intestine. One possible contributor to this straightening is the presence of subpopulations with different sensitivities as a function of cell-cycle phase. The a/b ratio is generally in the range 7–20 Gy for early reactions in tissues (10 Gy is commonly used) and 0.5–6 Gy for late reactions (3 Gy is commonly used).
Various chemical agents may alter the cell response to ionizing radiation, either reducing or enhancing the cell response (Hall and Giacca, 2006). Chemical agents that reduce cell response to radiation are called radioprotectors. They generally influence the indirect effects of radiation by scavenging the production of free radicals. Chemical agents that enhance the cell response to radiation are called radiosensitizers and they generally promote both the direct and indirect effects of radiation. Examples are halogenated pyrimidines that intercalate between the DNA strands and inhibit repair, hypoxic cell radiosensitizers that act like oxygen, and bioreductive agents that are activated under hypoxic conditions to kill hypoxic cells.
The presence or absence of molecular oxygen within a cell influences the biological effect of ionizing radiation. Only several micromolar units of oxygen are required to sensitize cells to radiation, and the maximum amount of sensitization on a dosage basis is about 2.5–3-fold, as occurs in well-oxygenated normal tissues and in oxic tumor cells. The sensitization effect is smaller at doses lower than 1 Gy, and when high-LET radiation is used, for example, neutrons, carbon ions. Cell sensitivity is increased with high-LET radiation, and the relative biological effectiveness (RBE) compares the dose of test radiation to the dose of standard radiation to produce the same biological effect. The RBE depends largely on the cell type and the dose per fraction being used. For therapeutic applications, the RBE in the era of neutron radiotherapy was about 3, and it is also around 3 for the newer dose-delivery patterns and protocols using carbon ions.
The time scale involved between the breakage of chemical bonds and the biological effect may be hours to years, depending on the type of damage. If cell killing is the result, it may happen in hours or much later when the damaged cell attempts to divide. Tissue and organ reactions can appear early or late depending on the renewal characteristics of the tissues. If the damage is oncogenic (cancer induction), then its expression may be delayed for years. If the damage is a mutation in a germ cell, the effects may be expressed after one or more generations.
Early reactions in tissues are characterized, for example, by inflammation, tissue edema, denudation of epithelia, and hemopoietic depression (Steel, 2002). Late reactions are characterized, for example, by teleangiectasia of blood vessels, dermal fibrosis, tissue atrophy, ulceration, and intestinal stenosis.
Acute total body radiation exposure can result in one of several radiation syndromes, leading to death, depending on the dose range. The bone marrow syndrome occurs in the dose range 1 Gy < dose < 10 Gy, the gastrointestinal (GI) syndrome occurs in the dose range 10 Gy < dose < 100 Gy, and the central nervous system (CNS) syndrome occurs after doses > 100 Gy. Partial body irradiation to doses at the low end of the dose range for the GI syndrome, of solely the kidneys, lung, or heart, can also result in late lethal reactions. Chronic irradiation over several years to accumulated doses of several Gy can also lead to a chronic radiation syndrome (CRS), characterized by persistent reactions in the hemopoietic, immune, and digestive systems.
The effects of radiation on tissue as a function of dose are measured via dose–response curves. Functional endpoints for various tissues are measured on a graded reaction scale or expressed as a proportion of cases in which reactions are greater than a specified level. Early tissue reactions can be alleviated by the application of radical scavengers before irradiation and/or growth factors after irradiation. These include hemopoietic growth factors in the case of bone marrow, epithelial growth factors (e.g., KGF) for mucosa and epithelium. The mechanism is for growth factors to accelerate the repopulation and differentiation of precursor cells. The lesser reactions imply that higher radiation doses can be tolerated, by up to double the radiation dose in the case of KGF and oral mucosa. These radioprotective effects may be very useful in accelerated radiotherapy in which early reactions are often more severe than in conventional treatments. Late reactions can also be modified by various vascular-associated compounds, such as essential fatty acids in skin, and angiotensin II enzyme inhibitors or receptor blockers in kidney. These agents at least delay the onset of functional injury, and may also reduce the incidence. Hence in principle, this might not only reduce late reactions that are dose limiting, but also allow the possibility of some dose escalation, which would increase tumor control rates.
