This sample Smallpox Research Paper is published for educational and informational purposes only. If you need help writing your assignment, please use our research paper writing service and buy a paper on any topic at affordable price. Also check our tips on how to write a research paper, see the lists of health research paper topics, and browse research paper examples.
Poxviruses And Orthopoxviruses (General Characteristics)
For the last two centuries, poxviruses and poxvirusassociated diseases have figured prominently in the inception of disease prevention and elimination strategies, many of which have since proven pivotal in the successful practice of public health. Poxviruses were also at the center of many of the twentieth century’s groundbreaking discoveries in the fields of virology and immunology. As early as 1913, Edna Steinhart and colleagues provided the first demonstration that a virus, the poxvirus Vaccinia virus, could be viably maintained (cultured) in tissue explants, in this case, rabbit corneas. Two years later, these investigators, again, were first in demonstrating the principle of virus neutralization, illustrating that exposure to convalescent serum could ablate subsequent infectivity of Vaccinia virus. Later, in 1938, a milestone in diagnostic virology was achieved when Ectromelia virus, a poxvirus pathogen of certain strains of inbred mice, became the first animal virus to be visualized using electron microscopy. These discoveries, of course, follow long after Edward Jenner’s famous experiments in 1796. Jenner’s experiments provided scientific justification for the practice of inoculation, whereby ‘cowpox’ (most likely Vaccinia virus) was scarified into the dermis as a preventive measure against virulent smallpox disease, thus laying the foundation for the field of immunization science.
Poxviruses have held the long-standing attention of the scientific community for a multitude of reasons. The family of poxviruses is genetically diverse and its members are capable of causing infections in a wide array of vertebrate and invertebrate taxa. There are 11 genera of viruses in the family Poxviridae, but only eight (subfamily Chordopoxvirinae) contain viruses capable of replication in vertebrate hosts (Figure 1). Among these, the genus Orthopoxvirus, which includes Variola virus, Cowpox virus, Monkeypox virus, and Vaccinia virus, holds the greatest importance to human health (Table 1). Variola virus, the causative agent of smallpox, is alone among the orthopoxviruses as a pathogen solely of humans. The remaining are zoonotic, which is to say transmissible from vertebrate animals to humans, and in some cases, the reverse.
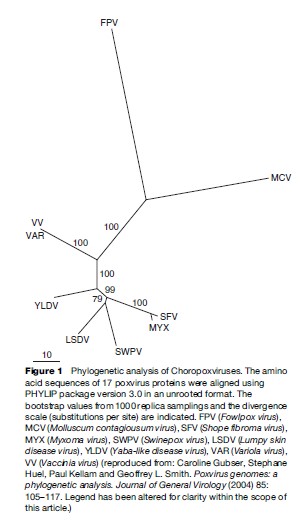
The distinctive features of poxviruses include their size, shape, nucleic acid composition, and method of cellular invasion and replication. Poxvirus virions are large (approximately 350 270 nm for Vaccinia virus), complex, and either ovoid or bricklike in shape as viewed by scanning or transmission electron microscopy. Their genomes – which at approximately 100–300 kilobase pair are large relative to most other viruses – consist of nonsegmented double-stranded DNA molecules, a single linear molecule per virion. In addition, and unlike most other DNA viruses, poxviruses replicate in the cytoplasm of host cells. These latter features render poxviruses attractive as tools for recombinant gene expression in mammalian cells and for heterologous antigen delivery (i.e., vaccine development for non-poxvirus-associated infections such as HIV and malaria).
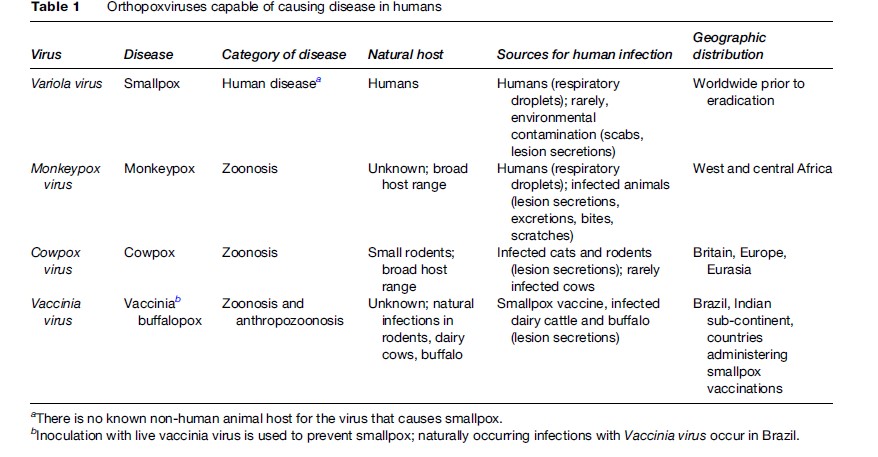
Orthopoxviruses also produce a characteristic pathophysiologic response upon infection of most animal species. The predominant feature of orthopoxvirus infections is the development of a characteristic disseminated vesicular-pustular rash (commonly associated with systemic disease) or focal lesion(s) at the site of virus introduction to the dermis (Figure 2). In humans, poxvirus infections are generally accompanied or preceded by pronounced fever, and in some cases, infections can result in the development of painful lesions on the nasal, oral, and respiratory mucosa, or can lead to ocular damage through irreversible scarring of the cornea. Variola virus, Monkeypox virus, Cowpox virus, and Vaccinia virus, the orthopoxviruses with most relevance to human health, are covered in greater detail in the sections that follow.
