This sample Toxoplasmosis Research Paper is published for educational and informational purposes only. If you need help writing your assignment, please use our research paper writing service and buy a paper on any topic at affordable price. Also check our tips on how to write a research paper, see the lists of health research paper topics, and browse research paper examples.
Introduction
Toxoplasma gondii is a zoonotic protozoan infection with a reservoir in mammals and birds. Transmission to humans is through ingestion of tissue cysts in poorly cooked meat of infested animals or ingestion of oocysts that are shed in the feces of the definitive host (felines) and contaminate the environment. Infection in healthy persons is usually subclinical or causes mild symptoms such as self-limited enlargement of lymph nodes and fever.
Infection in pregnant women with T. gondii may be transmitted to the fetus where it may cause permanent damage of the fetus including retinochorioditis and hydrocephalus. Congenital infection of the fetus in women infected just before conception is extremely rare and even during the first few weeks of pregnancy the maternal–fetal transmission rate is only a few percent (Dunn et al., 1999). Infection before pregnancy causes immunity against transmission to the fetus. The infection can reactivate after birth with attacks of retinochorioditis, and if lesions affect the macula, reduced eyesight. However, acquired toxoplasmosis after birth probably contributes more to the burden of toxoplasmosis than congenital infections (Gilbert et al., 1999; Gilbert and Stanford, 2000).
In immunocompromised patients, previous T. gondii infection may reactivate, or the parasites may be transferred to the host with an organ transplant, causing severe disease. Strategies for control and prevention of congenital toxoplasmosis include primary prevention – systematic prenatal screening for maternal seroconversion during pregnancy – and newborn screening to detect toxoplasma-specific immunoglobulin M (IgM) antibodies at birth. Management of T.gondii infection in transplant patients depends on regular screening for parasitemia by assays that employ the polymerase chain reaction (PCR).
The EUROTOXO project – a European Union-funded study, performed a systematic review of available data on prevention and treatment of congenital toxoplasmosis (EUROTOXO, 2006). This research paper focuses on the results from the EUROTOXO collaboration on congenital toxoplasmosis.
T. Gondii Infections In Humans: Historical Perspective
The first human case ascribed to infection with T.gondii was a child with hydrocephalus reported by Janku in 1923 ( Janku, 1923). Sabin reported the first case of encephalitis due to T. gondii (Sabin, 1941), and encephalitis due to T. gondii in immunocompromised patients was first reported from patients with Hodgkin’s disease during immunosuppressive treatment (Flament-Durand et al., 1967). During the 1940s there was improved understanding of the cause of maternal infection for congenital toxoplasmosis in newborns, and in 1953, Feldman reported a series of 103 children, 99% of whom had eye lesions, 63% had intracranial calcifications and 56% had psychomotor retardation (Feldman, 1953). This initiated interest in congenital infection among scientists in Europe (Couvreur, 1955).
In Gothenburg, Sweden, 50% of mothers had had previous infection with T. gondii and 2 out of 23 260 children had clinical toxoplasmosis during a 1948–51 study period (Holmdahl and Holmdahl, 1955). A study from Austria reported frequent symptoms in children with congenital toxoplasmosis (Eichenwald, 1957). A French study concluded that treatment prevented transmission from mother to child and reduced the clinical symptoms in children (Couvreur and Desmonts, 1962). A later study from France found that the seroprevalence in pregnant women in Paris was 85%, and there was a high risk of toxoplasma infection in seronegatives (Desmonts et al., 1965; Desmonts and Couvreur, 1974).
Following these studies, systematic prenatal screening programs were introduced in France and Austria in 1975 (Aspo¨ck and Pollak, 1992; Thulliez, 1992). The use of toxoplasma-specific IgM antibodies for neonatal diagnosis was proposed in 1968 (Remington et al., 1968), and systematic neonatal screening was piloted in New York (Kimball et al., 1971). The first neonatal screening program based on detection of IgM antibodies at birth was initiated in New England (U.S.) in 1988 (Guerina et al., 1994).
Prevalence Of T. Gondii Infection
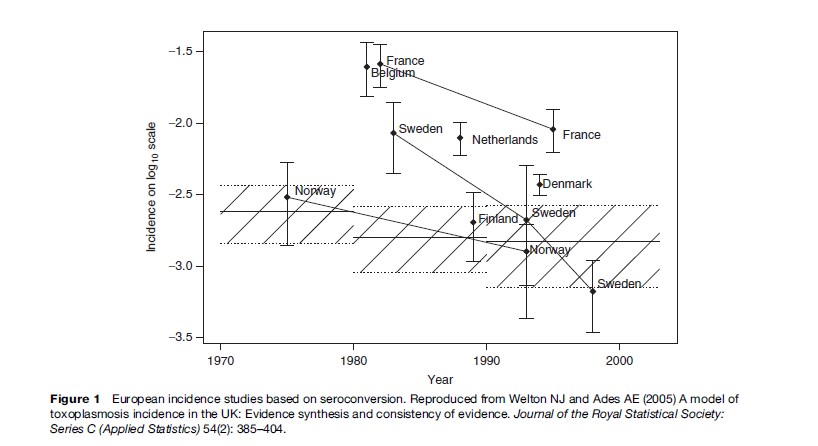
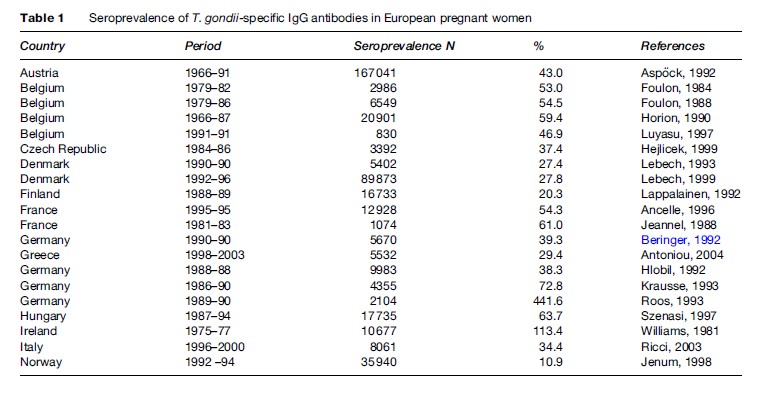
The prevalence of toxoplasma infection in Europe has recently been reviewed by Be´nard and Salmi (2006a), and data from different countries are shown in Table 1. The prevalence of infection has been decreasing in Europe over the past 3 to 4 decades (Horion et al., 1990; Forsgren et al., 1991; Krausse et al., 1993; Logar et al., 1995; Breugelmans et al., 2004; Welton and Aedes, 2005) (Figure 1). In the United States, data are collected regularly through the National Health and Nutrition Examination Study (NHANES) study. NHANES III 1999–2000 found a T. gondii seroprevalence of 15.8% in the age group 12–49 years. T. gondii seroprevalence was higher among non-Hispanic black persons than among non-Hispanic white persons (age-adjusted prevalence 19.2% vs. 12.1%). No statistically significant differences were found between T. gondii antibody prevalence in NHANES 1999–2004 and NHANES III (1988–94) ( Jones et al., 2003; McQuillan et al., 2004).
In South America, a study from Brazil found that seroprevalence was high in people living in poor socioeconomic conditions probably due to waterborne transmission (BahiaOliveira et al., 2003). Another study found a seroprevalence of 73% in slaughterhouse workers and suggested that fresh meat is a significant source of infection in Brazil (Dias et al., 2005). A study of children from Guatemala found that infection with T. gondii often took place before the age of 5 years at which age 43% were seropositive ( Jones et al., 2005). These data show that in countries where waterborne infections are prevalent, infection occurs at an early age.
A study from Korea found an immunoglobulin G (IgG) prevalence in pregnant women of 0.8% (Song et al., 2005), and a recent study of HIV-positive patients from Taiwan found a seroprevalence of 10.2% (Hung et al., 2005). A recent study from India found a seroprevalence of toxoplasma-specific IgG antibodies of 45% (Singh and Pandit, 2004), and a study of HIV-infected patients from Japan found an overall seroprevalence of 44.8%. The majority of these patients were in the age of 25 to 34 years (Nissapatorn et al., 2004). A study of 327 adult cat owners in Thailand found a seroprevalence of 6.4% (Sukthana et al., 2003), and a study from Malaysia found a high seroprevalence in Malays of 55.7% and people belonging to the Indian ethnic group of 55.3%, but low in ethnic Chinese, at 19.4% (Nissapatorn et al., 2003).
A study from Sao Tome´, West Africa, found a prevalence of 21.5% in children below 5 years of age (Fan et al., 2005) and a study from Sudan found a seroprevalence in pregnant women from Khartoum of 34.1% (Elnahas et al., 2003). Of 1828 HIV-positive patients from Bobo-Dioulasso, Burkina Faso, 25.4% had positive T. gondii serology (Millogo et al., 2000).