In general, reactions in tissues are greater when the volume irradiated is increased. This effect is most marked for very small volumes, and in that case it is due largely to migration of cells from the edges of the irradiation fields, which has a greater influence on healing small than large volumes. For larger irradiated volumes, most common in radiotherapy, the volume effect is less marked but still important. For skin, the tolerance of a larger irradiated region may be reduced although the reaction level may be little increased. This is because the likelihood of any area within the irradiated region not healing properly increases with an increase in the number of such areas irradiated. Also, the architecture of organ systems has an influence on the volume effect. Some organ systems comprise functional tissue subunits arranged in parallel, such as nephrons in the kidney and alveoli in the lung. In these cases, parts of the organ can be irradiated and injured without causing functional defects, because the other regions can compensate. In contrast, organs or tissues that comprise functional components in a serial arrangement – for example spinal cord, intestine, and blood vessels – can be functionally damaged to irreparable levels by injury in one small region. The presence of a volume effect is the rationale for new strategies in radiotherapy to reduce irradiated volumes. These include dynamic imaging used to reduce irradiated tumor margins, intensity-modulated radiotherapy used to shape the irradiated fields more closely around the tumor, and protons or carbon ions that can be used to provide a sharper edge to the irradiated volume than is possible using X-rays, gamma-rays, or electrons.
The aim of radiotherapy is to deliver enough radiation to the tumor to destroy it without irradiating normal tissue to a dose that will lead to serious complications (morbidity) (Hall and Giacca, 2006). The basis of dosefractionation practices is rooted in five primary biological factors called the ‘five Rs’ of radiotherapy: radiosensitivity (mammalian cells have different radiosensitivities), repair (mammalian cells can repair radiation damage), redistribution (of cells in the different phases of the cell cycle), repopulation (cells repopulate while receiving fractionated doses of radiation), and reoxygenation (of hypoxic cells during a fractionated course of treatment).
The different radiosensitivities between patients led to a search for biological or molecular markers that would allow the tailoring of different doses to patient subgroups and improve overall outcome (Steel, 2002). The best assay, that is, with the highest positive predictive value, in this case 90%, has been the use of the enzyme marker TGF-b to predict, in a high percentage of lung cancer patients, the progression of marked radiation-induced fibrosis. However, none of the assays has proved sufficiently robust to be able to implement this type of dose-prescriptionvariation strategy in regular practice. Hope is now pinned on the new molecular-based assays.
The biological mechanisms of conventional fractionation treatments are explained as follows. Each daily dose of 2 Gy kills around 50% of tumor cells through reproductive failure and apoptosis. The multiple daily fractions spare normal tissues through repair of sublethal damage between dose fractions and repopulation of normal cells. The former is of note for late-reacting tissues, and the latter applies to early-reacting tissues. The sparing of early reactions also prevents the consequence of an exacerbation of late reactions. Concurrently, dose fractionation increases tumor damage through redistribution of tumor cells into more-sensitive cell cycle phases (G2/M) and allows reoxygenation of hypoxic tumor cells. The differential (the therapeutic ratio) is increased as much as possible between the response of tumor and early and late-reacting normal tissues, so that small doses per fraction spare late reactions preferentially, and a reasonable schedule duration allows regeneration of early-reacting tissues, few consequential late reactions, tumor reoxygenation, and as little tumor cell repopulation as possible.
Current standard fractionation schedules are based on five daily treatments per week and the total treatment time of several to many weeks (Steel, 2002; Hall and Giacca, 2006). This regimen reflects practical aspects of dose delivery to patients, successful outcomes, and convenience to staff delivering the treatment. Other fractionation schemes are employed in particular cases with the aim of improving further the therapeutic ratio. The most common are accelerated fractionation (more than five daily fractions per week) and hyperfractionation (more than one fraction per day with a smaller dose per fraction, <1.8 Gy).