Smallpox
Clinical And Epidemiologic Features
Smallpox is considered one of the great epidemic disease scourges of human history. Frequently lethal, highly transmissible, and capable of leaving survivors with lifelong disfigurement, smallpox was greatly feared and its arrival in communities was cause for considerable alarm. Several distinct clinical forms of smallpox were recognized prior to eradication of the disease. These ranged in severity from relatively mild (‘modified-type,’ seen in previously vaccinated individuals) to very severe with high likelihood of fatal outcome (‘flat-type’ and ‘hemorrhagic-type,’ seen in those with predisposing immunocompromising conditions). The majority of infections resulted in a characteristic presentation referred to as ‘ordinary-type’ smallpox – it is this form that is generally thought of today in association with the disease.
‘Ordinary-type’ smallpox was characterized by a rapid onset of symptoms including fever, malaise, headache, and backache (‘prodrome’ phase) followed after 2 to 4 days by the appearance of a distinctive rash. The rash evolved slowly over the course of 2 to 3 weeks, typically beginning with macular lesions on the mucosal surfaces of the mouth and pharynx, appearing next on the face, forearms, and lower extremities, and finally the trunk (centrifugal distribution). The evolution of rash assumed a characteristic progression beginning with macules (spots) followed by papules (raised bumps), then vesicles (blisters), pustules (firm, pearlescent, lesions, deep-seated in the skin), and finally crusts (leathery scabs). In contrast to chickenpox, the disease most confused with smallpox at the earliest stages (caused by varicella zoster virus infection), smallpox lesions commonly occurred on the palms and soles and could be found at the same stage of evolution on affected parts of the body. Transmission of Variola virus between humans occurred principally through respiratory droplets, which were observed to be highly concentrated with virus, especially during early rash development when mucosal surfaces of the mouth and pharynx become colonized. Smallpox scabs were also demonstrated to harbor small amounts of infectious virus, and patients were considered competent to transmit infection until all scabs had disengaged. There are currently no drug therapies for treatment of smallpox that do not harbor an associated risk of toxicity. One compound that has shown pronounced in vitro activity against orthopoxviruses, however, is cidofovir (VISTIDE; a nucleotide analogue of cytosine). Because of the severity of illness and potential lasting complications of smallpox (namely, scarring), cidofovir has been proposed as an investigational therapy for treatment in emergency situations despite the fact that its use at therapeutic doses has been associated with significant nephrotoxicity. This is an active area of ongoing research.
Table 1 Orthopoxviruses capable of causing disease in humans
Fatality rates associated with ‘ordinary-type’ smallpox ranged from 10 to 62%, depending on various factors including virus genotype, availability of supportive health care, vaccination status, and so on. Other clinical types of smallpox were associated with predictably worse outcomes; ‘flat-type’ and ‘hemorrhagic-type’ smallpox, both of which were rare, were characteristically associated with rapid progression of an atypical rash, and subsequent death likely from sepsis syndrome or shock. These manifestations were nearly always fatal. Certain genotypes of Variola virus were historically associated with less severe clinical presentations, and concomitant decreased fatality rates. These strains are often referred to as variola minor – in contrast to variola major, reserved to describe strains associated with virulent smallpox. However, the historical descriptions that led to these characterizations were derived from globally dispersed epidemics, making it difficult to sort out precisely which epidemics of ‘variola minor’ were due to circulation of presumably less virulent strains, and which relatively mild epidemics might have been due to other mitigating factors such as the nutritional or immunologic status of the affected population. A virologically distinctive ‘variola minor,’ known as Alastrim minor, was characterized in Brazil. This genotype was associated with case-fatality rates of less than 1%.
Regardless of strain, Variola virus is capable of efficient human-to-human transmission. Secondary attack rates of 36 to 88% and 2 to 47% for unvaccinated and smallpox vaccinated persons, respectively, led in some parts of the world to sustained endemic presence of disease, as was experienced in Asia Minor, whereas in other parts of the world, smallpox appeared and disappeared, often sweeping through communities as unbridled and catastrophic outbreaks, as happened when fully virulent Variola virus from Europe was introduced to the New World in the fifteenth century.
Eradication
The history of smallpox and its impact on human society are not discussed here in detail. It is important, however, to emphasize the influence that the endeavor of smallpox eradication has had on public health practice, particularly in the areas of surveillance and cluster survey methodologies.
The first successful proposal for eradication was put before the World Health Assembly in 1958 by a delegate from the (former) Soviet Union. The proposal received near universal endorsement, but the extensive funds needed to support the personnel needs and logistical demands of mass vaccination, particularly in the underdeveloped countries where smallpox was endemic, were not dedicated in the regular budget of the World Health Assembly until 1966. The intensified campaign to eradicate smallpox began in January of the following year (Figure 3).