T. Gondii Genotypes And Clinical Disease
- gondii can be divided into three main genotypes (Sibley and Boothroyd, 1992; Grigg et al., 2001; Khan et al., 2005). It has been proposed that the different genotypes may be partly responsible for the different pathogenicity observed in the infection. In mice one T. gondii parasite of genotype I is lethal to mice, whereas the lethal dose of genotype II and III is about a thousand parasites (Boothroyd and Grigg, 2002). One study has reported an unusual abundance of type I and recombinant strains in patients with retinochorioditis (Grigg et al., 2001), and a recent study from Brazil of eyes with T. gondii lesions from necropsies found only type I and III strains and no type II strains (Vallochi et al., 2005). Recent work, however, suggests a more complicated picture in Brazil with both pathogenic and nonpathogenic isolates belonging to genotype I (Ferreira et al., 2006). A study of 86 pregnant women from France found predominantly genotype II (Ajzenberg et al., 2002), which confirms previous studies that also found primarily genotype II (Howe et al., 1997).
Recently, methods have been developed that allow T. gondii in patients to be at least partially typed using genotype specific markers (Kong et al., 2003), and this will be a valuable tool for assessing the geographical distribution of genotypes as well as the importance of genotype for pathogenicity. The phylogenic development over time of the different genotypes suggests that the ‘atypical’ or ‘exotic’ genotypes may be the ancestral types and genotype I, II, and III are more recent developments from the ancestral parasite (Su et al., 2003). A series of 16 cases with symptomatic T. gondii infection were reported from French Guyana, infected with T. gondii genotypes that did not belong to genotypes I, II, or III (Carme et al., 2002). The presently available data suggest the genotype II dominates in Europe, genotypes I and III dominate in South America, and all three genotypes can be found in the United States and Canada (Ajzenberg et al., 2004; Peyron et al., 2006).
Risk Factors For Infection With T. Gondii
Epidemiological surveys that examine the risk factors in infected and noninfected persons remain the most valid way of assessing the relative importance of different sources of T. gondii infection in humans (Leroy and Hadjichristodoulou, 2006). No biological test can distinguish infection from oocysts transmitted by felines from infection with tissue cysts in infected meats (Dubey, 2000; Hill and Dubey, 2002). Soil contact through gardening allows contact with infective oocysts deposited by cats. Oocysts take 1 to 5 days to become infective, but they can remain infective in soil and probably water for up to 1 year depending on ambient temperature and humidity (Frenkel et al., 1975).
A prospective, case-control study from Norway in 1992–94 found that eating raw or undercooked meat and meat products, poor kitchen hygiene, cleaning the cat litter box, and eating unwashed, raw vegetables or fruits were associated with a higher risk of T. gondii infection (Kapperud et al., 1996).
From 1991 to 1994 a prospective risk factor study in pregnant women infected during pregnancy and controls was performed in Italy. Eating cured pork or raw meat at least once a month was associated with a threefold higher risk of T. gondii infection (odds ratio [OR]: 3.1; 95% confidence interval [CI]: 1.6–6.0) (Buffolano et al., 1996). A case-control study from France found the following risk factors: poor hand hygiene (OR: 9.9; 95% CI: 0.8–125), consumption of undercooked beef (OR: 5.5; 95% CI: 1.1–27), having a pet cat (OR: 4.5; 95% CI: 1.0–19.9), frequent consumption of raw vegetables outside the home (OR: 3.1; 95% CI: 1.2–7.7) and consumption of undercooked lamb (OR: 3.1; 95% CI: 0.85–14) (Baril et al., 1999).
A European, multicenter, case-control study in Belgium, Denmark, Italy, Norway, and Switzerland included 252 cases and 708 controls (Cook et al., 2000). The study showed that contact with raw or undercooked beef, lamb, or other sources of meat, as well as with soil, were independent risk factors for T. gondii seroconversion during pregnancy. In addition, travel outside of Europe, the United States, and Canada was a risk factor for seroconversion. The population attributable fraction showed that 30 to 63% of seroconversions were due to consumption of undercooked or cured meat products and 6 to 17% were a result of soil contact, but ownership of a cat was not a risk factor (Cook et al., 2000). Information about how to avoid toxoplasmosis in pregnancy could be a cost-effective approach to preventing congenital toxoplasmosis (Conyn-van Spaedonck and van Knapen, 1992; Lopez and Dietz, 2000). Based on the knowledge of these identified risk factors for primary toxoplasmosis, pregnant women should be appropriately advised by their obstetricians and primary-care providers on how to lower the risk of congenital toxoplasmosis by avoiding risk factor exposure.
Transmission through surface water has been found to be important in Brazil, and this is probably an important source of transmission in poor socioeconomic societies in the tropics and subtropics (Bahia-Oliveiera et al., 2003).
Recommendations to prevent congenital toxoplasma infection in pregnant women are shown in Table 2 based on the EUROTOXO review of risk factors (Leroy and Hadjichristodoulou, 2006).

T. Gondii Infection In The Pregnant Woman And Newborn Child
Incidence Of Toxoplasma Infection In Pregnant Women
The burden of congenital toxoplasmosis in Europe has recently been reviewed (Be´nard and Salmi, 2006a). The lowest incidence of maternal infection was observed in the northern European countries (from 0.13% in Norway to 0.5% in Sweden), and the highest incidence was reported from France of 1.5% and 1.6% (Ancelle and Goulet, 1996; Jeannel et al., 1988), 1.4 and 2.6% in Belgium (Foulon et al., 1984), and 3.5% in Italy (Ricci et al., 2003). Low levels of toxoplasma-specific IgM antibodies may be found for several years after acute infection, and the mere demonstration of low levels of toxoplasma-specific IgM antibodies is therefore not regarded as a sign by itself of acute infection with T. gondii (Liesenfeld et al., 1997, 2001a,b; Robert et al., 2001; Gras et al., 2004; Leroy et al., 2006).
Maternal–Fetal Transmission Of T. Gondii
The risk of maternal–fetal transmission by trimester of pregnancy is dependent on gestational age and increases throughout pregnancy (Desmonts et al., 1965; Dunn et al., 1999; Gilbert et al., 2001, 2003; Thie´baut et al., 2006c).
Prevalence Of Congenital Toxoplasmosis At Birth
The prevalence of toxoplasmosis at birth varied from 0.7 per 10 000 births in Sweden (Fahnenhjelm et al., 2000; Evengard et al., 2001) and 2 per 10 000 births in Denmark (Lebech et al., 1999) to 7 per 10 000 births in the Poznan region, Poland (Paul et al., 2001).
The risk of pediatric complications varied according to complication types. The three complications that were the most frequently reported were retinochorioditis followed by intracranial calcifications and hydrocephalus. The prevalence of intracranial calcifications at birth varied from 6.3 to 10.6%, and the prevalence of hydrocephalus from 0 to 1.8%.
In the largest study with long-term follow-up the observed prevalence of eye lesions was 12.6% during infancy, increasing to 35% at 12 years (Binquet et al., 2003). Bilateral, visual impairment was extremely rare (Binquet et al., 2004).
The European Multicenter Study on Congenital Toxoplasmosis (EMSCOT) cohort has found that approximately 5% of infected children had neurological impairment or die due to congenital toxoplasmosis (Thie´baut et al., 2006c).
European Screening Programs For Congenital Toxoplasmosis
The national public health programs and recommendations to prevent toxoplasmosis that have been developed in Europe involve three kinds of control measures:
- Prenatal screening to detect as early as possible maternal toxoplasma infections (or suspicion of such infections) that might indicate a risk for congenital infection leading to a prenatal treatment.
- Newborn screening to detect infections as early as possible to enable early initiation of infected infants.
- Primary prevention programs to educate pregnant women on how to avoid infection (no official or national programs).
The different approaches to surveying congenital toxoplasmosis in Europe have been recently reviewed (Be´nard and Salmi, 2006b).
Prenatal Screening
Austria introduced a mandatory serological screening of pregnant women for toxoplasmosis in 1975 (Aspo¨ ck and Pollak, 1992; Aspo¨ ck, 2000, 2003). Every pregnant woman is tested for antibodies at the first antenatal clinical attendance. The test is repeated at no greater than 2-month intervals in the second and third trimester of pregnancy in seronegative women. In case of a low titer at the first visit, another test is carried out 3 weeks later and usually confirms an old infection (Aspo¨ ck, 2000). If a primary T. gondii infection of the pregnant woman is suspected because of seroconversion or significant rise of IgG titer (or primary high titer with IgM), prenatal treatment is carried out as soon as possible with spiramycin before the 16th week of gestation and pyrimethamin plus sulfadiazine after the 15th week of gestation. This program is subsidized and thus free of charge. Use of financial incentives guarantees an almost 100% testing of pregnant women, as a part of the ‘Mutter-Kind-Pass’ (mother-child-passport, or MKP); between 1975 and 1997, every woman who had all examinations received a sum of 1090€. However, in 1997, only women with a low income received an incentive (145€), and although the examinations remained free of charge, about 10% of women went untested. In 2002, a new regulation was introduced, ‘Kinderbetreuunsgeld,’ in which a daily sum of 15€ is provided from birth to the child’s third birthday, provided that all tests of the MKP have been performed. This measure resulted in an almost 100% participation in the screening program.
