This sample Sexually Transmitted Infections Research Paper is published for educational and informational purposes only. If you need help writing your assignment, please use our research paper writing service and buy a paper on any topic at affordable price. Also check our tips on how to write a research paper, see the lists of health research paper topics, and browse research paper examples.
Introduction
Sexually transmitted infections (STIs) are those conditions that are primarily spread through person-to-person sexual contact. They are of profound importance in terms of the immediate suffering caused to affected individuals, but also in terms of the public health impact and overall cost to humanity. Their magnitude is immense and underappreciated in policy-making circles. Even in areas where STIs are considered ‘under control’ they tend to persist in risk groups stratified by poverty, inequality, and general access to health and preventive services.
In general, experts distinguish between curable STIs caused by bacteria or protozoa (such as syphilis, gonorrhea, chlamydia, and trichomoniasis), and chronic STIs caused by viruses (such as genital herpes and human papillomavirus infection), which are treatable but not curable in the classic sense. Epidemiologically, the various STIs inhabit different sorts of infection networks that overlap to greater or lesser degree, but are slightly different from one another. Research investigators, clinicians, and public health officials now increasingly prefer the term STIs over the previously accepted and widely used term sexually transmitted diseases (STDs), since many STIs are asymptomatic and do not cause overt symptoms in the infected individual. The term STI captures the notion that persons may be infected, and therefore may represent a transmission risk to others, without suffering from a ‘disease’ in the classic sense.
By and large, Western, developed nations have developed and implemented STI surveillance and control programs to deal with community-level spread of infection, and these have been successful to greater or lesser degree depending on the particular STI and aspects of the social, cultural, and political environment in which they occur. Above all, STIs are malleable and adaptable to changing social norms of sexual contact and sexually associated behaviors.
Human immunodeficiency virus (HIV) is transmitted predominantly as an STI, but also is characterized by other routes of transmission such as blood borne exposure or through breastfeeding. Two types of HIV are distinguished, HIV-1, which has a worldwide distribution and is responsible for the great majority of HIV infections, and HIV-2, which is geographically linked to West African origins. Recent research continues to emphasize the ways in which genital ulcer and mucosal inflammatory diseases facilitate and reinforce HIV transmission and acquisition across populations at risk (Fleming and Wasserheit, 1999). For this reason, many experts see STI prevention and control as an important part of a comprehensive HIV prevention strategy.
History And Epidemiology Of STIs
Formerly called venereal diseases (after Venus, the goddess of love), STIs have afflicted human populations for centuries. Ancient Greek medical texts accurately described the clinical manifestations of gonorrhea (literally, ‘flowing seed’) and recognized a sexual route of transmission. Sixteenth-century Europe was ravaged by the Great Pox, later determined to be syphilis, and now widely believed to have been acquired from New World populations and transmitted across the Atlantic shortly after European contact. Military campaigns are intimately associated with STIs, often linked to young male soldiers acquiring infections through engagement with prostitution. Modern STI control strategies developed following World War I, as public health took a greater interest in community health preservation and the prevention of person-to-person spread. In the United States, this effort was most effectively spearheaded by Surgeon General Thomas Parran, whose monograph, Shadow on the Land (1937), helped establish surveillance systems for STIs and formal partner notification programs in local public health jurisdictions. Other countries also implemented surveillance and control programs for STIs, focusing on those conditions for which primary curative therapy could be implemented and distributed more widely. Bacterial STIs such as syphilis, gonorrhea, and chlamydia were added to the roster of reportable diseases, both to ensure that infected individuals receive appropriate therapy, and also to ensure that their sex partners are appropriately identified and treated as well.
Surveillance of STIs across time and space is a major public health responsibility. Tracking of STIs occurs through a combination of active and passive surveillance, and is most effectively implemented in developed countries with strong governmentally based public health structures in place. Nevertheless, STI incidence and prevalence remain incompletely tracked under current surveillance systems. In the United States, for example, chlamydia is the most commonly reported infectious condition, with nearly 1 million cases reported annually (Centers for Disease Control and Prevention [CDC], 2006a). However, this figure is a gross underestimate of the true burden of infection due to the asymptomatic nature of the disease, incomplete screening, and underreporting. A recent study estimated the incidence of STIs in the United States at approximately 18.9 million cases annually (Weinstock et al., 2004). Globally, the situation is much bleaker. Focusing solely on curable STIs (syphilis, gonorrhea, chlamydia, and trichomoniasis), the World Health Organization (WHO) estimated an incidence of 340 million infections occurring annually among men and women 15–49 years of age (WHO, 2001).
In general, viral STIs other than HIV are not reportable conditions in most jurisdictions, and surveillance for these infections is undertaken through population-based serologic testing. Viral STIs tend to be chronic, longstanding infections as they are not curable in the classic sense with antibiotic treatment. In the United States alone, prevalence estimates for genital tract infection with herpes simplex virus type 2 (HSV-2) and human papillomavirus (HPV) range from 11 to 33% of the sexually active population. Based on these figures, experts believe the true burden of viral STIs worldwide may exceed several hundred million persons. Evidence is also increasingly mounting that HSV-2 is a powerful cofactor for HIV transmission (Freeman et al., 2006), and studies are underway to determine the extent to which treatment of STIs (including genital herpes) serves to reduce sexual transmission of HIV.
Unfortunately, surveillance for STIs other than HIV in many developing countries is rudimentary, so the true prevalence of STIs in most areas of the world is unknown.