Though the inception of the intensified campaign had been delayed until difficult issues surrounding program costs, donor support, and vaccine sourcing could be resolved, the interval between program approval and launch of the intensified campaign was not wasted. Several major steps toward eradication were achieved during this time. Techniques for mass production of freeze-dried vaccine were standardized, resulting in increased availability of high-quality vaccine; the jet injector was adopted for use in mass vaccination campaigns, in the hopes of increasing the efficiency of large-scale vaccine administration (the jet injector was, however, ultimately rejected in favor of the bifurcated needle); and, in several countries, including India, ambitious, but fundamentally under-resourced, population-wide vaccination campaigns were instituted.
In India, where smallpox was considered endemic, an initial program target of 80% vaccination coverage across the population was recommended in order to interrupt smallpox transmission. But in 1964 this target was revised upward to 100% by the World Health Assembly’s expert committee on smallpox, based on observations of continuing transmission in regions of India that had purportedly achieved the target goal of 80%. Whether the failure to halt transmission in seemingly highly vaccinated populations was truly a function of insufficient coverage, or whether there might be a more proximal explanation (for example, having to do with varying degrees of efficiency of locally implemented vaccination programs), was the subject of active debate. Indeed, close analysis of vaccination records in India revealed a collection of failed vaccinations, multiple vaccinations administered to individual persons, and some apparent misrepresentations, indicating that 80% coverage had not, in fact, been achieved in many regions. This observation underscored what many believed to be the almost insurmountable difficulties faced by health authorities seeking to accomplish comprehensive vaccination programs without adequate supervision and resources. It also supported doubts held by many decision makers that mass vaccination alone was going to be a feasible strategy to effect smallpox eradication – thus opening the door to consideration of alternative strategies.
One such strategy, often dubbed ‘containment and control,’ became a critical component of eradication success. As part of the Intensified Program (begun in 1967), the Director General of the World Health Assembly advocated systematic enhancements to surveillance, case investigations, and active disease containment approaches (principally vaccination), as a means to curtail ongoing foci of transmission. This approach was originally spearheaded by Alexander Langmuir of the United States Centers for Disease Control, along with colleagues, and had been successfully employed to combat other infectious disease threats in the United States, and in North Africa. In many respects this concept was historically grounded in nineteenth-century smallpox control efforts in Leicester, England, where mass vaccination programs for smallpox had met with stiff resistance from community leaders. Against such a backdrop, active case identification and quarantine of persons exposed to smallpox became the mainstays of disease control.
Several further innovations to public health practice were developed and instituted during the intensified campaign for smallpox eradication. Some were developed principally because of need and later became standard features of epidemiologic science and public health practice. As an example, the need to efficiently monitor virus transmission potential (i.e., vaccination coverage) within communities of different sizes and compositions led to the development of rapid, standardized survey methodologies. Henderson and colleagues adapted a streamlined method for attribute sampling, outlined in 1965 by Serfling and Sherman, to facilitate rapid community-based smallpox vaccine coverage surveys in West Africa. This adapted method then served as a precursor to many of the systematic cluster sampling methodologies that are now common practice, such as the familiar EPI (Expanded Program of Immunization) survey.
Smallpox Post-Eradication
In May 1980, 31 months after the last naturally occurring case of smallpox was reported in Somalia in 1977, eradication was formally declared by the World Health Assembly. Although the disease no longer exists, stocks of Variola virus are still held in two locations sanctioned by the World Health Organization (WHO) and international member states – the Centers for Disease Control and Prevention in the United States, and the Russian State Research Center for Virology and Biotechnology in Siberia. The WHO actively monitors research activities involving Variola virus at both secured locations; no other institutions are known to be in possession of the virus. Some concern has been registered regarding the potential existence of additional unsecured stocks of the virus – although such stocks have never been documented. It is, however, conceivable that Variola virus could be used as an agent for bioterrorism given the absence of contemporary smallpox prevention programs and declining rates of population-level vaccine-derived immunity to the virus.
Because an intentional release of Variola virus could lead to widespread disease and massive civil disturbance, early detection of potential cases of smallpox is recognized as vital. Early detection can be facilitated through enhanced surveillance and reliable application of clinical and laboratory diagnostic algorithms (Figure 4). Few physicians and health-care providers practicing today have had significant, practical experience in recognizing and diagnosing smallpox. Clinical algorithms for smallpox diagnosis are generally based on the distinctive features of ordinary-type disease, which, as described earlier, include a febrile prodrome, a slowly evolving vesicular pustular rash, and multiple of what are considered to be major (e.g., prodromal symptoms including fever, well circumscribed deep-seated lesions, lesions in the same state of development on any one part of the body) and minor (e.g., lesions on the palms and soles, ‘toxic’ appearance, lesions initially on oral mucosa) clinical symptoms. The obvious drawback to this approach is that, while it may achieve a high degree of specificity for normal manifestations of Variola virus infection, it may be insensitive with respect to detection of the more rare manifestations of infection (flat-type or hemorrhagic). This trade-off presupposes both that clinical and epidemiologic patterns of smallpox ensuing from a deliberate release will mirror what was observed during past naturally occurring epidemics, and that early identification of the presumed normal and predominant manifestation of illness will be sufficient to trigger a rapid and appropriate response.