In France testing has been mandatory since 1978 and, since 1985, to screen toxoplasma infection during pregnacy. Premarital examinations are conducted to distinguish previously infected women from women who have not been previously infected. When a previously nonimmune woman or a woman with an unknown serological status becomes pregnant, testing is conducted at her first prenatal examination during the first trimester and at six additional examinations conducted monthly during her second and third trimesters, then testing is performed on cord blood at delivery. In addition, since 1983 a leaflet describing hygienic measures is given along with the laboratory results. The French program is free of charge.
There are no national guidelines on the management of seroconversions (Binquet et al., 2004), and actual practice varies from center to center.
In utero diagnosis is performed through amniocentesis using detection of T. gondii-specific nucleic acid (PCR) on amniotic fluid and ultrasound examinations of the fetus. If fetal infection is confirmed, pregnancy termination is offered. If the pregnancy is continued, treatment is changed to pyrimethamine and sulfadiazine or sulfadoxine with folinic acid. Newborns are tested to ensure early diagnosis and treatment of asymptomatic congenital toxoplasmosis with the goal of preventing later reactivation and late complications, especially ocular.
In practice, there is great variability between the specialized centers in France with regard to the indications for therapeutic abortion and amniocentesis, treatment protocols with pyrimethamine and sulfonamides, as well as in the frequency of sonographical monitoring (Binquet et al., 2004). Toxoplasma infection is not notifiable in France.
In Slovenia, there are national recommendations to educate pregnant women about preventing congenital toxoplasmosis, using leaflets and information during the first prenatal visit. Pregnant women are tested at the beginning of pregnancy and, in case of seronegativity, retested in the second and third trimesters of pregnancy (at 20–24 and 32–36 weeks, respectively) (Logar et al., 2002). Only a single review addresses the anxiety that systematic screening induces in pregnant women (Khoshnood et al., 2006).
Neonatal Screening
Neonatal screening for congenital toxoplasmosis is performed in New England in the United States, Denmark, and parts of South America (e.g., parts of Brazil and Colombia), by analyzing the blood samples obtained on filter paper with tests for specific IgM antibodies (Guthrie cards) day 5 postpartum (Guerina et al., 1994; Lebech et al., 1999; Sørensen et al., 2002; Neto et al., 2004). However, 15 to 55% of congenitally infected children do not have detectable toxoplasma-specific IgM antibodies at birth or early infancy (Decoster et al., 1992; Lebech et al., 1999; Leroy et al., 2006).
No Screening Policies
Currently, 21 European countries do not recommend screening for congenital toxoplasmosis. Eighteen countries officially recommend a primary prevention program alone, without any screening. These primary prevention programs are carried out at the first antenatal visit, instructions are given on how to avoid eating raw or undercooked meat, to avoid cross-contamination of other foods with raw or undercooked meat, and to use cat litter and practice soil-related hygienic measures. The rationale given by these countries for not recommending screening is diverse: unfavorable cost–benefit return, absence of satisfactory treatment (to be discussed later), program not feasible or too expensive, or incidence of toxoplasmosis infection too low (Janitschke 2003; Joynson 2003; Lappalainen 2003; Stray-Pedersen 2003; Be´nard and Salmi, 2006b).
Acquired Toxoplasma Infection In The Immunocompetent Individual
Symptomatic ocular infection with T. gondii is seen in immunocompetent persons who acquired infection after birth (Wilder, 1952; Holland, 2003). It was unclear for many years whether the burden of T. gondii ocular eye disease in adults was due to reactivation of congenital infection or to infection acquired after birth (Hogan, 1961). Initially, congenital infections were considered to be responsible for the majority of ocular disease in adults (Hogan et al., 1964), but more recent studies have shown that acquired T. gondii infections in adults are responsible for the majority of ocular T. gondii disease in adults (Gilbert et al., 1999; Gilbert and Stanford, 2000). A lifetime risk of 18 cases per 100 000 persons has been found in the United Kingdom (Gilbert et al., 1995). A study from Finland found an annual incidence of 0.4 cases per 100 000 persons with a cumulative prevalence of T. gondii ocular disease of 3 per 100 000 (Paivonsalo-Hietanen et al., 2000).
In the United States a study in 1972 found that 0.6% of the adult population had retinal scars compatible with previous T. gondii infection (Smith and Ganley, 1972), and the same prevalence was found in another study from Alabama 15 years later (Maetz et al., 1987). It is estimated that approximately 2% of the adult population in the United States has retinal findings compatible with T. gondii infections, but the majority do not experience reduced vision (Holland, 2003).
An outbreak of waterborne T. gondii at Vancouver Island (British Columbia, Canada) was associated with symptomatic retinochoroiditis (Bowie et al., 1997; Burnett et al., 1998). It is not precisely known how many people were infected, but 20 patients with retinochorioditis and 51 with adenopathy were reported. It was estimated that 0.5% of infected individuals developed retinochorioditis within a year (Burnett et al., 1998).
Retinal changes due to T. gondii appear to be much more common in southern Brazil than other parts of the world. One study found that 21.3% of persons above 13 years of age had retinochorioditis (Glasner et al., 1992). A follow-up study of 131 patients over 6 years found that 11 (8.3%) had developed typical T. gondii ocular lesions and the authors concluded that acquired T. gondii ocular lesions are common in immunocompetent adults in Brazil (Silveira et al., 2001).
One report from Sierra Leone found that 43% of adults with uveitis had T. gondii infections, indicating that eye disease due to T. gondii may also be common in Africa (Ronday et al., 1996).
It has been suggested that infection with T. gondii is associated with bipolar disease (Torrey and Yolken, 2003), but it is possible that people with bipolar disease place themselves at risk of infection through behavioral practices such as improper preparation of food.
Immunocompromised Patients
The majority of T. gondii infections in immunocompromised hosts are reactivations of previous infections (Mele et al., 2002).
HIV-Infected Patients
The high rate of toxoplasma encephalitis in patients with AIDS was reported soon after the start of the HIV epidemic (Luft et al., 1983; Roue et al., 1984; Enzensberger et al., 1985; Suzuki et al., 1988) and toxoplasma encephalitis was an important cause of death in HIV-infected patients before the introduction of highly active antiretroviral therapy (HAART) in 1996. In the pre-HAART era up to 30% of T. gondii, seropositive, HIV-infected patients developed T. gondii encephalitis when the immunosuppression progressed (McCabe and Remington, 1988), depending on the prevalence of T. gondii infection in the community. Trimethoprim-sulfamethoxazole prophylaxis for Pneumocystis jarovecii also reduced the risk of T. gondii encephalitis in HIV-infected patients (Schurmann et al., 2002).
Cardiac And Kidney Transplants
In one study of patients receiving a cardiac transplant, prophylaxis for 6 weeks with pyrimethamine reduced infection from 57% (4 out of 7) to 14% (5 out of 37) (Wreghitt et al., 1992). A review of 257 heart transplants 1985–93 and 33 heart–lung transplants found that 4.5% (n ¼ 13) donors were toxoplasma-positive/ recipient-negative in 4.5% (n ¼ 13) of cases; of these 9 were followed up and only one patient seroconverted. All patients received trimethoprim/sulfamethoxazole prophylaxis for P. jarovecii (Orr et al., 1994). A later study clearly showed the risk of infection in T. gondii-naive recipients receiving a cardiac transplant from a T. gondii -positive donor; 78% of recipients seroconverted (14 out of 16). In contrast, only 10% (6 out of 59) of donor-negative/ recipient-positive cases developed serological evidence of toxoplasma infection (Gallino et al., 1996). Toxoplasma infection has also been described after kidney transplants (Renoult et al., 1997; Aubert et al., 2000; Giordano et al., 2002; Wulf et al., 2005).
Bone Marrow Transplants (BMT)
An early review of 55 patients with allogeneic BMT complicated by T. gondii infection found that only 4% survived (Chandrasekar et al., 1997). The European Group for Blood and Bone Marrow Transplantation reported on 106 allogeneic, stem-cell transplants of which 55% of the donors were toxoplasma IgG-positive. All recipients received prophylaxis with trimethoprim and sulfamethoxazole for 6 months and 15% (16 out of 106; 95% CI: 8–21%) had at least one T. gondii, PCR-positive blood sample, and 6% (6 out of 106; 95% CI: 1–10%) experienced clinical disease due to T. gondii (Martino et al., 2005).