Transmission Dynamics Of STIs
A great conceptual leap forward in thinking about STIs was the development of the transmission dynamics model, first promoted in a series of articles in the late 1980s by Roy Anderson and Robert May (May and Anderson, 1987; Anderson and May, 1988). Building on prior work by Hethcote, Yorke, and other early modeling pioneers, the transmission dynamics model states that the reproductive rate of infection within a population (Ro) is a function of three parameters: the efficiency of transmission of the infectious organism during sexual contact (b), the rate of sex partner change among members of the population (c), and the duration of infectiousness (D). At equilibrium, Ro = 1, meaning that each case of infection generates one new case of infection and infection rates overall do not change. When the rate of infection is increasing within a population, then Ro > 1 (more people are entering the infected pool of individuals than are leaving it). Conversely, when infection rates are receding within a population, then Ro < 1 and more people are leaving the pool of infected individuals than are entering the pool (presumably through early detection, effective treatment, reducing risk behaviors, etc.).
The transmission dynamics model clarified two basic tenets which are essential to the public health approach to STIs: (1) reducing STI rates is achieved by implementing program interventions to reduce b, c, and D of the model components; and (2) when interventions are implemented, the infection will contract toward hard-to-reach populations, which are relatively resistant to the effects of the intervention and which serve to perpetuate and sustain infection within the community at large. These hard-to-reach populations are termed core groups, and the emergence of core group theory is specifically linked to the conceptual model that Anderson and May first formulated.
Public health structures focus primarily on minimizing b and D from the transmission dynamics model. In general, b is a measure of how efficiently STI pathogens are transmitted from an infected person to a susceptible partner within sexual partnerships. Using condoms correctly and consistently for all sexual contacts is a way of reducing the likelihood of transmission of STI pathogens during sexual partnerships, and thus serves to reduce b at the population level when this behavior is adopted widely throughout the population. Similarly, use of vaccination to prevent HPV acquisition effectively reduces b by decreasing the partner’s susceptibility to infection. D is reduced through early screening to detect indolent or asymptomatic infection, and provision of effective medication to cure or treat these infections. The screening of reproductive-age women to detect and treat chlamydial infection serves to reduce D when implemented broadly. Also, public awareness campaigns encouraging individuals to get tested for STI generally work to detect, and presumably treat, ongoing infections and reduce D by limiting these individuals’ ability to transmit infection to others. Tackling parameter c has been quite a different issue. The transmission dynamics model suggests that reducing the rate of partner change (later refined by Anderson and May to the effective mean rate of partner change) should lead to reduced spread of STI pathogens by limiting the number of susceptible individuals who are potentially exposed through sexual contact. However, public health officials have generally shied away from promoting what amounts to social engineering in the realm of sexual behavior and decision making, and have tended to avoid overt recommendations to reduce the rate at which persons acquire new partners.
The concept of core groups, facilitated by STI networks, helps explain how infections can persist within subsets of the population even as effective disease control and prevention strategies are implemented widely within a community. Aggressive screening, detection, and treatment of infection during an epidemic serves to drive the fewer remaining cases into harder-to-access populations, often marginal to mainstream society and with limited access to social service organizations. Core groups are subsegments of the general population for whom Ro > 1, that is, who generate more than one case of infection for each infected individual. Such persons may sustain sexual partnerships with high rates of transmission of infectious agents (elevated b), high rates of partner change (elevated c), and long durations of infectiousness (elevated D), thereby serving at the population level to keep Ro at equilibrium, even as the infection recedes throughout lower-risk segments of the community. Research in this area has also begun to clarify the nature of overlapping social and sexual networks, and the role of social networks in the spread of STI pathogens. Network studies of STI patients argue for the inclusion of social (nonsexual) network members in disease control investigations and outbreak interventions, presumably because these individuals are more likely to be engaged in similar sexual risk behaviors as the STI index case, even though they are not direct sexual contacts.
Classification Of STI Pathogens
More than 30 sexually transmitted pathogens have been described, although the majority of STIs are caused by just a few different agents. New pathogens (e.g., Mycoplasma genitalium) continue to be identified or confirmed as sexually transmitted organisms, based on advances in laboratory testing methodologies. A useful classificatory scheme is to distinguish between curable STIs, generally caused by bacteria or protozoa and which can be treated with antimicrobial therapy, and chronic STIs caused by viruses, which are often treatable but not curable in a classic sense. Some of the most important STIs are included in Table 1. Asymptomatic carriage is common, and infected individuals may or may not experience the full range of symptoms listed. Moreover, the data in Table 1 are incomplete inasmuch as a variety of other pathogens can be sexually transmitted.
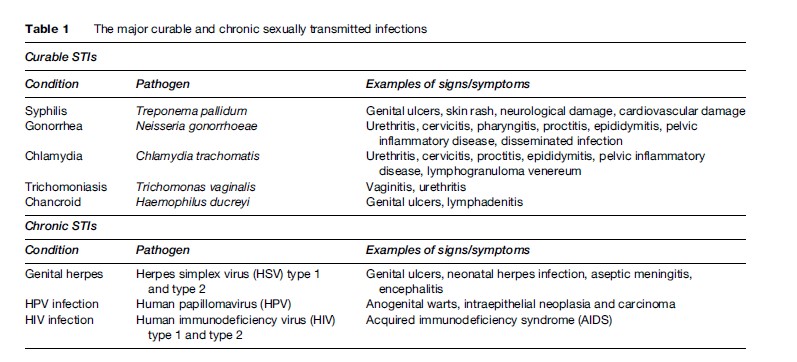
Complete discussion of all STI pathogens is beyond the scope of this research paper. Presented here is basic background information, common clinical manifestations, and long-term complications associated with the most common STIs: four bacterial infections (syphilis, gonorrhea, chlamydia, chancroid), two viral infections (genital herpes and HPV infection), and one parasitic infection (trichomoniasis). The reader is referred to a more comprehensive textbook source for additional information on these agents, and other STI pathogens not discussed here.
