This sample Sudden Cardiac Arrest Research Paper is published for educational and informational purposes only. If you need help writing your assignment, please use our research paper writing service and buy a paper on any topic at affordable price. Also check our tips on how to write a research paper, see the lists of health research paper topics, and browse research paper examples.
Definition
Sudden cardiac arrest (SCA) is a substantial public health problem that is challenging to investigate, prevent, and treat in part because there are no universally accepted criteria to readily classify the condition. The fundamental elements of the definition are comprised of (1) an unexpected event, (2) characterized by cardiovascular collapse, and (3) due to an underlying cardiac cause. Translation of these conceptual tenets to clinical circumstances can be problematic. For example, one consensus definition defines SCA as ‘natural death due to cardiac causes, heralded by abrupt loss of consciousness within one hour of the onset of acute symptoms; pre-existing heart disease may have been known to be present, but the time and mode of death are unexpected’ (Priori et al., 2002; Myerburg and Castellanos, 1997).
Even this well-reasoned definition has important limitations. Not all SCA is fatal and in fact hundreds of thousands of persons receive attempted resuscitation in North America and Europe each year (Rea et al., 2004a; Atwood et al., 2005). Though survival is modest, resuscitation has a measurable public health impact as tens of thousands of persons survive each year (Rea et al., 2003a, 2004; Atwood et al., 2005). The exact mode of the event may not be readily determined. Most patients do not receive medical monitoring during the event or an autopsy afterward. Symptoms such as dyspnea prior to collapse in an individual with both heart and lung disease may obscure the primary etiology. Moreover, many deaths potentially attributable to SCA are not witnessed so that symptoms and diagnostics are not available to help determine etiology. The lack of a witness can also make application of a discrete, time-based requisite such as 1 h often infeasible. (Whether 1 h has specific clinical or research importance is not certain.) As a consequence, supplemental information may be used to assess the etiology and time course. For example, death certificates that code cause of death as heart disease and location of death as occurring outside the hospital can be used as an alternative approach (Fox et al., 2005). However, death certificates alone tend to overclassify death due to heart disease (Narang et al., 1994; Iribarren et al., 1998; Lloyd-Jones et al., 1998; Chugh et al., 2004). Although the out-of-hospital location provides a surrogate for an unexpected event, some heart disease deaths outside the hospital are expected and/or gradual (Narang et al., 1994). Conversely, SCA also occurs in hospital, though the relative magnitude of in-hospital SCA is unclear (Peberdy et al., 2003).
Taken together, a universal and readily applied definition of SCA is not available. The choice of an SCA definition will be influenced by the aims of particular investigation or clinical program and the availability of resources to derive the SCA population of interest. Given the consequent heterogeneity of SCA definitions, one must appreciate the strengths and limitations of a particular definition when considering studies of SCA incidence and outcome.
Epidemiology
Attributable Mortality
Even with the limitations of the definition, SCA appears to be one of the most common causes of death worldwide. More deaths are attributed to SCA in Western, industrialized societies than other diseases such as stroke, lung cancer, breast cancer, or AIDS. Given the limitations inherent in the definition, estimates of the total mortality attributed to SCA vary from 5% to 15%, or one-quarter to one-half of heart disease death (Zheng et al., 2001; Chugh et al., 2004; Siscovick and Podrid, 2007). Applied to the US population for example, out-of-hospital SCA would account for between 150 000 and 450 000 deaths annually. The population burden of SCA in developing societies is not well investigated, although attributable mortality appears to be less but perhaps increasing. The lower attributable mortality is likely due to a number of factors including the younger populations and the greater competing causes of mortality in these countries.
Incidence
Incidence is the number of SCA events per population per year and similarly reflects the particular definition used to determine SCA. For example, incidence of SCA was 162 per 100 000 residents in the US using death certificate ascertainment (Zheng et al., 2001). In contrast, the incidence of SCA receiving attempted resuscitation was 54 per 100 000 person-years in the United States and 37 per 100 000 person-years in Europe (Rea et al., 2004a; Atwood et al., 2005). A third approach incorporating death certificate, clinical information, and emergency medical services information in an attempt to both accurately and comprehensively identify SCA produced an overall SCA incidence estimate of 190 per 100 000 person-years among persons age 50–79 in a large U.S. health maintenance organization (Rea et al., 2004b). Based on published reports from the World Health Organization, incidence of heart disease death varies greatly; for example, the age-adjusted mortality rate from CHD is sevenfold greater in Russia than in Japan (Rosamond et al., 2007). Such variation in total heart disease mortality likely translates to substantial variation in SCA incidence across countries.