Treatment Of T. Gondii Infections
Drugs Effective Against T. Gondii
The effectiveness of sulfonamides was demonstrated in 1942 by Sabin and Warren and confirmed in later studies (Eyles, 1953). Later, pyrimethamine was found effective against T. gondii (Eyles and Coleman, 1952) and synergy between sulfadiazine and pyrimethamine was demonstrated soon after (Eyles and Coleman, 1953). Spiramycin was shown to be effective against T. gondii in 1958 (Beverly, 1958; Garin and Eyles, 1958). These three drugs have ever since been the main treatment for T. gondii infections in pregnancy, congenital toxoplasmosis, and ocular toxoplasmosis. However, in 1976, it was shown that sulfadoxine combined with pyrimethamine was also highly effective against T. gondii (Garin et al., 1976), and some centers advocate postnatal treatment with sulfadoxine/ pyrimethamine for up to 2 years in infants with congenital toxoplasmosis (Villena et al., 1998). It should be emphasized that there are no comparative studies of treatment versus no treatment of T. gondii infections in pregnant women or newborns. The different drugs available have recently been reviewed (Daveluy et al., 2006a, b; Derouin, 2006).
Treatment Of T. Gondii Eye Disease
Perkins et al. (1956) randomized 164 persons with acute uveitis to treatment for 4 weeks with pyrimethamine or placebo and found a significant improvement of lesions among the recipients of pyrimethamine. A randomized, open-labeled clinical trial comparing the recurrence of retinochorioditis in 61 patients treated by sulfamethoxazole and trimethroprim (cotrimoxazole) every 3 days for up to 20 months (duration of the study) and 63 patients without treatment, found a significantly lower rate of recurrence in the treatment group ( p = 0.054; 6 out of 61 vs. 15 out of 63) (Silveira et al., 2002). In a prospective multicenter study of 149 consecutive patients with active toxoplasmic retinochorioditis who were randomly assigned to a treatment with pyrimethamine and sulfadiazine, clindamycin plus sulfadiazine or cotrimoxazole, found no difference in resolving of the eye lesion or recurrence over 2 years follow-up between the treated groups. The untreated group had only peripheral lesions so the only valid comparison is between treated groups (Rothova et al., 1993). A descriptive study with historical controls of the effect of an additional course of pyrimethamine and sulfadiazine compared to historical controls did not report a reduced rate of recurrence after additional treatment (Wallon et al., 2001). A study comparing pyrimethamine and sulfadiazine with pyrimethamine and azithromycin in adult patients with retinochorioditis found no difference in the clinical outcome (Bosch-Driessen et al., 2002). A recent systematic review found a lack of evidence to support routine antibiotic treatment for acute toxoplasmic retinochoroiditis (Stanford et al., 2003). Placebocontrolled randomized trials of antibiotic treatment in patients presenting with acute or chronic toxoplasmic retinochoroiditis arising in any part of the retina are required (Stanford et al., 2003).
A review of observational studies in the EUROTOXO collaboration on the effectiveness of postnatal treatment did not find evidence of a benefit of treatment of infants with congenital toxoplasmosis. However, without randomized, controlled trials the findings are difficult to interpret (Thie´baut et al., 2006a, b). Observational data from a cohort of severely infected children found improvement over time, which was interpreted as an effect of treatment (McLeod et al., 2006).
Treatment Of T. Gondii In Pregnant Women And Newborns With Congenital Toxoplasmosis
Prenatal treatment consists of spiramycin and sulfonamides combined with pyrimethamine (Charpiat et al., 2006a,b,c). The effectiveness of spiramycin is doubtful (Peyron et al., 2000; Charpiat et al., 2006d). Prenatal treatment has no effect on maternal–fetal transmission of T.gondii or on clinical manifestations in infants infected with congenital toxoplasmosis (Gilbert et al., 2001; Gras et al., 2001; European Multicentre Study on Congenital Toxoplasmosis, 2003; Gras et al., 2005). These findings have been confirmed by a recent individual patient data meta-analaysis of cohort studies from across the world (Thie´baut et al., 2006c).
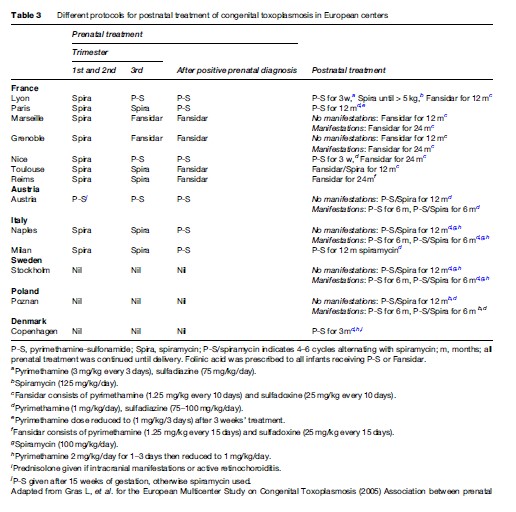
Table 3 shows postnatal treatment regimens used in different European centers. Postnatal treatment of congenital toxoplasmosis has recently been reviewed (Petersen and Schmidt, 2003). There are no randomized, controlled trials of treatment effect of either preor postnatal treatment.
New Drugs
The most promising new drug for the treatment of T. gondii is atovaquone, and studies in mice suggest that it may be partially effective against the tissue cyst (Huskinson-Mark et al., 1991). Azithromycin has also been found to have a partial effect on T. gondii tissue cysts (Derouin et al., 1992; Charpiat et al., 2006e).
Artemisinins have been tested in mice models and one study demonstrated that these drugs reduced brain cyst load (Sarciron, 2000). A study in the hamster model of T. gondii eye infections found no effect of atovaquone on the eye lesions but a 90% reduction in brain cyst numbers (Gormley et al., 1998). Other studies of atovaquone have also reported significant increased survival and reduction in brain cyst burden (Araujo et al., 1998; Alves and Vitor, 2005).
Prevention Of T. Gondii Infection
Infection with T. gondii is in theory preventable by interrupting the route of transmission of tissue cysts through meat and meat products and preventing infective (sporulated) oocysts in the environment from reaching humans. In practice, there are few data that show that health education significantly reduces rates of infection (Gollub et al., 2006).
In some places where prenatal screening has been implemented, a decline of congenital toxoplasmosis has been observed, as reported recently in Belgium, for instance (Breugelmans et al., 2004). The proportion of the decline specifically attributable to the program is unknown because no unscreened group of women exists for comparison, and there has been an overall decline in rates of seropositivity throughout Europe. Although established criteria for screening programs require evidence of effectiveness, no such evidence is available to support prenatal or neonatal screening.
Conclusion
The burden of T. gondii infection has been decreasing in Europe over the past 40 years, coinciding with the increased industrialization in farming.
The evidence for any effect of pre and neonatal screening programs rests on theoretical considerations and for programs started more than 30 years ago on data 40–50 years ago. With the declining risk in Europe and a lack of evidence of a treatment effect on maternal–fetal transmission, clinical disease in newborns, and a lack of evidence for a long-term reduction in eye disease in treated infants, there is a need for proper trials documenting the effectiveness of screening programs.
The small number of patients in each center, and the different approaches to diagnosis (screening or no screening) and treatment makes collaborative studies based on a common protocol mandatory to provide data that make rational decisions possible regarding diagnosis, treatment, and follow-up.
New methods of food production, especially organic meat where the animals are in contact with the environment, will increase the risk of T. gondii infection, which eventually will increase the risk of human infection.
Bibliography:
- Ahlfors K, Borjeson M, Huldt G, and Forsberg E (1989) Incidence of toxoplasmosis in pregnant women in the city of Malmo, Sweden. Scandinavian Journal of Infectious Diseases 21: 315–321.
- Ajzenberg D, Cogne´ N, Paris L, et al. (2000) Genotype of 86 Toxoplasma gondii isolates associated with human congenital toxoplasmosis, and correlation with clinical findings. Journal of Infectious Diseases 186: 684–689.
- Ajzenberg D, Banuls AL, Su C, et al. (2004) Genetic diversity, clonality and sexuality in Toxoplasma gondii. International Journal for Parasitology 34: 1185–1196.
- Alves CF and Vitor RW (2005) Efficacy of atovaquone and sulfadiazine in the treatment of mice infected with Toxoplasma gondii strains isolated in Brazil. Parasite 12: 171–177.
- Ancelle T and Goulet V (1996) La toxoplasmose chez la femme enceinte en France en 1995. Resultats d’une enquete nationale perinatale. Bulletin Epidemiologique Hebdomadaire 51: 227–229.
- Araujo FG, Khan AA, Bryskier A, and Remington JS (1998) Use of ketolides in combination with other drugs to treat experimental toxoplasmosis. Journal of Antimicrobiol Chemotherapy 42: 665–667.
- Aspo¨ ck H (2000) Prevention of congenital toxoplasmosis in Austria: Experience of 25 years. In: Ambroise-Thomas P and Petersen E (eds.) Congenital Toxoplasmosis, pp. 277–299. Paris, France: Springer-Verlag.
- Aspo¨ ck H (2003) Prevention of congenital toxoplasmosis in Austria. Archives de Pediatrie 10(supplement 1): 16–17.
- Aspo¨ ck H and Pollak A (1992) Prevention of prenatal toxoplasmosis by serological screening of pregnant women in Austria. Scandinavian Journal of Infectious Diseases. Supplementum 84: 32–37.