Syphilis
Syphilis is a bacterial infection caused by Treponema pallidum. It is one of the most studied STIs, particularly because of its rich historical background. Most experts agree that syphilis was unknown in Europe until the late fifteenth century, when it suddenly appeared as a major scourge affecting the European population. Syphilis is classified as a genital ulcer disease, and in its primary phases causes the development of painless ulcerations in the genital tract. The infection then progresses to a secondary phase, which results in cutaneous eruptions typically described as a rash but which can be very aggressive pustules or poxlike lesions. Untreated, the disease develops a latent state and can remain inactive for many years. However, a substantial proportion (25–35%) of those with latent infection may progress to develop long-term complications affecting the central nervous, cardiovascular, or other body organ systems. In the preantibiotic era, neurological syphilis was a major cause of long-term disability and early death. Upon invading the central nervous system, the organism affects the dorsal columns of the spinal cord leading to difficulties in walking and balance (tabes dorsalis), and also causes premature difficulties with mentation and cognition through the development of general paresis (also known as dementia paralytica). Syphilis can also be transmitted transplacentally from infected mother to fetus (congenital syphilis). Up to 50% of pregnancies in women with early syphilis may end in stillbirth; those infants born with syphilis suffer developmental abnormalities affecting the bones, teeth, brain, and other organ systems.
The Columbian theory of syphilis transmission holds that T. pallidum infections were prevalent in New World populations but generally absent from Europe prior to Columbus’s voyage in the late fifteenth century. These New World treponemal infections may have been nonvenereal dermatoses that afflicted native populations of North, Central, and South America and the Caribbean, and substantial paleopathological and historical evidence backs up the assertion that syphilis, or some variant thereof, was present in the New World for centuries prior to European contact. Upon arriving in the New World, Europeans quickly acquired venereal forms of syphilis and brought the infection back to Europe, where population-level herd immunity was absent. The infection spread quickly and virulently through European communities, often following lines of battle and the advancing armies of warring nations. A common clinical presentation was the development of aggressive, large, poxlike lesions affecting the face and body. These lesions were larger and different in character from other poxcausing diseases of the day, leading to the ascription of the term Great Pox to describe early pustular forms of secondary syphilis in the late fifteenth and early sixteenth centuries. Artistic renditions of persons with syphilitic lesions appear in woodcuttings and paintings starting at about this time, and are virtually unknown prior to this period. The current name by which we know this disease was ultimately taken from a poem by the early sixteenthcentury Italian poet Fracastoro, in which the protagonist named Syphilis offended the god Apollo and was afflicted with the disease as punishment.
Over the years, several treatments have been proposed for syphilis, including the use of heavy metals such as arsenic (salvarsan), or intentional infection of patients with malaria-causing organisms (championed by the Austrian physician Julius Wagner-Jauregg, who won the 1927 Nobel Prize in Physiology or Medicine for this work). All of these treatments were generally ineffective and were associated with long-term toxicities of their own. Syphilis treatment was revolutionized in the 1940s with the discovery and clinical use of penicillin. To this day, penicillin remains the drug of choice for treating syphilis.
In the United States, the history of syphilis is inextricably tied to the notorious Tuskegee Syphilis experiment, a natural history study of syphilis conducted by the U.S. Public Health Service from the mid-1930s to the mid-1960s. This was a nontreatment study of poor, rural, African-American men in Tuskegee, Alabama, ostensibly to monitor the long-term effects of untreated syphilis in a time when the treatments carried substantial side effects and toxicities of their own. Men who participated in the study were told they had ‘bad blood’ and were monitored serologically over an extended time period. Treatment was withheld from the study participants long after the curative effects of penicillin were well recognized. Today, reference to the Tuskegee study resonates strongly as a touchstone for governmental deception, abuse of power, and disenfranchisement of minority populations at the hands of a powerful medical/public health apparatus.
Today, syphilis continues to exert a major toll on human populations. Many developing populations continue to suffer from syphilis due to lack of surveillance systems, screening laboratory tests and infrastructure, and required treatment medication. The persistence of congenital syphilis is particularly unfortunate, inasmuch as serotesting and treatment of infected women during pregnancy could significantly reduce or eliminate this condition. In recent years, increasing rates of syphilis have been seen among men who have sex with men (MSM), and syphilis is increasingly implicated as a cofactor in sexual transmission of HIV among high-risk communities.
Gonorrhea
Gonorrhea is caused by infection with the bacterium Neisseria gonorrhoeae, a spherical-shaped organism (coccus) with Gram-negative staining characteristics. The bacterium is often referred to as the gonococcus, and the organisms appear in nature as paired sets of bacteria attached side-by-side, or diplococci. It is an ancient disease, with references to its clinical manifestations appearing in early texts from Greek, Roman, and biblical times. Among males, uncomplicated gonococcal infection often causes copious amounts of thick green or yellow urethral discharge, coupled with intense burning on urination (dysuria). This finding may have given rise to its name, which is derived from Greek roots (‘flow of seed’). In fact, the flowing material is a mixture of bacteria and pus, with the body’s immune system producing large amounts of infection-fighting white corpuscles (leukocytes) to combat the offending bacterial agent. Among females, gonorrhea typically infects the uterine cervix, rather than the urethra, and symptoms of uncomplicated infection are generally milder. Most women do not experience urinary symptoms; some may notice a mild vaginal discharge, and many are asymptomatic altogether.