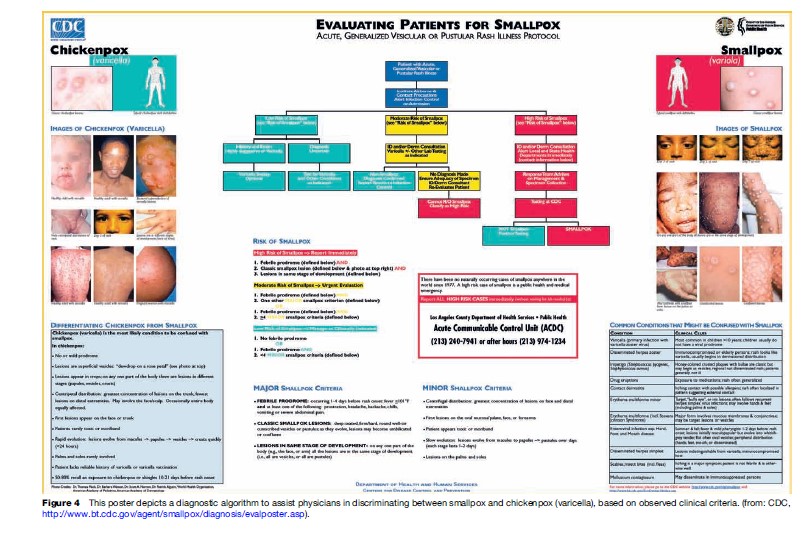
The inclusion of clinical and epidemiologic criteria in using decision algorithms to specify situations in which laboratory testing for Variola virus should proceed is also vital, in order to avoid adverse outcomes stemming from unnecessary testing. For example, a patient with vesicular-pustular rash confined to the trunk, who did not experience a febrile prodrome, and who has had recent contact with a child with chickenpox, would not be a good candidate to undergo laboratory testing to rule out smallpox. Testing clinical specimens from patients with a low index of suspicion for smallpox would have the effect of decreasing the overall positive predictive value (PPV) of Variola virus diagnostic assays, thus increasing the risk of a false-positive result and consequent unnecessary use of public health resources. The range of laboratory tests currently available for Variola virus diagnostics broadly encompasses nucleic acid and protein-based assays, immunologic tests, and particle imaging. Testing can be requested through the WHO; multiple nations have developed testing capacity through their respective national public health laboratories. It is possible in many laboratories to definitively identify Variola virus DNA in a clinical specimen within several hours of specimen arrival in the laboratory.
Mobilization of significant public health resources would be anticipated following detection of a genuine smallpox-associated event. Active surveillance for cases, contact identification and monitoring, patient isolation, enhanced infection control, and quarantine procedures would likely all have to be enacted in a relatively short period of time. In addition, any event triggering concern for the spread of smallpox would necessitate institution of policies and procedures for vaccination, and release of vaccine from national stockpiles. Regardless of whether the decision will be made to pursue mass vaccination or contain-and-control strategies (this will depend on the nature of the triggering event), monitoring of vaccination success (‘take’) and adverse event rates will be required. Screening of potential vaccinees for contraindications to vaccination, such as atopic dermatitis and immune compromising conditions, can significantly reduce the rate of adverse events following vaccination. Administration of vaccinia immune globulin (VIG) has been demonstrated to be effective in treating many serious complications arising from smallpox vaccination, including eczema vaccinatum, progressive vaccinia, severe generalized vaccinia, and possibly ocular vaccinia. But difficult choices may have to be made when individuals with known high-risk exposure to smallpox are found to have a contraindication to vaccination (see Vaccinia virus section below), or they refuse to be vaccinated. In these instances the potential risks associated with vaccination, and the shortcomings of compulsory quarantine, will have to be carefully weighed against the probability that the individual’s exposure will have resulted in a new infection. ‘Next generation’ vaccines with enhanced safety profiles are currently in clinical trials in the United States.
Epidemiologic modeling based on imagined smallpox release scenarios has, in recent years, served to assist the public health community in attempting to weigh the results and consequences of varied response strategies involving vaccination and quarantine. Simulation models of smallpox epidemics have, in general, reinforced the idea that a rapid, well-coordinated public health response will be required to halt an outbreak. But particular recommendations emerging from modeling efforts have tended to vary in line with the modeling approach (e.g., stochastic, socio-spatial, deterministic), and the assumptions and uncertainties inherent in each. No single advocated approach to epidemic control has emerged from epidemiologic modeling studies, but a wealth of hypothetical strategic decisions and outcomes have been catalogued, which may ultimately be useful if a smallpox event unfolds.
Monkeypox
In 1958 in Copenhagen, Denmark, two successive outbreaks of a smallpox-like illness in captive primates led to the identification of a novel orthopoxvirus, dubbed Monkeypox virus. The name monkeypox is, however, most likely a misnomer. Although the natural reservoir of Monkeypox virus has not been definitively identified, decades of observation and research point to the idea that the animal reservoir of this virus is probably a rodent and primates are merely incidental hosts. But, without the benefit of this acquired knowledge, Monkeypox virus was thought to be a naturally occurring virus of nonhuman primates in the years directly following its initial description. The discovery of asymptomatic viral infections in cynomologous monkeys (suggestive of virus tolerance) and the repeated occurrence of monkeypox outbreaks at primate facilities in the Netherlands and the United States reinforced this view.