- Aubert G, Maine GT, Villena I, et al. (2000) Recombinant antigens to detect Toxoplasma gondii-specific immunoglobulin G and immunoglobulin M in human sera by enzyme immunoassay. Journal of Clinical Microbiology 38: 1144–1150.
- Bahia-Oliveira LM, Jones JL, Azevedo-Silva J, Alves CC, Orefice F, and Addiss DG (2003) Highly endemic, waterborne toxoplasmosis in north Rio de Janeiro state, Brazil. Emerg Infect Dis 9: 55–62.
- Be´ nard A and Salmi R (2006a) Systematic review of published data on the burden of congenital toxoplasmosis in Europe. http://eurotoxo. isped.u-bordeaux2.fr/ (accessed January 2008).
- Be´ nard A and Salmi R (2006b) Survey on the programs implemented in Europe for the epidemiological surveillance of congenital toxoplasmosis. http://eurotoxo.isped.u-bordeaux2.fr/ (accessed January 2008).
- Beringer T (1992) Is toxoplasmosis diagnosis meaningful during prenatal care? Geburtshlf Frauenheilkund 52: 740–741.
- Beverly JKA (1958) A rational approach to the treatment of toxoplasma uveitis. Trans Ophthalmol Soc UK 78: 109–121.
- Binquet C, Wallon M, Quantin C, et al. (2003) Prognostic factors for the long-term development of ocular lesions in 327 children with congenital toxoplasmosis. Epidemiol Infect 131: 1157–1168.
- Binquet C, Wallon M, Metral P, Gadreau M, Quantin C, and Peyron F (2004) Toxoplasmosis seroconversion in pregnant women. The differing attitudes in France. Presse Med 33: 775–779.
- Boothroyd JC and Grigg ME (2002) Population biology of Toxoplasma gondii and its relevance to human infection: do different strains cause different disease? Curr Opin Microbiol 54: 38–42.
- Bosch-Driessen LH, Verbraak FD, Suttorp-Schulten MS, et al. (2002) A prospective, randomized trial of pyrimethamine and azithromycin vs pyrimethamine and sulfadiazine for the treatment of ocular toxoplasmosis. Am J Ophthalmol 134: 34–40.
- Bowie WR, King AS, Werker DH, et al. (1997) Outbreak of toxoplasmosis associated with municipal drinking water. The BC Toxoplasma Investigation Team. The Lancet 350: 173–177.
- Breugelmans M, Naessens A, and Foulon W (2004) Prevention of toxoplasmosis during pregnancy-an epidemiologic survey over 22 consecutive years. J Perinat Med 32: 211–214.
- Buffolano W, Gilbert RE, Holland FJ, Fratta D, Palumbo F, and Ades AE (1996) Risk factors for recent toxoplasma infection in pregnant women in Naples. Epidemiol Infect 116: 347–351.
- Burnett AJ, Shortt SG, Isaac-Renton J, King A, Werker D, and Bowie WR (1998) Multiple cases of acquired toxoplasmosis retinitis presenting an outbreak. Ophthalmol 105: 1032–1037.
- Carme B, Bissuel F, Ajzenberg D, et al. (2002) Severe acquired toxoplasmosis in immunocompetent adult patients in French Guiana. J Clin Microbiol 40: 4037–4044.
- Chandrasekar PH and Momin FJ Bone Marrow Transplant Team (1997) Disseminated toxoplasmosis in marrow recipients: a report of three cases and a review of the literature. Bone Marrow Transpl 19: 685–689.
- Charpiat B, Thie´ baut R, and Salmi LR (2006a) Systematic review of published pharmacokinetic data related to pyrimethamine. http:// eurotoxo.isped.u-bordeaux2.fr/ (accessed January 2008).
- Charpiat B, Thie´ baut R, and Salmi LR (2006b) Systematic review of published pharmacokinetic data related to sulfadiazine. http:// eurotoxo.isped.u-bordeaux2.fr/ (accessed January 2008).
- Charpiat B, Thie´ baut R, and Salmi LR (2006c) Systematic review of published pharmacokinetic data related to sulfadoxine. http:// eurotoxo.isped.u-bordeaux2.fr/ (accessed January 2008).
- Charpiat B, Thie´ baut R, and Salmi LR (2006d) Systematic review of published pharmacokinetic data related to spiramycine. http://eurotoxo.isped.u-bordeaux2.fr/ (accessed January 2008).
- Conyn-van Spaedonck MA and van Knapen F (1992) Choices in preventive strategies: experience with the prevention of congenital toxoplasmosis in The Netherlands. Scand J Infect Dis 84(Suppl.): 51–58.
- Cook AJ, Gilbert RE, Buffolano W, et al. (2000) Sources of toxoplasma infection in pregnant women: European multicentre case-control study. European Research Network on Congenital Toxoplasmosis. Br Med J 321: 142–147.
- Couvreur J (1955) E´ tude de la toxoplasmose congenitale a` propo de 20 observations. PhD thesis. Sorbonne, Paris.
- Couvreur J (1962) E´ tude de la toxoplasmose congenitale a` propo de 20 observation. These. Paris,1955. Sorbonne University.
- Couvreur J and Desmonts G (1962) Congenital and maternal toxoplasmosis. A review of 300 cases. Develop Med Chld Neurol 4: 519–530.
- Daveluy A, Haramburu F, Fourrier A, and Thie´ baut R (2006) Review of data related to side effects of drugs used in congenital yoxoplasmosis (2). http://eurotoxo.isped.u-bordeaux2.fr/ (accessed January 2008).
- Daveluy A, Haramburu F, Bricout H, et al. (2006a) Review of data related to side effects of drugs used in congenital yoxoplasmosis. http://eurotoxo. isped.u-bordeaux2.fr/ (accessed January 2008).
- Decoster A, Darcy F, Caron A, et al. (1992) Anti-P30 IgA antibodies as prenatal markers of congenital toxoplasma infection. Clin Exp Immunol 87: 310–315.
- Derouin F (2006) Systematic search and analysis of preclinical published data on in vitro and in vivo activities of antitoxoplasma drugs. http://eurotoxo.isped.u-bordeaux2.fr/ (accessed January 2008).
- Derouin F, Caroff B, Chau F, Prokocimer P, and Pocidalo JJ (1992) Synergistic activity of clarithromycin and minocycline in an animal model of acute experimental toxoplasmosis. Antimicrob Agents Chemother 36: 2852–2855.
- Desmonts G and Couvreur J (1974) Congenital toxoplasmosis: a prospective study of 378 pregnancies. N Engl J Med 290: 1110–1116.
- Desmonts G, Couvreur J, and Ben Rachid MS (1965) Le toxoplasme, la me` re et l’enfant. Arch Franc¸ Pe´diatr 22: 1183–1200.
- Dias RA, Navarro IT, Ruffolo BB, Bugni FM, Castro MV, and Freire RL (2005) Toxoplasma gondii in fresh pork sausage and seroprevalence in butchers from factories in Londrina, Parana State, Brazil. Rev Inst Med Trop Sao Paulo 47: 185–189.
- Dubey JP (2000) Sources of Toxoplasma gondii infection in pregnancy. until rates of congenital toxoplasmosis fall, control measures are essential. Br Med J 321: 127–128.
- Dunn D, Wallon M, Peyron F, Petersen E, Peckham CS, and Gilbert RE (1999) Mother to child transmission of toxoplasmosis: risk estimates for clinical counselling. The Lancet 353: 1829–1833.
- Eichenwald HF (1957) Congenital toxoplasmosis: a study of 150 cases. Am J Dis Chld 94: 411–412.
- Elnahas A, Gerais AS, Elbashir MI, Eldien ES, and Adam I (2003) Toxoplasmosis in pregnant Sudanese women. Saudi Med J 24: 868–870.
- Enzensberger W, Helm EB, Hopp G, Stille W, and Fischer PA (1985) Toxoplasma encephalitis in patients with AIDS. Dtsch Med Wochenschr 110: 83–87.
- European Multicentre Study on Congenital Toxoplasmosis (2003) Effect of timing and type of treatment on the risk of mother to child transmission of Toxoplasma gondii. BJOG 110: 112–120.
- Gilbert R and Chene G EUROTOXO (2006) http://eurotoxo.isped. u-bordeaux2.fr/ (accessed January 2008).
- Evengard B, Petersson K, Engman ML, et al. (2001) Low incidence of toxoplasma infection during pregnancy and in newborns in Sweden. Epidemiol Infect 127: 121–127.
- Eyles DE (1953) The present status of the chemotherapy of Toxoplasmosis. Am J Trop Med Hyg 2: 429–444.
- Eyles DE and Coleman M (1952) Tests of 2,4-diamonopyrimidines on toxoplasmosis. Pub Hlth Rep 67: 249–252.
- Eyles DE and Coleman M (1953) Synergistic effect of sulfadiazine and daraprim against Toxoplasmosis in mice. Antibiot Chemother 3: 483–490.