Unfortunately, untreated gonococcal infection can cause serious and significant complications. In males, the bacteria can ascend the urethral passage and establish infection in the epididymis and testicles (epididymitis and epididymo-orchitis). In females, gonorrhea can cause infection of the Bartholin’s and Skene’s glands near the vaginal introitus, leading to painful local abscess formation. Gonococcal infection (along with chlamydia) is also one of the most important causes of pelvic inflammatory disease (PID), characterized by upper genital tract infection of the uterus (endometritis), Fallopian tubes (salpingitis), and the abdominal-pelvic lining (peritonitis). Classically, gonococcal PID leads to irritation around the area of the liver in the right upper quadrant of the abdomen (perihepatitis or Fitz-Hugh-Curtis syndrome) and the development of adhesions in this area. The brisk inflammatory response triggered by gonococcal infection also causes scarring of other infected structures, particularly the Fallopian tubes, leading to tubal-factor infertility, ectopic pregnancy, and tubo-ovarian abscess as a result. Chronic pelvic pain is also a consequence of peritoneal scarring and adhesions. In both males and females, disseminated gonococcal infection (DGI) occurs when the bacterium gains access to the bloodstream and causes severe systemic disease, typically causing small-joint arthritis and the development of pustules on the skin surface (arthritisdermatitis syndrome). Infection of the heart valves (endocarditis) has also been described. Gonococcal infection is also an important cause of eye infection in the newborn. Neonatal conjunctivitis (also known as ophthalmia neonatorum) due to untreated maternal gonorrhea manifests as a purulent discharge from the conjunctival tissues developing within a few days after delivery, and can lead to blindness in the neonate if left untreated.
Antibiotic resistance has become, and continues to be, a major problem in treating gonorrhea. Over time, the organism has acquired resistance (both plasmid-mediated and chromosomally mediated) to many antibiotic classes, including penicillins, tetracyclines, and more recently fluoroquinolone antibiotics. Currently, quinolone-resistant Neisseria gonorrhoeae (QRNG) is a significant public health concern in developed as well as developing countries; rates of QRNG are high in Asian and Pacific Island settings, and are increasing across North America and Western Europe, further limiting the effective antibiotic armamentarium for treating these infections (CDC, 2006b). In general, cephalosporin antibiotics are considered the drugs of choice for treating gonorrhea. Coinfection with chlamydia is common, so antichlamydial therapy is also often prescribed for patients with gonorrhea.
Chlamydia
Chlamydia is caused by infection with Chlamydia trachomatis, an obligate intracellular pathogen that affects the mucus membrane tissues of the genital tract in males and females. In developing countries, the organism is also responsible for causing trachoma, a devastating ocular infection leading to corneal scarring and blindness; however, the chlamydial serovars (bacterial subspecies) that cause trachoma are different from those that cause genital tract infection. In many ways, sexually transmitted chlamydial infection behaves much like gonorrhea in terms of the body sites affected (male urethra and female cervix) as well as the long-term complications it causes (PID leading to tubal scarring and infertility). Chlamydia is a relatively newly recognized pathogen, coming to public health consciousness in the late twentieth century with the advent of effective laboratory testing procedures to detect its presence. In general, genital tract chlamydial infections tend to be less aggressive and less pyogenic than gonococcal infections, inasmuch as the inflammatory response is not as brisk and symptoms are generally milder. However, chlamydia is much more widely distributed than gonorrhea in developed countries and the numbers of infected individuals are much higher, such that chlamydia has become the single most important cause of tubal-factor infertility in developed countries. Implementation of widespread screening programs for chlamydia among reproductive-age women in developed nations is an important component of infertility prevention strategies. The true prevalence of genital tract chlamydial infection in developing countries is unknown, due to a lack of effective surveillance systems and laboratory testing strategies.
Many males and females with genital tract chlamydial infections are asymptomatic. Some males develop symptoms of urethritis, including burning on urination (dysuria) and a mucoid or sticky urethral discharge. Clinically, this condition looks much like gonococcal urethritis, although in general the discharge is less purulent and less aggressive, and microbiologic staining of the discharge material fails to reveal the classic Gram-negative diplococci of gonorrhea (hence the term NGU or nongonococcal urethritis to describe cases of chlamydial infection among symptomatic males). Females with cervical chlamydial infection are generally detected through screening, as symptoms are subtle (mild vaginal discharge or mild cervical bleeding) and may not be perceived by the infected individual. Infection of the newborn may occur when a child is born to a mother with untreated genital tract chlamydia. Neonatal chlamydia manifests as conjunctival infection (ophthalmia neonatorum) occurring within 1–2 weeks, or pneumonia that may not become clinically evident for several weeks after delivery.
Antibiotic treatment for chlamydia is achieved through the use of macrolide, tetracycline, or fluoroquinolone antibiotics. Single-dose treatment with azithromycin, a macrolide agent, is preferred by many clinicians for ease of treatment and likelihood of medication compliance. At the present time, antibiotic resistance is less of a concern for chlamydia than for gonorrhea, and widespread systematic resistance has not yet occurred. Infection rates remain high in North America and other developed nations despite aggressive screening of reproductive-age women.
Chancroid
Chancroid is an infectious disease caused by the bacterium Haemophilius ducreyi. Individuals with acute infection develop lesions that resemble the ‘chancre’ of primary syphilis, hence the name chancroid. In fact, chancroid is markedly different from syphilis, causing the development of deep genital ulcerations that frequently are complicated by infection of the inguinal lymph nodes with subsequent nodal suppuration.
Transmission of chancroid is exclusively by way of sexual contact, with the development of clinical symptoms occurring approximately 3–10 days after exposure. Infected individuals generally develop painful ulcerations in the genital tract, associated with swelling or pain in the inguinal region due to infection of regional lymph nodes. Chancroid ulcers typically have ragged, irregular edges with undermined borders (soft chancre), and the ulcer base is generally necrotic and purulent. The ulcers may mimic those of syphilis, but they typically lack the firm, indurated border that is characteristic of syphilitic lesions (hard chancre). Infected inguinal lymph nodes (buboes) may ultimately rupture as a consequence of ongoing infection, and pus can often be expressed from ruptured nodes on direct palpation.