The era during which monkeypox was discovered marked a period of increased use of primates for laboratory investigation. Many of the animals involved in the monkeypox outbreaks had been brought to Europe and the United States for purposes of polio vaccine development, having been captured in the wild in heavily forested areas of West Africa and subsequently processed through export facilities in South-East Asia. Because of this indirect route of transfer which involved intermediate holding facilities, a correct determination of the origin of the virus(es) as African was not made for several years. And it was not until 1964, when a large outbreak occurred at the Rotterdam Zoo, that it became apparent that the Monkeypox virus was capable of causing disease in animals other than primates. (The ability of Monkeypox virus to cause disease in a broad array of mammalian taxa has been subsequently confirmed; see the paragraphs on the outbreak in the United States later in this section.) No humans were reported to have become infected due to contact with ill or asymptomatic primates during this time.
Human infections with Monkeypox virus were not observed until 1970, during the intensification of the smallpox eradication campaigns. Heightened surveillance for smallpox-like illnesses in areas declared free of smallpox led to the first identification of human monkeypox disease in northern Democratic Republic of the Congo (DRC, former Zaire); additional cases were subsequently identified in Liberia and Sierra Leone. The initial case in DRC occurred in a 9-month-old child who had a clinical illness consistent with smallpox. There had not been a significant outbreak of smallpox in the region for 2 years and the virus isolated from this patient had biologic characteristics consistent with Monkeypox virus – the virus isolated decades previously from captive primates – rather than Variola virus. Other cases continued to be observed, mainly, though not exclusively, in children living in West and Central Africa who had not been vaccinated for smallpox. Studies conducted at the time indicated that smallpox vaccination could provide up to 85% protection against Monkeypox virus infection.
The clinical description of human monkeypox, derived from examination of patients from DRC, suggested an illness highly similar to ordinary-type or modified smallpox, albeit with a potentially lower case-fatality proportion (though overall case-fatality proportions reported for the two diseases at this time period in DRC were largely comparable at 9.6% and 9.0% for smallpox and monkeypox, respectively). Without the benefit of laboratory testing, monkeypox and ordinary smallpox are considered clinically indistinguishable. Both are known to be associated with a febrile prodrome, a centrifugal rash distribution (including palms and soles), and characteristic deep-seated pustular lesions. When investigators performed a thorough accounting of symptoms present during illness, however, a feature unique to monkeypox became apparent – the presence of pronounced lymphadenopathy, which has been observed to occur early during infection in 70 to 80% of non-smallpox-vaccinated persons infected with Monkeypox virus.
Investigators observed another, less pronounced, difference between smallpox and monkeypox, which was the apparent lack of efficiency with which Monkeypox virus passed from one person to another, relative to Variola virus. Secondary attack rates for monkeypox among nonsmallpox-vaccinated household contacts were observed in DRC to be on the order of 10%, whereas, for smallpox, secondary attack rates of 25 to 40% were common within the same population. Epidemiologic modeling studies performed in the 1980s suggested that, given the low inter-human transmission potential, Monkeypox virus was unlikely to be a self-sustaining pathogen in human communities, especially against the backdrop of a highly smallpox-vaccinated population. Instead, a pattern of sporadic disease incidence and abbreviated outbreaks (rather than sustained epidemics) was predicted, with each instance touched off by virus introduced from a primary zoonotic (sylvan) source.
And, indeed, this seems largely to have been the case during the two decades following the discovery of monkeypox as a human illness; sporadic cases and outbreaks were reported infrequently, and all occurred within the confines of areas predicted to be endemic for the virus (Figure 5). However, since the mid-1990s, sizable outbreaks of monkeypox have been recorded in DRC, (more than 500 cases reported in total) and in the past 6 years, human infections have been newly reported in three countries that had never before reported cases: the Republic of the Congo, situated within the predicted endemic range of the virus; and Sudan and the United States, both outside the predicted endemic area.
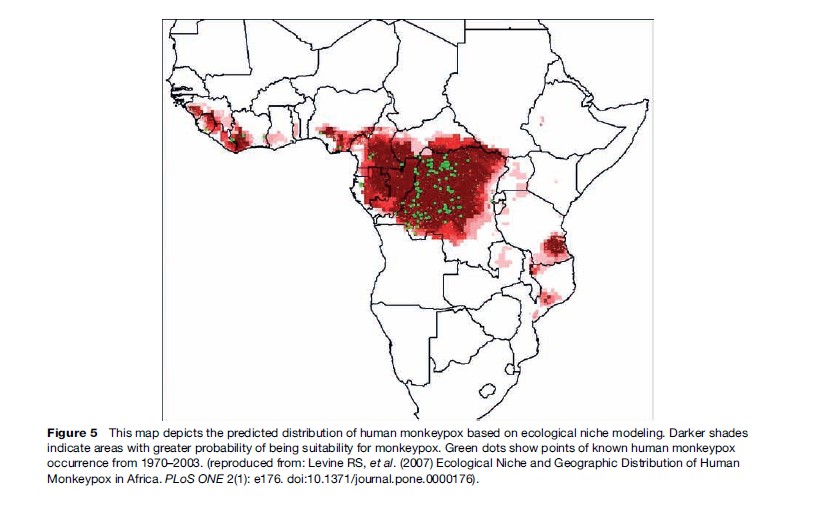
Evidence of Monkeypox virus circulation in sylvan animals has also emerged from Ghana, a country that has had no reports of human disease. This evidence originated as a consequence of the outbreak of human monkeypox in the United States. Monkeypox virus entered the United States in 2003 in a consignment of exotic animals (mainly rodents) intended for sale as pets. The virus was transmitted from African rodents in the consignment to a number of susceptible non-African species with which they were co-housed; chief among these were prairie dogs. Forty-seven confirmed and probable human Monkeypox virus infections were documented during the outbreak, each stemming from direct or indirect contact with Monkeypox-virus-infected prairie dogs. The public health investigation ensuing from the U.S. outbreak led to identification of Ghana as the country of origin of a consignment of monkeypox-infected rodents imported to the United States.