- Fan CK, Hung CC, Su KE, et al. (2005) Seroprevalence of Toxoplasma gondii infection among pre-school children aged 1–5 years in the Democratic Republic of Sao Tome and Principe, Western Africa. Trans R Soc Trop Med Hyg 101: 1157–1158.
- Feldman HA (1953) Congenital toxoplasmosis – a study of 103 cases. Am J Dis Child 86: 487.
- Ferreira Ade M, Vitor RW, Gazzinelli RT, and Melo MN (2006) Genetic analysis of natural recombinant Brazilian Toxoplasma gondii strains by multilocus PCR-RFLP. Infect Genet Evol 6: 22–31.
- Flament-Durand J, Coers C, Waelbroeck C, Geertruyden J, and van Tousaint C (1967) Toxoplasmic encephalitis and myositis during treatment with immunodepressive drugs. Acta Clin Belg 22: 44–54.
- Forsgren M, Gille E, and Ljungstrom I (1991) Toxoplasma antibodies in pregnant women in Sweden in 1969, 1979 and 1987. The Lancet 337: 1413–1414.
- Foulon W, Naessens A, Volckaert M, Lauwers S, and Amy JJ (1984) Congenital toxoplasmosis: a prospective survey in Brussels. Br J Obstet Gynaecol 91: 419–423.
- Frenkel JK, Ruiz A, and Chinchilla M (1975) Soil survival of toxoplasma oocysts in Kansas and Costa Rica. Am J Trop Med Hyg 24: 439–443.
- Gallino A, Maggiorini M, Kiowski W, et al. (1996) Toxoplasmosis in heart transplant recipients. Eur J Clin Microbiol Infect Dis 15: 389–393.
- Garin JP and Eyles DE (1958) Le traitement de la toxoplasmose expe´ rimentale de la souris par la spiramycine. Nouv Presse Me´d 66: 254–260.
- Garin JP, Sung RTM, Mojon M, and Paillard B (1976) Gue´ rison de la toxoplasmose expe´ rimentale de la souris par l’association sulfadoxine-pyrimethamine. Propositions d’application a` l’homme. Lyon Med 236: 19–23.
- Gilbert RE and Stanford MR (2000) Is ocular toxoplasmosis caused by prenatal or postnatal infection? Br J Ophthalmol 84: 224–226.
- Gilbert RE, Stanford MR, Jackson H, et al. (1995) Incidence of acute, symptomatic Toxoplasma retinochoroiditis in south London according to country of birth. Br Med J 310: 1037–1040.
- Gilbert RE, Dunn DT, Lightman S, et al. (1999) Incidence of symptomatic toxoplasma eye disease: aetiology and public health implications. Epidemiol Infect 123: 283–289.
- Gilbert RE, Dunn D, Wallon M, et al. (2001) Ecological comparison of the risks of mother-to-child transmission and clinical manifestations of congenital toxoplasmosis according to prenatal treatment period. Epidemiol Infect 127: 113–120.
- Gilbert RE and Gras L the European Multicentre, Study on Congenital, Toxoplasmosis (2003) Effect of timing and type of treatment on the risk of mother to child transmission of Toxoplasma gondii. BJOG 110: 112–120.
- Giordano LFCM, Lasmar EP, Tavora ERF, and Lasmar MF (2002) Toxoplasmosis transmitted via kidney allograft: case report and review. Transplant Proc 34: 498–499.
- Glasner PD, Silveira C, Kruszon-Moran D, et al. (1992) An unusually high prevalence of ocular toxoplasmosis in southern Brazil. Am J Ophthalmol 114: 136–144.
- Gollub EL, Leroy V, Gilbert R, Cheˆ ne G, and Wallon M (2006) Effectiveness of health education approaches for primary prevention of congenital toxoplasmosis. http://eurotoxo.isped.u-bordeaux2.fr/ (accessed January 2008.
- Gormley PD, Pavesio CE, Minnasian D, and Lightman S (1998) Effects of drug therapy on Toxoplasma cysts in an animal model of acute and chronic disease. Invest Ophthalmol Vis Sci 39: 1171–1175.
- Gras L, Gilbert RE, Ades AE, and Dunn DT (2001) Effect of prenatal treatment on the risk of intracranial and ocular lesions in children with congenital toxoplasmosis. Int J Epidemiol 30: 1309–1313.
- Gras L, Gilbert RE, Wallon M, Peyron F, and cortina-Borja M (2004) Duration of the lgm response in women acquiring Toxoplasma gondii during pregnancy: implications for clinical practice and crosssectional incidence studies. Epidemiology and Infection 132: 541–548.
- Gras L, Wallon M, Pollak A, et al. for The European, Multicenter Study on Congenital, Toxoplasmosis (2005) Association between prenatal treatment and clinical manifestations of congenital toxoplasmosis in infancy: a cohort study in 13 European centers. Acta Pediatrica 94: 1721–1731.
- Grigg ME, Bonnefoy S, Hehl AB, Suzuki Y, and Boothroyd JC (2001) Success and virulence in Toxoplasma as the result of sexual recombination between two distinct ancestries. Science 294: 161–165.
- Guerina NG, Hsu HW, Meissner HC, et al. (1944) Neonatal serologic screening and early treatment for congenital T. gondii infection. The New England Regional Toxoplasma Working Group. N Engl J Med 330: 1858–1863.
- Guerina NG, Hsu HW, Meissner HC, et al. (1994) Neonatal serologic screening and early treatment for congenital Toxoplasma gondii infection. The New England Regional Toxoplasma Working Group. New England Journal of Medicine 330: 1858–1863.
- Hejlicek K, Literak I, et al. (1999) Toxoplasma gondii antibodies in pregnant women in the Ceske Budejovice District. Epidemiol Mikrobiol Imunol 48: 102–105.
- Hill D and Dubey JP (2002) Toxoplasma gondii: transmission, diagnosis and prevention. Clin Microbiol Infect 8: 634–640.
- Hlobil H and Gultig K (1992) Congenital toxoplasma infections in BadenWurttemberg. Klinisches Labor 38: 679–686.
- Hogan MJ (1961) Ocular toxoplasmosis in adult patients. Surv Ophthalmol 6: 935–951.
- Hogan MJ, Kimura SJ, and O’Connor GR (1964) Ocular toxoplasmosis. Arch Ophthalmol 72: 592–600.
- Holland GN (2003) Ocular toxoplasmosis : A global reassessment. Part I: Epidemiology and course of disease. Am J Ophthalmol 136: 973–988.
- Holmdahl SC and Holmdahl K (1955) The frequency of congenital toxoplasmosis and some viewpoints on the diagnosis. Acta Paediatr 44: 322–329.
- Horion M, Thoumsin H, Senterre J, and Lambotte R (1990) 20 years of screening for toxoplasmosis in pregnant women. The Liege experience in 20,000 pregnancies. Rev Med Liege 45: 492–497.
- Howe DK, Honore S, Derouin F, and Sibley LD (1997) Determination of genotypes of Toxoplasma gondii strains isolated from patients with toxoplasmosis. J Clin Microbiol 35: 1411–1414.
- Hung CC, Chen MY, Hsieh SM, Hsiao CF, Sheng WH, and Chang SC (2005) Prevalence of Toxoplasma gondii infection and incidence of toxoplasma encephalitis in non-haemophiliac HIV-1-infected adults in Taiwan. Int J STD AIDS 16: 302–306.
- Huskinson-Mark J, Araujo FG, and Remington JS (1991) Evaluation of the effect of drugs on the cyst form of Toxoplasma gondii. J Infect Dis. 164: 170–171.
- Janitschke K (2003) Official recommendations and strategy for prevention of congenital toxoplasmosis in Germany. Arch Pediatr 10 (Suppl 1): 15.
- Janku J (1923) Pathogenesa a patologicka anatomie tak nazvaneho vrozenehonalezem parasitu v sitnici. Cas Lek Ces 62: 1021–1027.
- Jeannel D, Niel G, Costagliola D, Danis M, Traore BM, and Gentilini M (1988) Epidemiology of toxoplasmosis among pregnant women in the Paris ares. Intl J Epidemiol 17: 595–602.
- Jeannel D, Niel G, Costagliola D, Danis M, Traore BM, and Gentilini M (1998) Prevalence of Toxoplasma gondii specific immunoglobulin G antibodies among pregnant women in Norway. Epidemiol Infect 120: 87–92.
- Jenum PA, Stray-Pedersen B, Melby KK, et al. (1998) Incidence of Toxoplasma gondii infection in 35,940 pregnant women in Norway and pregnancy outcome for infected women. J Clin Microbiol 36: 2900–2906.
- Jones JL, Kruszon-Moran D, and Wilson M (2003) Toxoplasma gondii infection in the United States, 1999–2000. Emerg Infect Dis 9: 1371–1374.
- Jones JL, Lopez B, AlvarezMury M, et al. (2005) Toxoplasma gondii infection in rural Guatemalan children. Am J Trop Med Hyg 72: 295–300.