Following irradiation and recovery of normal tissues, there is some residual injury. For example, skin is generally more susceptible to mechanical trauma, and bone marrow may be compromised in stem-cell content and hence more susceptible to subsequent cytotoxic agents. This indicates that retreatment using radiation may be possible, but by using lower doses. There are now many cases of reasonably successful treatments of recurrences in patients and many data in experimental animal systems, including kidney and spinal cord, showing the gradually increasing tolerance to retreatment at later times after the first course of irradiation.
Medical Radiation Physics
Therapeutic medical radiation physicists are scientists who are involved in the application of radiation in the clinical environment. Clinicians define the treatment volume, the surrounding critical structures, and prescribe the radiation dose and fractionation regime (Halperin et al., 2004; Smith and McKenna, 2004; Price and Sikora, 2005). Medical physicists, on the other hand, are responsible for planning the course of treatment, defining the technique that will be used to deliver the radiation to the specified volume, and assuring that the equipment is performing according to strict quality standards.
In order to assist in localizing disease relative to surrounding radio-sensitive anatomy, radiological tools are used. Several new emerging technologies in the diagnostic arena have resulted in a greater utilization of imaging modalities in the therapeutic environment. In addition to providing anatomical localization, physiological and metabolic tools are now also widely available to support the accurate definition of a treatment volume. Figure 1 is an X-ray image of a breast that presents three-dimensional anatomical information in two dimensions via a traditional gray-scale film record.
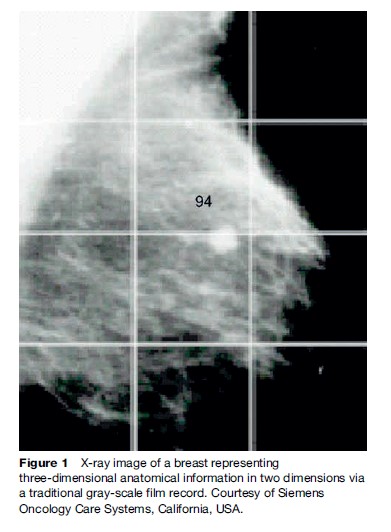
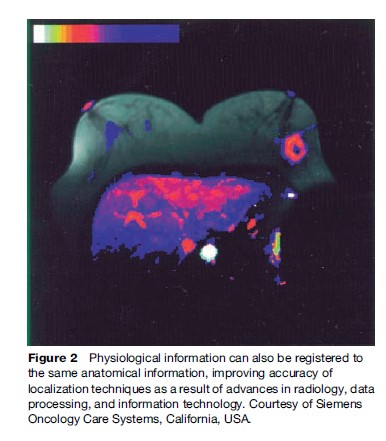
Figure 2, however, shows how physiological information can also be registered to the same anatomical information. This improves accuracy of localization techniques as a result of advances in radiology, data processing, and information technology.
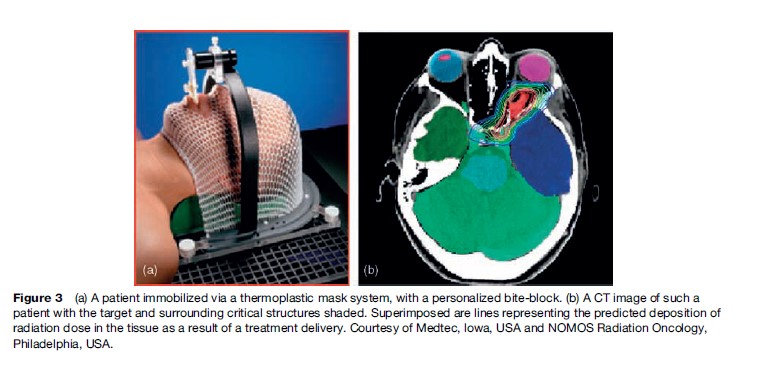
The precise localization of a target relative to surface anatomy has always been the challenge in treatment planning of radiation fields, and in the reproducible positioning of a patient on a treatment unit for a course of fractionated radiotherapy. Most treatment machines are isocentric, which means that they can be maneuvered relative to a known reference point in space. The treatment volume within a patient is also located relative to the isocenter, and the radiation delivery is then focused around the isocenter. Radiation fields are conformed to the shape of the treatment volume. Wide field margins are intuitively known to result in higher morbidity. In some anatomical sites, for example the brain, immobilization of the patient is achieved by using external fixation systems, and reproducible positioning of a cooperative patient is then reassured. Figure 3 shows a patient immobilized by using a thermoplastic mask system with a personalized bite-block. A computed tomography (CT) image of such a patient is also shown, with the target and surrounding critical structures shaded. Superimposed are lines representing the predicted deposition of radiation dose in the tissue as a result of a treatment delivery.