Chancroid is more common in developing-country settings, or among individuals with a recent travel history to endemic areas. Studies have documented higher rates of infection in marginalized populations such as commercial sex workers and their clients. Uncircumcized men have also been shown to have a higher risk of acquisition of chancroid than circumcized men, presumably because the foreskin serves as a bacterial reservoir that can harbor infectious bacteria after exposure. Localized urban outbreaks in developed countries are often linked to illicit drug use or commercial sex work. Chancroid and other genital ulcers have been shown to be important cofactors that facilitate HIV acquisition and transmission in developing countries.
Diagnosing chancroid can be difficult. The causative organism (H. ducreyi) is a small, facultative, anaerobic coccobacillus that requires hemin (X-factor) for growth, and clinical samples often grow poorly in culture even using selective media. DNA amplification methods to detect chancroid, while promising, are not yet clinically available. Therefore, a high index of clinical suspicion for chancroid is required to ensure that appropriate antibiotic therapy is rendered in a timely fashion to persons who may be infected. A number of antibiotic agents are effective against chancroid, including members of the macrolide, cephalosporin, and fluoroquinolone classes. Buboes may require drainage by aspiration if large or painful, and extended treatment courses may be required to ensure complete bubo resolution. Higher rates of treatment failure have been reported among uncircumcized men, and close clinical follow-up is required to ensure treatment adequacy, particularly among HIV-infected individuals.
Genital Herpes
Genital herpes is caused by herpes simplex virus (HSV). The virus is classically divided into two viral types (type 1 and type 2), which can be distinguished molecularly, and which historically have been held to infect different parts of the body. HSV-1 commonly causes infection of the mouth, lips, and oral mucous membranes (orolabial herpes), while HSV-2 commonly causes infection of the penis, scrotum, labia, vulva, vagina, and other genital tract structures (genital herpes). Serologic and virologic testing demonstrates a significant degree of overlap with regard to herpes types and body sites infected: some orolabial infections are caused by HSV-2, and increasing numbers of genital tract infections are caused by HSV-1. Type-specific serologic tests for HSV are now available in many developed-country settings and are being increasingly used to identify individuals with HSV infection. By and large, HSV-2 infections are generally held to be sexually transmitted, and serologic studies of HSV-2 infection confirm high rates of infection in general population samples. Recent studies estimate overall age-adjusted seroprevalence of HSV-2 in the United States at 17.0% of the general population (Xu et al., 2006).
Clinical manifestations of genital herpes can range from aggressive, painful blisters and ulcerations (clinical infection), to subtle but perceivable irritations, rashes, fissures, and other manifestations (subclinical infection), to a complete absence of identifiable clinical symptoms (asymptomatic infection). The virus is believed to enter the body through microabrasions that occur during sexual contact. Clinical symptoms, which occur in up to 20% of persons with genital tract HSV-2 infection, may develop within a few days of exposure and include painful genital lesions (blisters, ulcerations, rashes), local edema, and systemic symptoms such as fever, malaise, and fatigue. The virus is neurotropic and ascends local nerve roots, establishing latency in the dorsal root ganglion of the spinal column; infection is lifelong and is not curable in the classic sense. Viral reactivation is common, and causes local recurrence of genital lesions, usually in a unilateral (dermatomal) distribution.
Subclinical infection and asymptomatic infection are actually more common than classic clinical infection. Many individuals experience recurrent rashes, irritations, fissures, or other genital tract lesions which they do not recognize or perceive as herpes, but which can later be so identified through laboratory testing. Infants born to infected mothers are at risk for developing neonatal herpes, a devastating condition affecting the newborn that is associated with serious, long-term, neurological consequences. Pregnant women with active genital-tract herpes lesions at the time of labor are delivered abdominally by cesarean section to prevent or limit neonatal exposure to herpes virus in the birth canal. Research studies have identified the highest risk of neonatal herpes among infants whose mothers acquire primary genital tract herpes infection during pregnancy (Brown et al., 2003).
Untreated clinical infection ultimately resolves over several days or weeks. Early treatment with oral acyclovir (or related medications such as famciclovir or valacyclovir) significantly reduces the duration of clinical lesions and reduces viral shedding in primary infection as well as in outbreaks of recurrent infection. Management of recurrent genital herpes infections includes patient education to identify and recognize clinical symptoms, coupled with antiviral treatment to reduce or prevent morbidity. Episodic treatment of recurrences involves use of antiviral medication at the time lesions begin to develop, or as soon thereafter as possible, so as to limit the duration of the clinical recurrence. Suppressive treatment involves daily use of antiviral medication to prevent the onset of recurrent clinical lesions, and is generally recommended for persons with frequent or traumatic recurrences. Some studies suggest that antiviral medications may be beneficial for preventing sexual transmission of HSV within serodiscordant partnerships. Recent data suggest a strong link between genital-tract herpes infection and the spread of HIV in areas of high HIV prevalence such as sub-Saharan Africa (Freeman et al., 2006). Research studies are underway to determine whether herpes treatment reduces the likelihood of HIV acquisition in high-prevalence settings.
Human Papillomavirus (HPV) Infection
More than 80 different viral types of human papillomavirus (HPV) have been identified, and more than 20 of these have been found to infect the human genital tract. HPV has long been recognized as the cause of external warts in the anogenital area, and aggregations of warts are commonly referred to as condylomata acuminata. These manifestations are typically caused by HPV types 6 and 11, although other HPV types can also cause anogenital warts. Warts are generally treated with local therapies designed to eliminate the wart itself, rather than the viral infection. After treatment, wart recurrence rates are high because the virus remains viable within dermal and subdermal tissues.