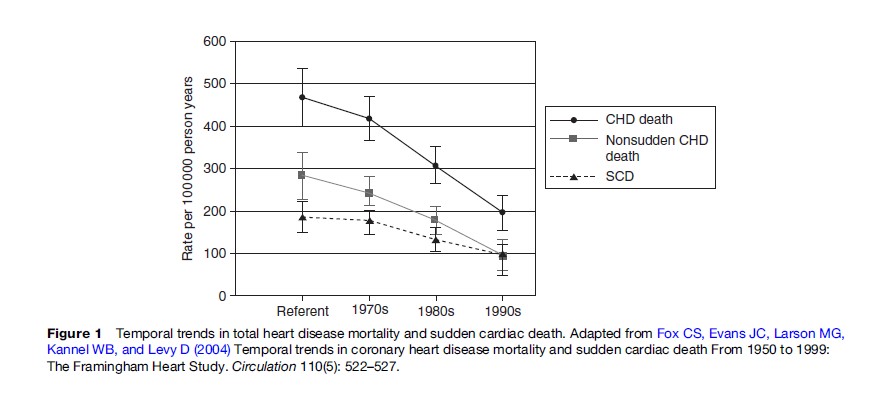
Importantly, the incidence of SCA appears to be declining over time in many developed nations (Goldberg, 1989; de Vreede-Swagemakers et al., 1997; Kuisma et al., 1999; Herlitz et al., 2000; Zheng et al., 2001; Rea et al., 2004b). The absolute rate of decline is not clear, but multiple studies evaluating different populations and using distinct methodologies have consistently indicated a temporal decline in SCA incidence. The pattern to some extent mirrors the overall decline in total mortality due to heart disease (Figure 1) (Fox et al., 2004). The reasons for the decline are not completely understood but likely reflect better health behaviors and clinical treatments aimed at both primary and secondary prevention of coronary heart disease. Conversely, the incidence of total heart disease mortality in the developing portions of the world appears to be increasing; such that the incidence of SCA worldwide may be expected to increase (Leeder et al., 2004; Yach et al., 2004; Zipes, 2005).
Pathophysiology And Risk Factors
The pathophysiology that manifests as SCA is complex and not fully explained. More than 90% of persons who suffer SCA have evidence of structural heart disease, approximately 80% have coronary artery disease with or without cardiomyopathy, another 10–15% have nonischemic cardiomyopathy such as dilated or hypertrophic cardiomyopathy, with the remaining 5–10% having no evidence of structural disease (Huikuri et al., 2001). The prevalent pathophysiology can depend on geography or clinical population; for example Southeast Asia Sudden Unexpected Death Syndrome is a condition where apparently healthy young and middle-aged men unexpectedly die, typically at night, without an identifiable cause. In most cases, however, structural heart disease provides the substrate for an arrhythmic focus that in some instances requires an additional trigger such as acute coronary ischemia, electrolyte abnormality, stimulant exposure, or physical exertion. For example, scarred myocardium from prior infarction can cause reentry circuits that result in autonomous, abnormal conduction in the ventricle. Most commonly in SCA, a ventricular tachyarrhythmia is produced, which typically presents clinically as ventricular fibrillation (Figure 2) (Weiss et al., 2000).