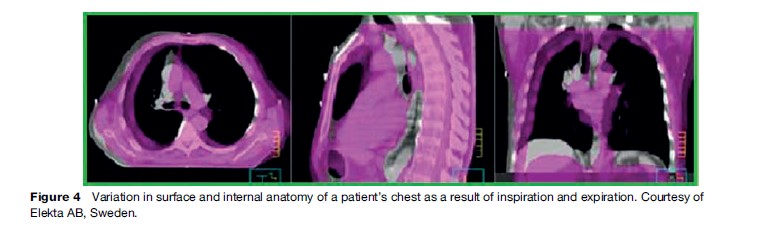
In 1999, the International Commission of Radiation Units and Measurements published an updated report that endeavored to establish consistency in the definitions of radiation therapy targets and in the recording and reporting of dose delivery. The internal target volume was defined, in which a margin around a radiotherapy target could be visualized that accounted for variations in the treatment volume as a result of involuntary patient motion. Modern high-speed imaging modalities allow scanning of patients within a few seconds, so the movement of internal organs relative to surface anatomy can now be quantitatively measured. As a result, margins around treatment volumes can be more precisely defined. Figure 4 indicates the variation in surface and internal anatomy of a patient’s chest as a result of inspiration and expiration.
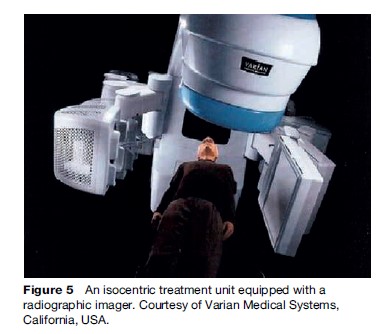
The resultant challenge to tailor daily treatment to the exact location of the target requires knowledge of inter and intra-fraction organ motion. This is a new technique known as adaptive radiotherapy. Adaptive radiotherapy involves confirming target positioning online, and several different techniques have been developed to assist in this process. An example of this is the use of radiographic imaging in the treatment position. Figure 5 shows an isocentric treatment unit equipped with a radiographic imager. Radio-opaque markers, implanted into the patient or onto his or her skin surface, can then also be used to enhance daily target localization in these systems.
Another example of adaptive therapy is the use of an integrated gating system in which the radiation beam is activated in response to equipment that monitors or controls the breathing pattern.
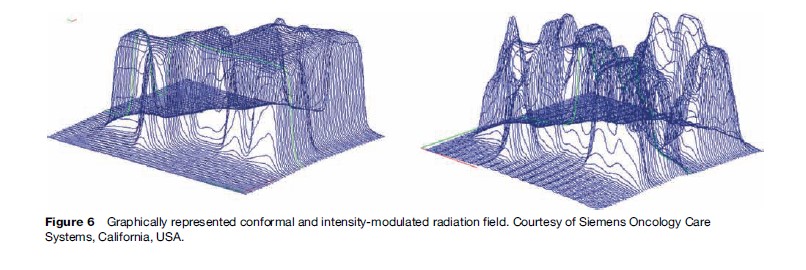
Blocking, shaping, or conforming radiation beams to the shape of a target volume is achieved with multileaf collimators or customized shielding. Multileaf collimators are generally embedded into the head of a treatment machine. Providing a nonuniform beam distribution within a radiation field is also achievable by varying the thickness of this collimation inside the radiation field. This is known as intensity modulated radiotherapy (IMRT). Deliberate nonuniformity in the radiation dose delivery, as well as treatment of targets that encompass a critical structure, are achievable with this type of technology. A conformal and intensity-modulated radiation field are graphically represented in Figure 6.