Other HPV types are oncogenic and have been closely linked to development of cancer of the cervix, vulva, vagina, and anus. These ‘high-risk’ HPV types include 16 and 18, and to a lesser extent several others (e.g., 31, 33, 35, 45, 56), and are implicated as the cause of most cervical and anal cancers. For this reason, aggressive screening for high-risk HPV cervical infection has been advocated among reproductive-age women in developed countries for early detection and treatment of HPV-related dysplasias and neoplasias, in an effort to prevent cervical cancer morbidity and death. (In practice, this generally takes the form of routine cervical screening for dysplasia by Papanicolaou (Pap) smear, followed by reflex HPV testing of Pap smear specimens only if dysplastic or suspicious cells are identified.) By and large, the wart-causing HPV types 6 and 11 are not oncogenic, and individuals with external genital warts caused by HPV are not necessarily at risk for the development of cervical or other genital tract neoplasia. However, coinfection with other high-risk HPV types can occur, so individuals with anogenital warts should be counseled to follow routine screening guidelines for cervical cancer prevention. Two preventive vaccines against HPV have been recently developed and are being introduced for clinical use. One is a quadrivalent vaccine targeting cancer-causing HPV types 16 and 18 as well as wart-causing HPV types 6 and 11, while the other is a bivalent vaccine targeting HPV types 16 and 18 alone. Widespread use of the vaccine among adolescent and young adult females may help prevent further spread of HPV and the development of HPV-associated diseases (Bosch et al., 2006).
Trichomoniasis
Trichomoniasis, also known as trichomonal infection, is a clinical condition caused by infection with the parasite Trichomonas vaginalis. It is commonly diagnosed in women, in whom it typically causes a profuse, malodorous vaginal discharge with vaginal inflammation. Male infection also occurs but is much more likely to be asymptomatic. Trichomonal infection is the most common curable STI worldwide, accounting for more than half of the estimated 340 million bacterial or parasitic infections occurring annually (WHO, 2001). Trichomoniasis has been linked to premature rupture of membranes in pregnancy and preterm delivery, and has been identified in epidemiological studies as a cofactor in HIV transmission.
The causative agent, T. vaginalis, is a flagellated anaerobic protozoan that is transmitted during sexual contact. Up to 50% of infected women are initially asymptomatic, although many of these will proceed to develop clinical symptoms within several months of infection. Because the organism can survive for months or years in epithelial crypts and periglandular areas, subclinical infection may occur and diagnosis may be delayed. Most commonly, women develop a frothy gray or yellowgreen vaginal discharge, associated with vaginal itching (pruritus) indicative of a brisk vaginal inflammatory response. Some women classically develop small petechial hemorrhages on the cervix, which may be visible on inspection during a pelvic examination (‘strawberry cervix’), although this occurs in a minority of cases. Infection in males is most commonly asymptomatic, although symptomatic urethritis may occur in a minority of cases.
Most cases of trichomonal vaginitis are diagnosed by direct visualization of the parasitic organisms on a microscopic examination of vaginal secretions. Unfortunately, this method of diagnosis is relatively insensitive (40–70%) and is dependent on the technique of specimen collection as well as the experience of the microscopist. Diagnostic accuracy can be increased by incubating vaginal secretions in commercially available culture media, although these tests are not widely available in many clinical sites. Diagnosis in resource-poor settings is generally based on syndromic presentation and clinical history.
Metronidazole or tinidazole are the treatments of choice for trichomonal infection. Asymptomatic male partners must also be treated to prevent reinfection of treated females. Drug resistance, although unusual, should be suspected in cases of trichomoniasis that do not respond to standard antimicrobial therapy. Individuals should be counseled not to drink alcohol while taking these medications, because they can cause a disulfiramtype reaction (flushing, shortness of breath, and severe nausea and vomiting).
Consequences Of STIs
Sexually transmitted infections have immediate as well as long-term consequences. Many STIs cause pain, discomfort, and immediate suffering to infected individuals. Genital herpes causes painful, recurrent ulcerations in the genital tract. Early treatment with antiviral medication can reduce the intensity and duration of the lesions, but is not curative of the infection. Trichomonal infection often causes an uncomfortable, malodorous vaginal discharge in women, and is known to cause urethral irritation and inflammation in some men as well. Gonorrhea in men classically causes intense burning on urination and copious amounts of urethral discharge, and both syphilis and chancroid cause ulcerations in the genital tract along with other associated symptoms. The immediate pain and suffering of STIs is an important public health consequence, for these symptoms often trigger a health-seeking response among affected individuals in need of care for their medical condition.
In addition to causing substantial physical discomfort, STIs are often stigmatizing conditions in society, and commonly lead to feelings of shame, guilt, or regret among infected individuals. Substantial research is underway to clarify the extent to which stigma and shame help structure the STI experience at the level of the individual. For example, persons at risk for STI may be reluctant to seek care if they believe their privacy or confidentiality could be compromised through the care-seeking process. As a result, lack of early detection and of early treatment can further facilitate the transmission of infection by prolonging the duration of infectiousness. Chronic infections, such as genital herpes, may be more stigmatizing than curable infections, although limited data are available to address the magnitude of these effects.
From a public health standpoint, the long-term medical and economic consequences of STIs are profound, particularly with regard to reproductive tract infections in women. STIs are the most important preventable cause of human infertility: in industrialized countries, untreated chlamydial and gonococcal infections are a major cause of PID, resulting in infertility from Fallopian tube scarring, as well as other complications such as ectopic (tubal) pregnancy, tubo-ovarian abscess, and chronic pelvic pain. Untreated STIs among pregnant women are a major cause of spontaneous abortion, stillbirth, premature labor, and low birth weight, and can lead to serious infections in the newborn including neonatal herpes, congenital syphilis, and ophthalmia neonatorum. STIs are important facilitators of HIV transmission as well. Studies in developed and developing countries demonstrate that STIs, particularly those causing genital ulcerations, enhance the likelihood of HIV acquisition among HIV-negative individuals (Fleming and Wasserheit, 1999) and also promote genital-tract shedding of HIV among persons who are already HIV-infected. Moreover, STIs have oncogenic potential: oncogenic strains of HPV are widely recognized as the most important cause of anogenital cancers, particularly cancer of the cervix in women. And hepatitis B, which like HIV may be transmitted through sexual contact as well as through bloodborne routes, is a major cause of liver cancer worldwide. Finally, from an economic perspective, research suggests that STIs other than HIV account for up to 5% of the total discounted healthy life-years lost in sub-Saharan Africa. STIs consistently rank among the top disease categories for which adults seek health care in developing countries, and remain among the top causes of disease, death, and healthy life lost among women of childbearing age.