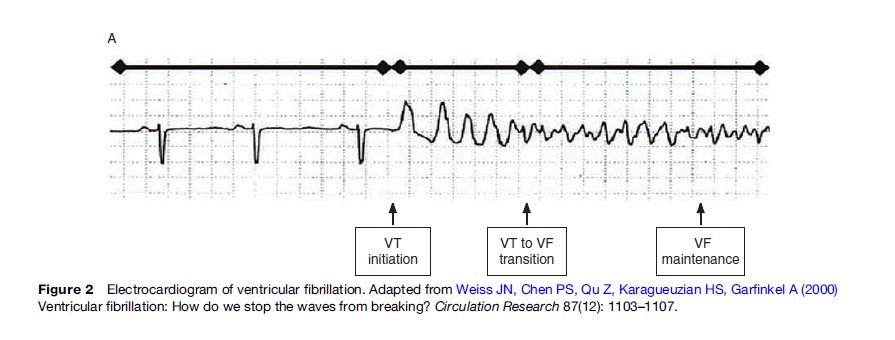
Ventricular fibrillation is characterized electrically by chaotic but measurable electrical activity (Callaway and Menegazzi, 2005). The underlying electrical basis for ventricular fibrillation is not yet completely defined. Processes that may contribute include (1) reentrant mechanisms whereby anatomically specific mother rotors produce scroll waves, (2) nonreentrant mechanisms such as rapid triggered activity or automaticity, or (3) a combination of reentrant and nonreentrant mechanisms (Chen et al., 2005; Thomas et al., 2005; Nash et al., 2006). Both chronic local cardiac tissue heterogeneity (in diseased myocardium) and dynamic factors (cellular membrane voltage and calcium ion cycling between sarcoplasmic reticulum and cytoplasm) may promote fibrillation (Weiss et al., 2005). Over minutes, the electrical organization of the ventricular fibrillation signal deteriorates into asystole (Callaway and Menegazzi, 2005).
Risk Factors For SCA
Our understanding of why some individuals experience SCA but other clinically comparable persons do not is incomplete and constitutes a major public health challenge. Collectively, risk factors consist of both chronic or persistent and acute or transient factors. Since underlying coronary heart disease is common in SCA, most research indicates that traditional chronic risk factors for coronary artery disease such as older age, African American race, hypertension, diabetes, dyslipidemia, and smoking are also risk factors for SCA (Kannel and Schatzkin, 1985; Gillum, 1989; Cupples et al., 1992; Becker et al., 1993; Kannel et al., 1998; Albert et al., 2003; Goldenberg et al., 2003; Rea et al., 2004b). Decreased left ventricular function, regardless of ischemic or nonischemic etiology, is one of the strongest predictors of SCA such that those with ejection fraction less than 30% have a risk that is fiveto tenfold greater than the age-matched population (Myerburg and Castellanos, 2005).
Other risk (or protective) factors appear to be more specific for SCA rather than coronary heart disease. Male sex appears to be a particularly strong risk factor, with men at twoto threefold higher risk of SCA than women. A greater prevalence of coronary artery disease does not explain this sex-specific risk difference. Based on the results of family studies, genetic differences also contribute to SCA risk (Friedlander et al., 1998; Jouven et al., 1999). Several autosomal conditions that increase SCA risk have been identified and mapped to particular chromosomes. Persons with one of these rare, monogenetic traits have a functional abnormality in ion channels that predispose to ventricular tachyarrhythmia (Shah et al., 2005). More common genetic variation, with population prevalence typically greater than 5% and hence with potential to influence public health, may also influence SCA risk; though whether and how such an understanding is to be incorporated into clinical use for risk stratification and prevention is not yet clear (Arking et al., 2004, 2006; Sotoodehnia et al., 2006).
Health behaviors also affect SCA risk. The relationship between exercise and SCA is complex and may reflect the balance of sympathetic and parasympathetic tone. Overall regular exercise substantially lowers SCA risk, though risk is transiently elevated during and immediately following the period of exercise (Siscovick et al., 1984; Lemaitre et al., 1999; Albert et al., 2000). Moderate alcohol consumption is associated with a lower risk of SCA compared to nondrinkers, while heavy consumption has been associated with increased risk (Siscovick et al., 1986; Wannamethee and Shaper, 1992; Albert et al., 1999). A diet that includes fatty fish such as salmon once or twice per week and is rich in n-3 polyunsaturated fatty acids (PUFA) has been associated with a lower risk of SCA. Molecular evidence suggests that n-3 PUFA modulate sodium and calcium ion channels in cardiac myocytes to stabilize action potentials (Leaf et al., 2003). In some studies, acute psychological stress has been linked to an increase in SCA risk (Leor et al., 1996; Hemingway et al., 2001; Whang et al., 2005). Finally, in some circumstances, medications can increase risk. Particular antiarrhythmics, high-dose (compared to low-dose) thiazide diuretics, and antipsychotic medications have been associated with elevated SCA risk (Siscovick et al., 1994; Pratt and Moye, 1995; Hennessy et al., 2002).