Treatment planning computers have traditionally been used with forward-planning approaches. The optimal radiation field arrangements and beam-modifying devices are modeled by algorithms, which predict the resultant radiation transport and deposition into a patient. Monte Carlo techniques are now being introduced for this purpose, given the availability of computational power. Monte Carlo techniques model the transport of radiation through the patient geometry at the particle level, using physics first principles. Inverse treatment planning methods have been adopted for sophisticated techniques such as IMRT, which may involve numerous, complex radiation field sequences. The calculation of the best possible dose distribution from many IMRT fields also requires embedded iterative optimization routines. Currently, the challenge lies in remodeling pretreatment plans online to the information retrieved from image-guided radiotherapy techniques. This remodeling would then need to be translated into a modified delivery at the time of treatment.
Interstitial, intracavitary, and intraluminal brachytherapy is a highly localized treatment modality in which a physically small radioactive source is delivered directly into a tumor bed. Implantation of the sources can be permanent or fractionated by using remote after loading systems, which are automated. Figure 7 shows the variety of applicators and introducers available to this treatment modality. These devices guide the source placement relative to the tumor.
Comprehensive quality assurance in the radiation oncology environment remains a challenge for the team of health-care professionals involved in managing a patient. New recommendations and protocols are developed all the time to ensure safety in treatment delivery.
Dosimetry in radiation medical physics is the measurement of radiation-absorbed dose with reference to an international, primary standard that is based on water calorimetry. Most centers participate in international dosimetry audits, in which external checks of absorbed dose in the clinic are encouraged. The most common and accurate method to establish radiation dose in the clinical environment is by derivation by using the response of a calibrated air ionization chamber placed into a water phantom. Improvements in the uncertainty of such measurements are ongoing.
Similarly, all advances in tools for target localization, treatment planning, and treatment delivery in radiation oncology require the concurrent development of effective dosimetry tools to verify equipment performance, confirm the integrity of information exchange, and assure quality in service. Figure 8 shows a modern tool for online beam dosimetry, for instance, capable of wireless transmission of beam data during patient treatment. The device is inserted into the accessory holder of the treatment machine and is designed to minimally perturb the radiation beam.

Radiation medical physicists play a significant role in the health professional team in clinical radiation oncology. Continued professional and curriculum development is essential in this dynamic environment to ensure the safe and effective application of radiation to health care.
Clinical Science Of Radiation Oncology
Although radiation therapy is frequently given as a single treatment modality, it is also used in combination with other treatment modalities. When combined with surgery, radiation therapy can be given either as preoperative or postoperative radiation therapy. The potential advantages of preoperative radiation therapy include eradication of subclinical or microscopic disease beyond the margins of the surgical resection, diminished tumor implantation by decreasing the number of viable cells within the operative field, sterilization of metastases in lymph nodes inside and/or outside of the operative field, decrease in the potential for dissemination of viable clonogenic tumor cells, and an increase in the possibility of resectability. The main disadvantage of preoperative radiation therapy is possible interference with the process of normal healing of tissues affected by irradiation. The potential advantages of postoperative radiation therapy include elimination of subclinical disease remaining in the tumor bed, the possibility to administer higher doses of radiation therapy for overt disease found in surgical specimens, and the possibility to tailor the irradiation fields according to the pathological staging. The delay in initiation of postoperative radiation therapy until wound healing is completed is a potential disadvantage of postoperative radiation therapy. In addition, radiation effects may be impaired by vascular changes occurring post-surgery in the operative field.