STI Control And Prevention
The public health approach to STIs is based on the twin tenets of control and prevention. Control is the term used to describe the implementation of basic programmatic strategies at the national level to detect infections and limit their spread throughout the wider population. Prevention speaks to the systematic application of public health principles to inhibit the primary acquisition of STIs, and to limit further spread of infection in the event that primary acquisition does occur. Often, control and prevention go hand in hand. For example, treatment of an incident bacterial STI such as gonorrhea serves a control function by eliminating the infection within the affected individual, and also serves a prevention function by eliminating the opportunity for the individual to spread the infection to others in the future.
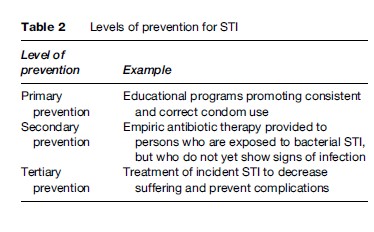
In general, three different levels of prevention for STIs can be distinguished (Table 2). Primary prevention is the implementation of programmatic activities to prevent direct exposure to an STI pathogen. Educational programs promoting safer sex and correct and consistent condom use, or programs promoting delay of first sexual contact among teenagers, are examples of primary STI prevention programs. (Vaccination against STI pathogens such as HPV constitutes another example of primary prevention.) Secondary prevention is the implementation of strategies to prevent the onset of disease in those who have been exposed and may be infected with an STI pathogen. Provision of empiric antibiotic therapy to sex partners of persons with a bacterial STI such as syphilis is an example of secondary STI prevention. Tertiary prevention is the effort to limit morbidity and restore health to those who are infected. This is the most basic form of STI control, and is really a form of harm-reduction inasmuch as treatment is designed to prevent further tissue damage or long-term complications from an ongoing clinical condition. Clinical care for symptomatic curable STIs is an example of tertiary prevention. (Some argue that STI treatment also serves a primary prevention function by reducing the number of infected individuals within the community, thereby reducing the risk for others as well.)
Partner Notification And Referral
Identifying, tracking down, and providing treatment to the ‘source case’ of the infection, as well as to secondary cases who may also have been infected by the index case, is another aspect of STI control. In developed countries, this process is typically implemented through systems of partner notification and referral for medical treatment. Two types of partner notification have been described. The first form, patient referral, occurs when an STI patient is instructed to notify his or her own partners that they have been exposed to an STI, and that they should seek medical care for testing and treatment. The second form, provider referral, occurs when a health-care provider or health agency member interviews an STI patient to elicit the names and contact information of recent sex partners, and then notifies these partners of their STI exposure and need for testing and treatment. Unfortunately, these methods of partner notification are inadequate and incomplete. Individuals with an STI are often unwilling or unable to identify their sex partners or release contact information to a health department investigator when asked. Some individuals do not wish to notify sex partners who they believe infected them in the first place. And STI network studies suggest that infections occur among social as well as sexual network members, such that only following direct chains of sexual contact inevitably misses some cases of STI occurring in social (but not sexual) networks of interaction with infected individuals.
Recently, advocates of expedited partner therapy (EPT) have demonstrated enhanced outcomes in partner notification and referral through the provision of antimicrobial therapy to partners outside of the usual channels of notification and referral (Golden et al., 2005). These sorts of novel strategies for partner treatment often obviate the need for a physical examination of the partner, since treatment is offered outside of clinical care settings. In some cases, EPT can be achieved through patient delivered mechanisms, whereby persons with an STI are given extra doses of medication to give to their partners. In other settings, pharmacy-delivered mechanisms have been used, whereby partners are referred to local pharmacies who then give antimicrobial treatment. Advocates point to increased numbers of partners treated by way of EPT strategies compared with traditional partner notification and referral patterns, and also to decreased rates of reinfection or persistent infection of the initial STI patient, presumably due to effective treatment of sex partners through EPT who otherwise would remain untreated and infectious.
Passive and active surveillance systems are also important aspects of STI control and prevention programs in developed and some developing countries. Passive surveillance occurs when health authorities track and take note of case reports of disease that are submitted by treating clinicians and laboratory-testing facilities. These case reports form the basis of STI intervention planning, for they direct health authorities to areas of ongoing concern or changing epidemiologic patterns. Active surveillance implies the intentional use of screening or directed testing among large sections or subsections of the population, with the intent of finding asymptomatic or subclinical infection that might otherwise remain undetected without aggressive outreach. The implementation of chlamydia screening among women of reproductive age is an example of an active surveillance system. Both methods are important components of comprehensive STI control and prevention strategies.
The Future Of STIs
New developments in STI research and programmatic activities hold promise for reducing the public health burden of these infections. The use of field-based test strips for diagnosing syphilis is one such development. Historically, syphilis has been diagnosed using standard serologic tests, which require a certain amount of laboratory processing that is often unavailable in resource-poor settings. Blood samples must be collected and centrifuged to separate blood cells from serum, and then testing is performed on these serum samples. Recently, several manufacturers have developed field-based, immunochromatographic, syphilis test strips that can be used with whole blood samples, thereby obviating the need for additional specimen processing in the laboratory. A number of these test strips have been evaluated by the WHO and other health authorities to determine sensitivity, specificity, and performance characteristics. Production and distribution of the test strips at low cost will advance syphilis detection and may serve to facilitate early treatment, particularly among pregnant women for prevention of congenital syphilis infection.