Prevention
Population-Attributable Risk
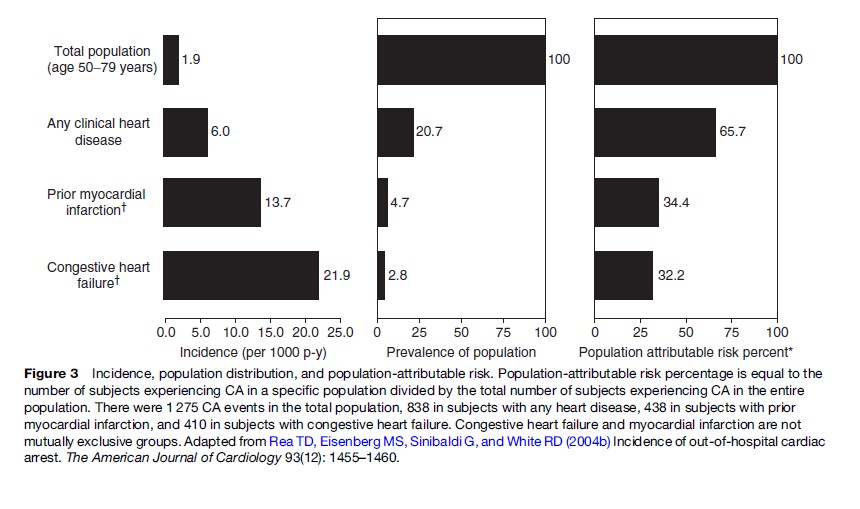
A strategy to efficiently decrease the public health burden of SCA requires an understanding of SCA risk factors, their distribution in the population, approaches to identify individuals at risk, and therapies to reduce risk (Figure 3) (Rea et al., 2004b). Our current understanding is not sensitive and/or specific enough to accurately identify those who will (or will not) suffer SCA. As a consequence, efforts directed at prevention in particular high-risk clinical groups will not capture the majority of persons who suffer SCA. Though such focused strategies can improve public health, the approach will not address the largest portion of the SCA burden. Moreover, the prevention modality for one clinical group may not be appropriate for another, necessitating that prevention strategies be tailored to the individual risk profile.
Prevention
Since most SCA events occur in individuals with underlying structural heart disease, most often coronary artery disease and/or cardiomyopathy, identification and treatment of conditions causing coronary artery disease and/or congestive heart failure provide a cornerstone for SCA prevention. Lifestyle choices that include regular exercise, weight control, a low-saturated-fat and cholesterol diet, abstinence from tobacco, and limited alcohol consumption are recommended regardless of SCA risk profile and hence should be incorporated as part of broad-based, population efforts to reduce SCA. Evidence-based and consensus guidelines direct screening and treatment of clinical conditions such as hypertension, diabetes, and dyslipidemia associated with coronary heart disease (Smith et al., 2006; Mosca et al., 2007). For those with clinically established coronary heart disease, additional pharmacologic treatments including antiplatelet therapy, lipid-lowering treatment, and beta blockers are often indicated to lower the risk of SCA. Coronary revascularization in selected persons can also reduce SCA risk. Finally, persons with ischemic or nonischemic cardiomyopathy, characterized by decreased left ventricular function, experience an especially high risk of SCA. Risk of total mortality including death from SCA can be reduced in this clinical group with pharmacologic therapies including angiotensin converting enzyme inhibitors, beta blockers, angiotensin receptor blockers, and aldosterone inhibitors (Hunt et al., 2005). Taken together, a multifaceted approach suited to the individual that addresses health behaviors, medication treatment, and coronary revascularization can lower risk of coronary heart disease and congestive heart failure and in turn lower risk of SCA.