Radiation therapy and chemotherapy are combined in order to improve effects on both local/regional and distant levels (Halperin et al., 2004; Smith and McKenna, 2004). There are four possibilities that this combination may explore: spatial cooperation (drugs affect tumor cells outside the radiation therapy field), independent cell kill (drugs can independently affect tumor cells within the radiation therapy field), enhancement (drugs can enhance effects in radiation therapy field), and protection of normal tissues (Steel, 2002; Hall and Giacca, 2006). Whereas the first two mechanisms do not require interaction, the latter two depend on the interaction of these two treatment modalities. One mechanism does not necessarily exclude employment of another mechanism; for example, an active agent can act on a local level as well as affect micro metastasis outside the radiation therapy field. There are several generations of agents used in combined radiation therapy and chemotherapy, and clinical research in this field is one of the most important research areas in human oncology. Recent years also brought improvements in molecular oncology that ultimately led to the introduction of targeted therapy. Agents such as those targeting epidermal growth factor receptor hold promise of improving loco regional tumor control at least in some tumor types, such as head and neck, and another class of agents is targeting tumor microvasculature.
Timing of combined radiation therapy and chemotherapy can include, first, chemotherapy followed by radiation therapy given with the main goal of addressing the issue of micro metastasis control and local or regional tumor decrease or down staging, as well as using reduced irradiation fields, thus decreasing the radiation morbidity (Halperin et al., 2004; Smith and McKenna, 2004; Price and Sikora, 2005). It can also include, second, adjuvant or consolidation timing when chemotherapy follows radiation therapy. A third possibility is concurrent radiation therapy and chemotherapy, aimed mostly at an improvement on a local or regional level. A fourth possibility involves alternating radiation therapy with chemotherapy in order to increase therapeutic benefit by decreasing toxicity, which can occur with any concurrent regimen, by giving both modalities separated by short periods of time. In addition to these, many hybrid combinations exist that explore different mechanisms of the combined treatment approach. Combination radiation therapy and chemotherapy is nowadays considered the standard of treatment in many tumor types, especially for locally advanced, inoperable cases.
A combined treatment modality approach may also include all treatment methods. Examples of such combination may include postoperative treatment of breast cancer or gastric cancer. Frequently, less radical surgery followed by radiation therapy with or without chemotherapy yields the same local tumor control at the primary tumor site and survival as do radical procedures such as is the case in soft tissue sarcomas or breast cancer. This approach offers organ preservation and is frequently coupled with an improvement in functioning and quality of life.
In addition to these, hormonotherapy is frequently used in hormone-sensitive tumors such as those of breast or prostate. Its use and timing largely depends on initial and/or postsurgical tumor volumes, hormone receptor status, and administration of other treatment modalities (surgery and/or chemotherapy).
Impact Of Biology And Technology On Clinical Science Of Radiation Oncology
The most important contribution of radiobiology to clinical radiation oncology is in the field of fractionation. Although conventional fractionation regimen is a debatable term, altered fractionation regimens (hyper fractionation, accelerated fractionation, hypo fractionation) have been employed in a number of tumor sites with success, improving local tumor control and overall survival, albeit with occasionally observed increase in acute adverse events (Steel, 2002; Halperin et al., 2004; Smith and McKenna, 2004; Price and Sikora, 2005; Hall and Giacca, 2006). Increasing the total dose with hyper fractionation, by using a higher number of smaller fraction sizes, improved survival in head and neck cancer and lung cancer, among others, whereas accelerated fractionation, aiming to overcome accelerated repopulation of tumor clonogens, did so in lung cancer. Both types of fractionation use at least partially (e.g., in concomitant boost regimen) two fractions per day. Besides altered fractionation, hypoxia was addressed adequately in a number of studies, especially in head and neck cancer and cervix cancers, thus showing that its existence adversely influences overall treatment outcome. In addition, special chemical compounds, namely radiosensitizers and radioprotectors, hold great promise to enable differential radiosensitization of tumor cells but not normal cells (sensitizers) or preferentially protecting normal tissue (protectors) while not doing so on tumor cells/tissues, respectively. More clinical research is needed to optimize these approaches, a quest frequently identified as the ‘Holy Grail’ of radiation oncology.