- Joynson DH (2003) Congenital toxoplasma infection in the UK. Arch Pediatr 10(Suppl 1): 27–28.
- Kapperud G, Jenum PA, Stray-Pedersen B, Melby KK, Eskil A, and Eng J (1996) Risk factors for Toxoplasma gondii infection in pregnancy. Results of a prospective case-control study in Norway. Am J Epidemiol 144: 405–412.
- Khan A, Taylor S, Su C, et al. (2005) Composite genome map and recombination parameters derived from three archetypal lineages of Toxoplasma gondii.. Nucleic Acids Res 33: 2980–2992.
- Khoshnood B, Vigan D, de Goffinet F, and Leroy V (2006) Prenatal screening and diagnosis of congenital toxoplasmosis: A review of safety issues and psychological consequences for women who undergo screening. http://eurotoxo.isped.u-bordeaux2.fr/ (accessed January 2008).
- Kimball AC, Kean BH, and Fuchs F (1971) Congenital toxoplasmosis: a prospective study of 4,048 obstetric patients. Am J Obstet Gynaecol 111: 211–218.
- Kong JT, Grigg ME, Uyetake L, Parmley S, and Boothroyd JC (2003) Serotyping of Toxoplasma gondii infections in humans using synthetic peptides. J Infect Dis 187: 1484–1495.
- Krausse T, Straube W, Wiersbitzky S, Hitz V, and Kewitsch A (1993) Screening for toxoplasmosis in pregnancy – a pilot program in Northeast Germany. Geburtshilfe Frauenheilkd 53: 613–618.
- Lappalainen M (2003) Current situation regarding toxoplasmosis in Finland. Arch Pediatr 10(Suppl 1): 19.
- Lappalainen M, Koskela P, Hedman K, et al. (1992) Incidence of primary toxoplasma infections during pregnancy in southern Finland: a prospective cohort study. Scand J Infect Dis 24: 97–104.
- Lebech M, Petersen E, and Larsen SO (1993) Prevalence, incidence and geographical distribution of Toxoplasma gondii antibodies in pregnant women in Denmark. Scand J Infect Dis 25: 751–756.
- Lebech M, Andersen O, Christensen NC, et al. the Danish Congenital, Toxoplasmosis Study, Group(1999) Feasibility of neonatal screening for toxoplasma infection in the absence of prenatal treatment. The Lancet 353: 1834–1837.
- Leroy V and Hadjichristodoulou C (2006) Systematic review of risk factors for Toxoplasma gondii infection in pregnant women. http://eurotoxo.isped.u-bordeaux2.fr/ (accessed January 2008).
- Leroy V, Harambat J, Perez P, Rudin C, Gilbert R, and Petersen E (2006) http://eurotoxo.isped.u-bordeaux2.fr/ (accessed January 2008).
- Liesenfeld O, Press C, Montoya JG, et al. (1997) False-positive results in immunoglobulin M (IgM) toxoplasma antibody tests and importance of confirmatory testing: the Platelia Toxo IgM test. J Clin Microbiol 35: 174–178.
- Liesenfeld O, Montoya JG, Kinney S, Press C, and Remington JS (2001) Effect of testing for IgG avidity in the diagnosis of T. gondii infection in pregnant women: experience in a U.S. reference laboratory. J Infect Dis 183: 1248–1253.
- Liesenfeld O, Montoya JG, Kinney S, Press C, and Remington JS (2001) Confirmatory serological testing for acute toxoplasmosis and rate of induced abortions among women reported to have positive Toxoplasma immunoglobulin M antibody titers. Am J Obstet Gynecol 184: 140–145.
- Logar J, Novak-Antolic Z, and Zore A (1995) Serological screening for toxoplasmosis in pregnancy in Slovenia. Scand J Infect Dis 27: 163–164.
- Logar J, Petrovec M, Novak-Antolic Z, et al. (2002) Prevention of congenital toxoplasmosis in Slovenia by serological screening of pregnant women. Scand J Infect Dis 34: 201–204.
- Lopez A, Dietz VJ, Wilson M, Navin TR, and Jones JL (2000) Preventing congenital toxoplasmosis. MMWR Morbodity and Mortality Weekly Report 49: 59–68.
- Luft BJ, Conley F, and Remington JS (1983) Outbreak of central nervous system toxoplasmosis in Western Europe and North America. The Lancet ii: 781–784.
- Luyasu V, Robert A, Lissenko D, Bertrand M, Bohy E, Wacquez M, and De Bruyere M (1997) A seroepidemiological study on toxoplasmosis. Acta Clin Belg 52: 3–8.
- Maetz HM, Kleinstein RN, Frederico D, and Wayne J (1987) Estimated prevalence of ocular toxoplasmosis and toxocariasis in Alabama. J Infect Dis 156: 414.
- Martino R, Bretagne S, Einsele H, et al. and the Infectious Disease, Working Party of the European, Group for Blood, Marrow Transplantation (2005) Early detection of Toxoplasma infection by molecular monitoring of T. gondii in peripheral blood samples after allogenic stem cell transplantation. Clin Infect Dis 40: 67–78.
- McCabe R and Remington JS (1988) Toxoplasmosis: the time has come. N Engl J Med 318: 313–315.
- McLeod R, Boyer K, Karrison T, et al. Toxoplasmosis Study, Group (2006) Outcome of treatment for congenital toxoplasmosis, 1981–2004: the National Collaborative Chicago-Based, Congenital Toxoplasmosis Study. Clin Infect Dis 42: 1383–1394.
- McQuillan GM, Kruszon-Moran D, Kottiri BJ, Curtin LR, Lucas JW, and Kington RS (2004) Racial and ethnic differences in the seroprevalence of 6 infectious diseases in the United States: data from NHANES III, 1988–1994. Am J Public Health 94: 1952–1958.
- Mele A, Paterson PJ, Prentice HG, Leoni P, and Kibbler CC (2002) Toxoplasmosis in bone marrow transplantation: a report of two cases and systematic review of the literature. Bone Marrow Transplant 29: 691–698.
- Millogo A, Ki-Zerbo GA, Traore W, Sawadogo AB, Ouedraogo I, and Peghini M (2000) Toxoplasma serology in HIV infected patients and suspected cerebral toxoplasmosis at the Central Hospital of BoboDioulasso (Burkina Faso). Bull Soc Pathol Exot 93: 17–19.
- Neto EC, Rubin R, Schulte J, and Giugliani R (2004) Newborn screening for congenital infectious diseases. Emerg Infect Dis 10: 1068–1073.
- Nissapatorn V, Noor-Azmi MA, Cho SM, et al. (2003) Toxoplasmosis: prevalence and risk factors. J Obstet Gynaecol 23: 618–624.
- Nissapatorn V, Lee C, Quek KF, Leong CL, Mahmud R, and Abdullah KA (2004) Toxoplasmosis in HIV/AIDS patients: a current situation. Jpn J Infect Dis 57: 160–165.
- Orr KE, Gould FK, Short G, et al. (1994) Outcome of T. gondii mismatches in heart transplant recipients over a period of 8 years. J Infect 29: 249–253.
- Paivonsalo-Hietanen T, Tuominen J, and Saari KM (2000) Uveitis in children: Population-based study in Finland. Acta Ophthalmol Scand 78: 84–88.
- Paul M, Petersen E, and Szczapa J (2001) Prevalence of congenital Toxoplasma gondii infection among newborns from the Poznan region of Poland: validation of a new combined enzyme immunoassay for Toxoplasma gondii-specific immunoglobulin A and immunoglobulin M antibodies. J Clin Microbiol 39: 1912–1916.
- Perkins ES, Schofield PB, and Smith CH (1956) Treatment of uveitis with pyrimethamine (daraprim). Br J Ophthalmol 40: 577–586.
- Petersen E and Schmidt DR (2003) Sulfadiazine and pyrimethamine in the postnatal treatment of congenital toxoplasmosis: what are the options? Expert Rev Anti Infect Ther 1: 175–182.
- Peyron F, Lobry J, Muret K, et al. (2006) Serotyping of Toxoplasma gondii in pregnant women. Predominance of type II in the old world and type I and III in the new world. Microbial Infections 8: 2333–2340.
- Peyron F, Wallon M, Liou C, and Garner P (2000) Treatments for toxoplasmosis in pregnancy. Cochrane Database Syst Rev 2: CD001684.
- Remington JS, Miller MJ, and Brownlee I (1968) IgM antibodies in acute toxoplasmosis. I. Diagnostic significance in congenital cases and a method for their rapid demonstration. Pediatr 41: 1082–1091.
- Renoult E, Georges E, Biava MF, et al. (1997) Toxoplasmosis in kidney transplant recipients: report of six cases and review. Clin Infect Dis 24: 625–634.
- Ricci M, Pentimalli H, Thaller R, Rava L, and DiCiommo V (2003) Screening and prevention of congenital toxoplasmosis: An effectiveness study in a population with a high infection rate. J Matern Fetal Neonatal Med 14: 398–403.