Some prevention measures are associated with specific SCA risk reduction. In several epidemiological studies, diets rich in n-3 PUFA have been associated with a lower risk of SCA but not other fatal heart disease (Siscovick et al., 1995; Albert et al., 1998; Siscovick et al., 2003). The observational relationship was strengthened by the results of a large randomized trial of postmyocardial infarction patients, demonstrating a reduction in SCA for persons treated with n-3 PUFA versus placebo. However, other randomized trials in especially high-risk patients have not demonstrated a benefit of n-3 PUFA supplementation (Mozaffarian, 2007).
The implantable cardioverter defibrillator (ICD) is a device that monitors the heart rhythm continuously and provides a shock typically designed to terminate a sustained ventricular tachyarrhythmia. Clinical indications for ICD placement have expanded as a result of findings from randomized clinical trials, supporting its use in secondary and primary prevention of SCA (Goldberger and Lampert, 2006). Typically in these trials, ICD therapy was compared to treatment with antiarrhythmic medication such as beta-blockers or amiodarone. In the secondary prevention setting (persons successfully resuscitated following ventricular fibrillation SCA), ICD reduces the risk of SCA by 50% and risk of death by 25%. Randomized trials also support a mortality benefit of the ICD in primary prevention among specific clinical groups characterized by ischemic or nonischemic cardiomyopathy with decreased systolic left ventricular function. Although these indications continue to evolve, frequently mentioned indications for ICD among heart failure patients includes New York Heart Association class II and III heart failure with ejection fraction less than 35%, and postmyocardial infarction with ejection fraction less than 30%. In these clinical groups, the ICD reduces mortality by 25–50%, with most of the mortality reduction due to a decrease in SCA. Placement of ICDs is also currently recommended in consensus guidelines for relatively rare conditions such as hypertrophic cardiomyopathy right ventricular dysplasia, long QT syndrome, and Brugada syndrome; such recommendations are supported by some case-series data since these conditions are too uncommon to make large prospective trials feasible (Begley et al., 2003; Corrado et al., 2003; Maron et al., 2003). The lack of definitive evidence can produce controversies regarding precise indications.
Over the past two decades, ICD indications have broadened initially from patients with inducible arrhythmias in electrophysiology studies, to SCA survivors, to postmyocardial infarction patients with heart failure, to patients with ischemic or nonischemic systolic heart failure. The expanding indications have produced an exponential increase in ICD placement (Figure 4). The increasing use of this relatively expensive therapy yields questions of cost and cost-effectiveness. Some but not all studies suggest ICDs meet traditional cost-effectiveness benchmarks; such evaluations are typically sensitive to the clinical group’s risk of SCA and the cost of the ICD (Lynd and O’Brien, 2003). However, cost may make ICD placement impractical in some areas of the globe where other health conditions have public health priority or economic resources are more limited.
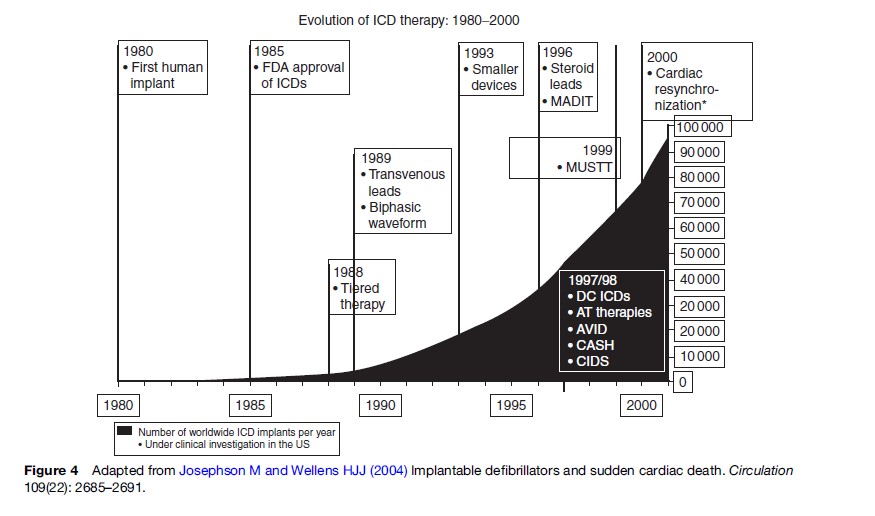
Ongoing investigation and clinical improvements may limit complications and enhance the effectiveness of the ICD. For example, complications associated with ICDs include device infection (2%), lead fracture (3%), dislodgement (1%), and bleeding (1%) and can cause substantial morbidity and rarely even death (Kron et al., 2001). Some ICDs are now equipped with biventricular pacing to synchronize right and left ventricular contraction, which may improve cardiac function, ameliorate symptoms, and potentially improve the cardiac outcomes (Bristow et al., 2004; Goldberger and Lampert, 2006). Thus the ICD has an established and yet evolving role in SCA prevention, though current ICD indications may account for only about one-quarter to one-third of the population suffering SCA (Stecker et al., 2006). Improved approaches for SCA risk stratification could better allocate ICD distribution as well as other SCA prevention therapies.