Attributing an epidemiologic basis for the observation of monkeypox in Sudan is more difficult, as the cases may have arisen either from virus importation from neighboring DRC or as a consequence of ecologic range expansion of the virus into neighboring territory. However, if we assume that the natural endemic range of the virus is constrained by the ecologic requirements of the animal host, range expansion for this virus is not apt to increase without a concomitant expansion of the ecology suitable for its sylvan host. Alternatively, the geographic boundaries of monkeypox could increase through increasing efficiency of inter-human spread – for example, longer chains of uninterrupted person-to-person transmission, combined with travel. Given the discontinuation of smallpox vaccination, the likelihood of this latter outcome is inevitably increased, due principally to increasing densities of susceptible human hosts. Nosocomial spread of monkeypox in hospitals has also been observed in Africa, raising the specter of iatrogenic disease amplification, highlighting the need for enhanced infection control practices reminiscent of those used during the smallpox era.
Substained efforts will be needed to fully comprehend the impact of smallpox eradication and the discontinuation of vaccination programs on the emergence of Monkeypox virus including the inception of active surveillance programs, ready access to laboratory diagnostics in endemic areas, and increased understanding of the principal risk factors for primary zoonotic and personto-person transmission of the virus. The U.S. outbreak revealed that several taxa of African rodent are capable of propagating Monkeypox virus and spreading it to other animals (e.g., giant Gambian rats, African dormice, rope squirrels). These, as well as primates and possibly other species, could be sources for primary introduction of Monkeypox virus to humans. Preventing such introductions into human communities and controlling any opportunities for person-to-person spread (including nosocomial) will be important, immediate, public health goals to prevent the emergence of this virus as a significant pathogen of humans.
Cowpox
Cowpox is another zoonotic disease resulting from infection with a rodent-associated orthopoxvirus. However, the virological characteristics of Cowpox virus(es), their geographic range, and the nature of the illness they cause in humans are very distinct from those of Monkeypox virus.
Recognition of human and animal cowpox predates Edward Jenner’s smallpox inoculation experiments, and though his writings name ‘‘cowpox virus’’ as the agent used to prevent smallpox, it has been hypothesized that a now extinct (or exceedingly rare) orthopoxvirus of horses resembling Vaccinia virus may have been the virus used in his experiments.
The application of modern genetic analyses – particularly genome-level analyses – to the study of Cowpox virus phylogeny has revealed that, rather than consisting of a single unified lineage, cowpox viruses actually represent a broad array of genetically distinct entities. These viruses have been found throughout the United Kingdom (excluding Ireland), Europe, and western Eurasia, and have been traditionally grouped together as a single species because of similar biological properties (e.g., particle morphology by electron microscopy, pock growth characteristics on chorioallantoic [egg] membrane, formation of cytoplasmic inclusions in host cells), epidemiologic features, and geographic range.
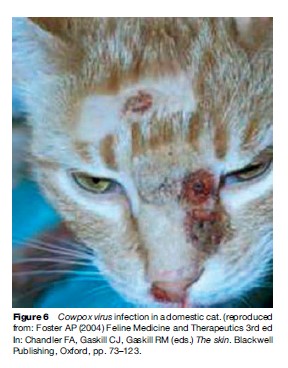
Though not yet definitively identified for every cowpox virus variant, the natural reservoir hosts for cowpox in Britain and Eurasia have been identified as bank voles and wood mice, and great gerbils, respectively. The ecology of Cowpox virus in Britain has been extensively studied by Bennett and colleagues, who have demonstrated that while wood mice may perpetuate local virus transmission cycles, this species cannot do so indefinitely without the presence of bank voles – which are more competent virus hosts. They further theorize that the absence of bank voles from Ireland provides an explanation for why cowpox virus is not found on the island. Rodent association accounts for two notable facets of cowpox disease epidemiology in Britain. The first is the marked seasonality of human cases in Britain, which occur mainly during the months from July to October when sylvan rodents are expected to be most active and in greatest number, and second is the frequent infection of domestic cats. Cats are efficient predators of small rodents and they have proven to be susceptible to severe (though rarely fatal) cowpoxassociated illness, characterized for dermal eruptions and sometimes pneumonia (Figure 6). Domestic cats have also been implicated as transmission hosts to humans, typically their owners but also veterinary workers (infrequently). Other companion animals (e.g., dogs, pet rats) have occasionally been suspected of transmitting the virus to humans, and numerous exotic animal species in zoos (including elephants and cheetahs) have been affected during sporadic outbreaks at zoologic parks, but zookeepers have not yet been reported to have developed illness as a consequence. Cowpox virus infection in bovids is now quite rare.