The impact that new technology made upon the clinical science of radiation oncology was first observed through better imaging. With the use of CT and magnetic resonance imaging (MRI) scans, it was possible to better visualize tumors and embark on more effective treatment planning. This was followed by the wider introduction of positron emission tomography (PET) technology in pretreatment diagnosis and staging of cancer, but also in evaluations of response and follow-up efforts. Finally, PET-CT is increasingly being used for treatment planning. Besides imaging, substantial improvement in computerized sciences enabled wide introduction of powerful software programs that made treatment planning system more sophisticated and faster. Radiation oncology has slowly but definitely moved from 2D to 3D and recently to 4D treatment planning, by taking into account not only volume but also temporal (movement in time) aspects of a tumor tissue in a host. These improvements enabled more conformal radiation therapy to be performed than ever before. It became possible to precisely tailor the radiation therapy dose to the tumor and its immediate vicinity in order to raise the dose to higher levels, while protecting normal tissues. A number of dose escalation studies reconfirmed an important premise of radiation therapy that increased tumor dose should lead to an improvement in local tumor control and ultimately overall survival (Halperin et al., 2004; Smith and McKenna, 2004; Price and Sikora, 2005).
Intensity-modulated radiation therapy is an especially advanced form of 3D conformal radiation therapy that incorporates sophisticated computer-controlled radiation beam delivery and computer-optimized treatment planning design. This is achieved by varying the beam intensity within each beam portal, as opposed to the uniform beam intensities used in conventional 3D conformal radiation therapy. Although there is a huge interest in this technology, clinical experience is still confined to single institutional data, and major evidence coming from big prospective, preferably randomized, studies is lacking. This technique was mostly used in cancers of the prostate and head and neck.
In addition to intensity-modulated radiation therapy, other technological advances such as tom therapy or a special device called ‘cyber knife’ are increasingly being used in the clinic. These tools hold promise for better therapeutic ratio in various tumor types, an ultimate goal of radiation therapy.
Biology and technology meet through clinical studies, which are seen as a necessary pathway toward adoption of a method or a treatment technique, largely based on existing evidence. Practicing evidence-based oncology is one of the ‘musts’ in the contemporary setting, since it represents the conscientious, explicit, and judicious use of current best evidence in making decisions about the care of individual patients. The practice of evidence-based oncology means integrating individual clinical expertise with the best available external clinical evidence from systematic research. The major component of this exercise comes through existing meta-analyses and prospective randomized clinical trials offering strong support pro or contra one technique, method of delivery, or treatment regimen. Radiation oncologists are largely involved in the design and performance of clinical studies, although much clinical research still needs to be done to identify and address important questions in radiation oncology.
Bibliography:
- Hall EJ and Giacca AJ (2006) Radiobiology for the Radiologist, 6th edn. Philadelphia, PA: Lippincott.
- Halperin EC, Schmidt-Ullrich RK, Perez AC, and Brady LW (2004) The discipline of radiation oncology. In: Halperin EC, Schmidt-Ullrich RK, Perez AC, and Brady LW (eds.) Principles and Practice of Radiation Oncology, 4th edn., pp. 1–95. Philadelphia, PA: Lippincott.
- Price P and Sikora K (2005) Treatment of Cancer. 4th edn. London: Arnold.
- Smith RP and McKenna WG (2004) The basics of radiation oncology. In: Abeloff MD, Armitage JO, Niederhuber JE, Kastan MB, and McKenna WG (eds.) Clinical Oncology, pp. 535–578. Philadelphia, PA: Elsevier, Churchill Livingstone
- Steel GG (2002) Basic Clinical Radiobiology. London: Arnold.
See also:
Free research papers are not written to satisfy your specific instructions. You can use our professional writing services to buy a custom research paper on any topic and get your high quality paper at affordable price.