Challenges Of Risk Stratification
A number of clinical tests have been proposed to help identify individuals at high risk for SCA ( Josephson and Wellens, 2004). Electrocardiogram findings that correlate with increased risk of SCD include increased QRS width, left bundle branch block, left ventricular hypertrophy, T wave alternans, and QT dispersion. Signal averaged electrocardiograms use special techniques to further improve prediction. Interventional electrophysiology studies may also provide additional risk stratification in clinical subsets. Many of these measures, recorded as part of ICD trials, have been used to derive SCA prediction models and require prospective validation (Bailey et al., 2007). These measures may improve specificity within an already high-risk population but may have a much more limited role in the larger, lower-risk general population. Thus ongoing efforts are needed to refine SCA risk prediction in order to better allocate interventional and intensive therapies among high-risk groups and to identify novel risk predictors among the general population to better target limited resources and treatments among those who might otherwise not be considered at-risk.
Resuscitation
Epidemiology
Despite progress with prevention, hundreds of thousands of persons suffer SCA each year in North America and Europe and receive attempted resuscitation (Rea et al., 2004a; Atwood et al., 2005). In one study in the out-of-hospital setting, for example, approximately one-third of persons suffered heart disease death without an organized emergency response aimed at resuscitation, another third received an emergency response but without attempted resuscitation given that death had progressed, making a resuscitation attempt futile, and the final third received an emergency response and attempted resuscitation by emergency medical services (EMS) (Rea et al., 2003b). Taken together, the incidence of out-of-hospital SCA with attempted resuscitation has been estimated between 38 per 100 000 in Europe and 51 per 100 000 in the United States. The incidence of in-hospital SCA with attempted resuscitation is uncertain but these events also appear to have an important public health impact, while the frequency of resuscitation efforts in developing countries has not been defined (Peberdy et al., 2003).
The outcome of resuscitation is poor. In many communities, only 5% of treated SCA victims are successfully resuscitated and survive to return home (Rea et al., 2004a; Atwood et al., 2005). Importantly, however, survival of treated SCA varies substantially across communities. For example, survival to hospital discharge following ventricular fibrillation SCA approaches 40% in some communities with organized EMS and hospital care, far exceeding survival following ventricular fibrillation SCA in most settings (Cobb et al., 1999; White et al., 2005; Rea et al., 2006). These findings indicate that improvement in resuscitation care across communities could have public health implications and potentially translate to thousands of additional lives saved each year. Importantly, long-term prognosis of persons surviving SCA to be discharged from hospital appears to be improving over time, with evidence indicating median survival following hospital discharge of at least 7 years, nearly twice the expected survival compared to survivors from two decades earlier (Rea et al., 2003a). Most persons who survive appear to enjoy satisfactory functional status and quality of life (Rea and Paredes, 2004).