Human infections with Cowpox virus remain localized to the skin in otherwise healthy people. Infections generally manifest as large (0.5–2 cm in diameter), painful, ulcerative skin lesions, on the hands or sometimes the face. The virus enters through an existing or concurrent break in the skin and the lesion evolves slowly over the course of several weeks, terminating in a characteristic eschar. Dermal scarring is common, and corneal deficits may persist for months following ocular infections. Typically, persons who are infected with cowpox virus experience systemic symptoms at the time the lesion becomes apparent; these include fever, malaise, headache, and sometimes vomiting.
Because human cowpox infections are lesionproducing and usually affect persons living in rural settings where cats, cattle, and sheep are all common, other zoonotic infections are often considered in the diagnosis, for example, anthrax and parapoxvirus infections. Herpes virus infection may also be considered if patients are evaluated early during illness when the lesion characteristics associated with the two agents are similar. Definitive determination of human Cowpox virus infections therefore relies on laboratory diagnostic testing. Virus culture, electron microscopy, PCR, and immunohistochemical analysis of clinically derived specimens are all useful diagnostic techniques for assessing suspected Cowpox virus infections.
The risk factor profile for severe outcomes from Cowpox virus infection is similar to that for Vaccinia virus (i.e., contraindications for smallpox vaccination). Atopic dermatitis (eczema), immune compromise, and extended use of topical or systemic steroids are risk factors for serious outcomes including disseminated Cowpox virus infection, which in rare cases may result in death. Person-to-person transmission of Cowpox virus has not been documented, even in the context of disseminated infection.
It remains unclear whether there is any protective effect of smallpox vaccination against Cowpox virus infection, but this effect, if it exists, would likely be nominal at best. The public health focus surrounding Cowpox virus infections remains to increase awareness among rural health-care providers and veterinarians, as well as to promote appropriate diagnostic testing and case reporting. The development of effective drug therapies would support these endeavors by encouraging healthcare-seeking behaviors in persons who think they might be infected.
Vaccinia-Like Viruses And Buffalopox
As described earlier, Vaccinia virus forms the basis of current and past smallpox vaccines. Smallpox vaccine (live Vaccinia virus) is administered by scarification (scratching) into the superficial epidermis using specialized bifurcated needles. Lyophilized virus is stable, dried, and can be reconstituted for use on demand – for example, in an emergency situation, or for routine vaccination of first-responders or laboratory workers who manipulate live orthopoxviruses during the course of their work. Typically, inoculation results in the development of a single lesion at the site of vaccine introduction, which evolves over the course of several weeks. Vaccinees are considered competent to transmit the virus until their vaccination scab has separated.
Although several new candidate smallpox vaccines are under development, the various vaccine formulations currently stockpiled or in (limited) use across the globe are based on non-attenuated Vaccinia virus and are associated with similar risk profiles for adverse events. Persons with active or dormant atopic dermatitis are actively discouraged from receiving smallpox vaccine due to risk of a severe life-threatening complication, eczema vaccinatum. Ocular infections from inadvertent auto-inoculation of the virus (vaccinia keratitis), generalized vaccinia, myopericarditis, progressive vaccinia, post-vaccinal encephalitis, fetal vaccinia, and secondary transmission to social contacts are all potential adverse events associated with smallpox vaccine. During the era of eradication, the rate of severe adverse events in the United States was estimated at 142–523/100 000 vaccinees, far higher than that associated with any contemporary vaccine currently in widespread use. For this reason, modern-day vaccination programs have faced significant challenges in situations where public perception of the risk of smallpox-related bioterrorism is small. However, complications of vaccinations can be avoided by careful screening of potential vaccinees for contraindications to vaccination and by educating vaccinees about how to prevent transmission to social contacts and family members.
Though it has been propagated and maintained by humans for over two centuries, the biologic origins of Vaccinia virus are obscure; its natural host is unknown, and at various times has been speculated to be horses, rodents, or bovid species. As with cowpox and monkeypox viruses, Vaccinia virus is capable of infecting a broad range of mammalian taxa, including humans. But vaccinia is unique as an agent of anthropozoonosis (disease commonly transmissible from man to animal). During the era of smallpox, transmission of Vaccinia virus from recently vaccinated dairy workers to cows – and subsequently from cows to other milkers – occurred seemingly wherever there was dairy production. These outbreaks were at times substantial, affecting hundreds of animals and scores of dairy workers, with considerable negative impact on production. Vaccinia virus infection in cows typically manifested itself as ulcerating mastitis with conspicuous weeping lesions on the teat. In the absence of appropriate hygienic precautions, virus was easily spread via the process of milking (in non-mechanized dairies), spreading throughout the herd and workforce in repeated cycles of human-to-animal-to-human, or sometimes direct animal-to-animal, transmission.
The cessation of routine smallpox vaccinations and the advent of mechanized dairy production led to precipitous declines in anthropozoonotic outbreaks of vaccinia. However, two probable foci of Vaccinia virus transmission persist in southern Brazil and the Indian subcontinent. The epidemiologic features of the outbreaks differ between the two areas, but outbreaks in both regions have been attributed to orthopoxviruses genetically and are virologically undistinguishable from Vaccinia virus.