Additional research is needed in the realm of STI treatment, particularly with regard to effective, singledose, oral therapies. Such treatments, if available at low cost, could be distributed widely to populations at risk in developing and developed-country settings alike to reduce STI-associated suffering. Unfortunately, few such agents are currently available which meet these criteria. Single-dose azithromycin has activity against chlamydia (and at higher doses, gonorrhea) but recent studies have demonstrated high levels of resistance to azithromycin among syphilis strains in North America and Europe, and have cast a shadow on the utility of this agent for oral treatment of syphilis. Fluoroquinolone antibiotics, once a mainstay of treatment for gonorrhea, are now limited in their utility by antimicrobial resistance to quinolone antibiotics. STI control and prevention will depend to a great degree on the availability of field-based treatments that can be administered easily and safely by public health personnel in a variety of clinical and nonclinical settings.
Finally, the use of social and sexual network paradigms will continue to advance STI prevention goals by shedding light on the ways in which sex partners are selected within and outside of networks of social interaction. Degrees of network embeddedness, overlap, and separation affect STI risk of network members, and additional research in these areas is warranted. Network analyses provide insight with regard to the dynamic nature of human sexual behavior, and the relationship between sexual behavior and STI risk. As public health incorporates network methods into standard STI control activities, enhanced case finding and disease prevention goals are advanced.
Conclusion
STIs are widely distributed across human societies and are a significant cause of suffering worldwide. A variety of microbial agents can be sexually transmitted, including bacteria, viruses, and parasites. Bacterial and parasitic STIs are curable with antibiotic therapy, and viral STIs can now be effectively treated with appropriate antiviral agents. STIs represent an important public health problem because of their propensity to spread asymptomatically across sectors of the population and because of their substantial impact on reproductive and neonatal health. Surveillance and testing of populations at risk are essential elements of STI control and prevention in developed and developing countries alike. Further research is needed in STI biology, epidemiology, and prevention to identify more effective tools to prevent the spread of STI pathogens and reduce the burden of these pathogens on human populations.
Bibliography:
- Anderson RM and May RM (1988) Epidemiological parameters of HIV transmission. Nature 333: 514–519.
- Bosch FX, Cuzick J, Schiller JT, et al. (eds.) (2006) HPV vaccines and screening in the prevention of cervical cancer. Vaccine 24 (Suppl3): S251–S261.
- Brown ZA, Wald A, Morrow RA, et al. (2003) Effect of serologic status and cesarean delivery on transmission rates of herpes simplex virus from mother to infant. Journal of the American Medical Association 289: 203–209.
- Centers for Disease Control and Prevention (CDC) (2006a) Sexually transmitted disease surveillance, 2005. Atlanta, GA: U.S. Department of Health and Human Services.
- Centers for Disease Control and Prevention (CDC) (2006b) Sexually transmitted diseases treatment guidelines, 2006. MMWR Morbidity and Mortality Weekly Report 55: No. RR-11.
- Fleming DT and Wasserheit JN (1999) From epidemiological synergy to public health policy and practice: The contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sexually Transmitted Infections 75: 3–17.
- Freeman EE, Weiss HA, Glynn JR, et al. (2006) Herpes simplex virus 2 infection increases HIV acquisition in men and women: Systematic review and meta-analysis of longitudinal studies. AIDS 20: 73–83.
- Golden MR, Whittington WLH, Handsfield HH, et al. (2005) Effect of expedited treatment of sex partners on recurrent or persistent gonorrhea or chlamydial infection. New England Journal of Medicine 352: 676–685.
- May RM and Anderson RM (1987) Transmission dynamics of HIV infection. Nature 326: 137–142.
- Parran T (1937) Shadow on the Land. New York: Reynal and Hitchcock.
- Weinstock H, Berman S, and Cates W, Jr. (2004) Sexually transmitted diseases among American youth: Incidence and prevalence estimates, 2000. Perspectives on Sexual and Reproductive Health 36: 6–10.
- World Health Organization (WHO) (2001) Global Prevalence and Incidence of Selected Curable Sexually Transmitted Infections: Overview and Estimates. Geneva, Switzerland: World Health Organization.
- Xu F, Sternberg MR, Kottiri BJ, et al. (2006) Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. Journal of the American Medical Association 296: 964–973.
- Anderson RM and May RM (1991) Infectious Diseases of Humans: Dynamics and Control. Oxford, UK: Oxford University Press.
- Brandt AM (1987) No Magic Bullet: A Social History of Venereal Disease in the United States since 1880. New York: Oxford University Press.
- Holmes KK, Sparling PF, Mardh PA, et al. (eds.) (1999) Sexually Transmitted Diseases, 3rd edn. New York: McGraw-Hill.
- Jones JH (1981) Bad Blood: The Tuskegee Syphilis Experiment. New York: The Free Press.
- Stoner BP (2007) Current controversies in the management of adult syphilis. Clinical Infectious Diseases 44: S130–S146.
- World Health Organization (2005) Sexually Transmitted and Other Reproductive Tract Infections – a Guide to Essential Practice. Geneva, Switzerland: World Health Organization.
- World Health Organization (2007) Global Strategy for the Prevention and Control of Sexually Transmitted Infections: 2006–2015. Geneva, Switzerland: World Health Organization.
See also:
Free research papers are not written to satisfy your specific instructions. You can use our professional writing services to buy a custom research paper on any topic and get your high quality paper at affordable price.