- Robert A, Luyasu V, Zuffrey J, Hedman K, and Petersen E; European Network on Congenital Toxoplasmosis (2001) Potential of the specific markers in the early diagnosis of Toxoplasma-infection: A multicentre study using combination of isotype IgG, IgM, IgA and IgE with values of avidity assay. Eur J Clin Microbiol Infect Dis 20: 467–474.
- Ronday MJ, Stilma JS, Barbe RF, et al. (1996) Aetiology of uveitis in Sierra Leone, west Africa. Blindness from uveitis in a hospital population in Sierra Leone. Br J Ophthalmol 80: 956–961.
- Roos T, Martius J, Gross U, and Schrod L (1993) Systematic serologic screening for toxoplasmosis in pregnancy. Obstet Gynecol 81: 243–250.
- Rothova A, Meenken C, Buitenhuis HJ, et al. (1993) Therapy for ocular toxoplasmosis. Am J Ophthalmol 115: 517–523.
- Roue R, Debord T, Denamur E, et al. (1984) Diagnosis of Toxoplasma encephalitis in absence of neeurological signs by early computerised tomography scanning in patients with AIDS. The Lancet 2(8417–18): 1472.
- Sabin AB (1941) Toxoplasmic encephalitis in children. J Am Med Assoc 116: 801–807.
- Sarciron ME, Saccharin C, Petavy AF, and Peyron F (2000) Effects of artesunate, dihydroartemisinin, and an artesunate-dihydroartemisinin combination against Toxoplasma gondii. Am J Trop Med Hyg 62: 73–76.
- Schurmann D, Bergmann F, Albrecht H, et al. (2002) Effectiveness of twice-weekly pyrimethamine-sulfadoxine as primary prophylaxis of Pneumocystis carinii pneumonia and toxoplasmic encephalitis in patients with advanced HIV infection. Eur J Clin Microbiol Infect Dis 21: 353–361.
- Sibley LD and Boothroyd JC (1992) Virulent strains of Toxoplasma gondii comprise a single clonal lineage. Nature 359: 82–85.
- Silveira C, Belfort R Jr, Muccioli C, et al. (2001) A follow-up study of Toxoplasma gondii infection in southern Brazil. Am J Ophthalmol 131: 351–354.
- Silveira C, Belfort R Jr, Muccioli C, et al. (2002) The effect of long-term intermittent trimethoprim/sulfamethoxazole treatment on recurrences of toxoplasmic retinochoroiditis. Am J Ophthalmol 134: 41–46.
- Singh S and Pandit AJ (2004) Incidence and prevalence of toxoplasmosis in Indian pregnant women: a prospective study. Am J Reprod Immunol 52: 276–283.
- Smith RE and Ganley JP (1972) Ophthalmic survey of a community. I. Abnormalities of the ocular fundus. Am J Ophthalmol 74: 1126–1130.
- Song KJ, Shin JC, Shin HJ, and Nam HW (2005) Seroprevalence of toxoplasmosis in Korean pregnant women. Korean J Parasitol 43: 69–71.
- Sørensen T, Spenter J, Jaliashvili I, Christiansen M, Nørgarrd-Pedersen B, and Petersen E (2002) An automated time-resolved immunofluometric assay for detection of Toxoplasma gondii specific IgM and IgA antibodies in filterpaper samples from newborns. Clin Chemistry 48: 1981–1986.
- Stanford MR, See SE, Jones LV, and Gilbert RE (2003) Antibiotics for toxoplasmic retinochoroiditis: an evidence-based systematic review. Ophthalmol 110: 926–931.
- Stray-Pedersen B (2003) Prevention of congenital toxoplasmosis in Norway. Arch Pediatr 10(Suppl 1): 23–24.
- Su C, Evans D, Cole RH, Kissinger JC, Ajioka JW, and Sibley LD (2003) Recent expansion of Toxoplasma through enhanced oral transmission. Science 299: 353–354.
- Sukthana Y, Kaewkungwal J, Jantanavivat C, Lekkla A, Chiabchalard R, and Aumarm W (2003) Toxoplasma gondii antibody in Thai cats and their owners. South East Asian J Trop Med Public Health 34: 733–738.
- Suzuki Y, Israelski DM, Dannemann BR, Stepick-Biek P, Thulliez P, and Remington JS (1988) Diagnosis of toxoplasmic encephalitis in patients with acquired immunodeficiency syndrome by using a new serological method. J Clin Microbiol 26: 2541–2543.
- Szenasi Z, Ozsvar Z, Nagy E, et al. (1997) Prevention of congenital toxoplasmosis in Szeged, Hungary. Int J Epidemiol 26: 428–435.
- Thie´ baut R, Bricout H, Costanzo S, and di, Mouillet E (2006a) Systematic review of published studies evaluating postnatal treatment effect. http://eurotoxo.isped.u-bordeaux2.fr/ (accessed January 2008).
- Thie´ baut R, Leroy V, Alioum A, et al. (2006b) Biases in observational studies of the effect of prenatal treatment for congenital toxoplasmosis. Eur J Obstet Gynecol Reprod Biol 124: 3–9.
- Thie´ baut R, et al. on behalf of SYROCOT (Systematic, Review on Congenital, Toxoplasmosis) Investigators (2006c) Individual patient data meta-analysis of prenatal treatment effect for congenital toxoplasmosis. http://www.isped.u-bordeaux2.fr/RECHERCHE/ SYROCOT/SYROCOT.pdf (accessed January 2008).
- Thulliez P (1992) Screening programme for congenital toxoplasmosis in France. Scand J Infect Dis 84(Suppl.): 43–45.
- Torrey EF and Yolken RH (2003) Toxoplasma gondii and schizophrenia. Emerg Infect Dis 9: 1375–1380.
- Vallochi AL, Muccioli C, Martins MC, Silveira C, Belfort R Jr, and Rizzo LV (2005) The genotype of Toxoplasma gondii strains causing ocular toxoplasmosis in humans in Brazil. Am J Ophthalmol 139: 350–351.
- Villena I, Aubert D, Leroux B, et al. (1998) Pyrimethamine-sulfadoxine treatment of congenital toxoplasmosis: follow-up of 78 cases between 1980 and 1997. Reims Toxoplasmosis Group. Scand J Infect Dis 30: 295–300.
- Wallon M, Cozon G, Ecochard R, Lewin P, and Peyron F (2001) Serological rebound in congenital toxoplasmosis: long-term followup of 133 children. Eur J Pediatr 160: 534–540.
- Welton NJ and Ades AE (2005) A model of toxoplasmosis incidence in the UK: Evidence synthesis and consistency of evidence. Journal of the Royal Statistical Society: Series C (Applied Statistics) 54(2): 385–404.
- Wilder HC (1952) Toxoplasma chorioretinitis in adults. Arch Ophthalmol 48: 127–136.
- Williams KA, Scott JM, Macfarlane DE, Williamson JM, Elias-Jones TF, and Williams H (1981) Congenital toxoplasmosis: a prospective survey in the West of Scotland. J Infect 3: 219–229.
- Wreghitt TG, Gray JJ, Pavel P, et al. (1992) Efficacy of pyrimethamine for the prevention of donor-acquired Toxoplasma gondii infection in heart and heart-lung transplant patients. Transpl Int 5: 197–200.
- Wulf MWH, Crevel R, van, Portier R, et al. (2005) Toxoplasmosis after renal transplantation: Implications of a missed diagnosis. J Clin Microbiol 43: 3544–3547.
- Antoniou M, Tzouvali H, Sifakis S, et al. (2004) Incidence of toxoplasmosis in 5532 pregnant women in Crete, Greece: Management of 185 cases at risk. European Journal of Obstetrics, Gynecology, and Reproducitve Biology 117: 138–143.
- Baril L, Ancelle T, Goulet V, Thulliez P, Tirard-Fleury V, and Carme B (1999) Risk factors for Toxoplasma infection in pregnancy: A casecontrol study in France. Scandinavian Journal of Infectious Diseases 31: 305–309.
- Berger R, Merkel S, and Rudin C (1995) Toxoplasmosis and pregnancy – findings from umbilical cord blood screening in 30,000 newborn infants. Schweizerische medizinische Wochenschrift 125: 1168–1173.
- Foulon W, Naessens A, Lauwers S, DeMeuter F, and Amy JJ (1988) Impact of primary prevention on the incidence of toxoplasmosis during pregnancy. Obstetrics and Gynecology 72: 363–366.
- Signorell LM, Seitz D, Merkel S, Berger R, and Rudin C (2006) Cord blood screening for congenital toxoplasmosis in northwestern Switzerland 1982–1999. Pediatric Infectious Disease Journal 25: 123–128.
- Wallon M, Liou C, Garner P, and Peyron F (1999) Congenital toxoplasmosis: Systematic review of evidence of efficacy of treatment in pregnancy. British Medical Journal 318: 1511–1514.
See also:
Free research papers are not written to satisfy your specific instructions. You can use our professional writing services to buy a custom research paper on any topic and get your high quality paper at affordable price.