In Brazil, genetically diverse vaccinia viruses have been isolated from humans, cows, and rodents in affected areas. Outbreaks occur seasonally ( June–September) on small, traditional (non-mechanized) dairy farms, and rodents are hypothesized to serve as interim reservoirs, although this has not been definitively demonstrated. Considerable human and animal morbidity is associated with seasonal illness and medical and veterinary public health efforts are currently underway to educate at-risk populations about how to prevent transmission (through enhanced hygienic practices) or mitigate the effects of virus introduction on small family farms.
The vaccinia-associated disease that occurs on the Indian subcontinent is know as buffalopox and was reported most recently in Pakistan in 2005. Reports of buffalopox emerged as early as 1934 from India, with subsequent descriptions of disease from Italy, Indonesia, Pakistan, Egypt, and the former Soviet Union. These outbreaks involved water buffalo or dairy cows and were described either as zoonotic (virus transmission occurred from animals to humans) or veterinary (restricted to animals). Little is known about the current epidemiologic pattern or geographic distribution of buffalopox, but the recent human infections reported from Pakistan occurred among hospitalized patients, suggesting a novel transmission pattern.
Additional research is needed to determine whether these foci in Brazil and India represent ongoing circulation of vaccine strain viruses (in the absence of additional spillover from human society) or natural circulation of vaccine progenitor strains. Given their broad host range, potential for long-term persistence in varied ecologies, and medical and veterinary significance, Vaccinia viruses remain a public health concern, and steps should be taken to better understand their continued circulation in nature as well as to develop means to mitigate negative impacts of infection.
Concluding Remarks
Eradication of smallpox was a crowning achievement of public health during the latter half of the twentieth century. This success rested on a foundation of scientific ingenuity, dedicated effort, and purposeful international cooperation. Because there was no animal reservoir or other persistent environmental source for Variola virus, extirpation of smallpox could be achieved by interruption of virus transmission among humans. Other orthopoxviruses that cause human illness – Monkeypox virus, Cowpox virus, and Vaccinia virus – are transmissible from animals to humans. It is conceivable that one or more of these viruses may be able to exploit the ecologic (immunologic) niche left vacant by eradication of Variola virus and cessation of smallpox vaccination. The capacity for these agents to emerge as increasingly significant pathogens of humans remains an ongoing public health concern.
Bibliography:
- Baxby D, Bennett M, and Getty B (1994) Human cowpox 1969–93: A review based on 54 cases. British Journal of Dermatology 131: 598–607.
- Bennett M and Baxby D (1996) Cowpox. Journal of Medical Microbiology 45: 157–158.
- Breman JG (2000) Monkeypox: An emerging infection for humans? In: Scheld WM, Craig WA and Hughes JM (eds.) Emerging Infections vol. 4, pp. 45–67. Washington, DC: ASM Press.
- Breman JG and Henderson DA (2002) Diagnosis and management of smallpox. New England Journal of Medicine 346: 1300–1308.
- da Fonseca FG, Trindade GS, Silva RL, Bonjardim CA, Ferreira PC, and Kroon EG (2002) Characterization of a vaccinia-like virus isolated in a Brazilian forest. Journal of General Virology 83: 223–228.
- Dumbell K and Richardson M (1993) Virological investigations of specimens from buffaloes affected by buffalopox in Maharashtra State India between 1985 and 1987. Archives of Virology 128: 257–267.
- Fauquet CM, Mayo MA, Maniloff J, Desselberger U, and Ball LA (2005) Virus Taxonomy: VIIIth Report of the International Committee on Taxonomy of Viruses. New York: Academic Press.
- Fenner F (1948) The pathogenesis of the acute exanthems. The Lancet 2: 915–920.
- Fenner F, Henderson DA, Arita I, Jezek Z, and Ladnyi ID (1988) Smallpox and Its Eradication. Geneva, Switzerland: World Health Organization.
- Hutin YJ, Williams RJ, Malfait P, et al. (2001) Outbreak of human monkeypox, Democratic Republic of Congo 1996 to 1997. Emerging Infectious Diseases 7: 434–438.
- Hutson CL, Lee KN, Abel J, et al. (2007) Monkeypox zoonotic associations: Insights from laboratory evaluation of animals associated with the multi-state U.S. outbreak. American Journal of Tropical Medicine and Hygiene 76: 757–768.
- Jezek Z, Szczeniowski M, Paluku KM, and Mutombo M (1987) Human monkeypox: Clinical features of 282 patients. Journal of Infectious Diseases 156: 293–298.
- McFadden G (2005) Poxvirus tropism. National Review of Microbiology 3: 201–213.
- Reed KD, Melski JW, and Graham MB (2004) The detection of monkeypox in humans in the Western Hemisphere. New England Journal of Medicine 350: 342–350.
- Vaccinia (smallpox) vaccine (1991) Recommendations of the Immunization Practices Advisory Committee (ACIP). MMWR Morbidity and Mortality Weekly Report RR-40, 1–10.
See also:
Free research papers are not written to satisfy your specific instructions. You can use our professional writing services to buy a custom research paper on any topic and get your high quality paper at affordable price.