Links In The Chain Of Survival
Hence, efforts to improve SCA survival are necessary. Investigation and evaluation have established at least some of the important prognostic determinants of SCA and provide the basis for efforts to improve outcomes following SCA. The health service determinants have been collectively termed the links in the chain of survival and include early activation of emergency response, early cardiopulmonary resuscitation (CPR), early defibrillation (electrical shock), and timely advanced medical care (AHA, 2005). For example, observational studies have consistently demonstrated that the chance of survival decreases as the interval from collapse to defibrillation increases for ventricular fibrillation SCA (Valenzuela et al., 1997). The Public Access Defibrillation randomized trial rigorously confirmed this time-to-shock – survival relationship and highlighted one strategy – public access defibrillation – to improve survival by evaluating the effects of equipping laypersons with automated external defibrillators (AEDs). The AED is a device typically the size of a laptop computer that enables accurate cardiac rhythm assessment and appropriate shock by persons not trained in rhythm interpretation, and hence may be widely disseminated to enable the potential for early defibrillation (Rho and Page, 2007). In the Public Access Defibrillation Trial, SCA survival was doubled in select public locations where laypersons were equipped with AEDs as well as CPR training compared to locations where laypersons were trained in CPR but not equipped with AEDs (Hallstrom et al., 2004). Although layperson defibrillation appears promising, this strategy is challenged by the reality that most SCA occurs in the home so that large public health impacts of this approach will require far greater dissemination of AEDs and their accompanying training requirements and support resources (Culley et al., 2004). Novel approaches that deliver defibrillation earlier after collapse also have promise. Such programs enlist and equip nontraditional medical responders such as police, security officers, or flight attendants with lifesaving skills including AEDs (Page et al., 2000; Valenzuela et al., 2000; White et al., 2005).
Cardiopulmonary resuscitation, or CPR, consists of a series of chest compressions often alternating with ventilations (AHA, 2005). These rescuer actions provide some measure of oxygenated blood circulation to vital organs (the brain and heart) until native circulation can be restored. Observational studies generally indicate that early CPR provided by laypersons prior to arrival of EMS can improve the relative chances of survival, especially as the interval from collapse to EMS arrival increases (Cobb et al., 1999; AHA, 2005; Gilmore et al., 2006). In most SCA events, however, the victim does not receive layperson CPR. Ongoing approaches may address this gap by increasing layperson CPR training, simplifying CPR techniques, and enhancing emergency dispatcher programs to assist CPR. Additional research aims to identify (1) the ideal composition of CPR with regard to CPR duration, the ratio of chest compressions and ventilations, as well as the depth and rate of chest compression and volume of ventilations; (2) whether mechanical devices can improve CPR and affect survival; and (3) the best interface between CPR and other components of resuscitation such as defibrillation (AHA, 2005a, 2005b).
Advanced therapies also potentially have an important role in improving survival following SCA. Specifically, based on the results of randomized trials, active induction and maintenance of hypothermia following restoration of native circulation improves survival following ventricular fibrillation SCA (Bernard et al., 2002; Hypothermia After Cardiac Arrest Study Group, 2002). Hypothermia moderates reperfusion processes that occur when critically ischemic tissues receive oxygen. Pathologic reperfusion produces oxidative radicals that damage cells, a process that seems especially relevant to the brain (Ambrosio and Tritto, 1999; Becker, 2004). The timing, extent, duration, modality, and ease of implementation are all potentially important aspects of hypothermia therapy that may influence its effectiveness and in turn continue to require ongoing investigation. Additional advanced therapy also may improve outcome following SCA. For example, timely coronary artery revascularization may be appropriate in select SCA patients (Spaulding et al., 1997). Additional ongoing research tries to understand if treatments aimed at sepsis syndromes might be relevant for patients early on following resuscitation (Laurent et al., 2005).
These health service measures likely only account for a portion of the variability in outcome, indicating that other characteristics influence the likelihood of survival (Hallstrom et al., 1996). Evidence indicates that patient characteristics such as underlying chronic health conditions influence the likelihood of resuscitation (Hallstrom et al., 1996; Carew et al., 2007). This relationship suggests that individual characteristics influence the pathophysiology of SCA and in turn may guide the choice of specific resuscitation treatments. In some studies, higher levels of socioeconomic status are associated with a greater chance of survival (Hallstrom et al., 1993; Clarke et al., 2005). Whether socioeconomic status is simply a surrogate for health status or identifies disparity in health delivery is not clear, but again provides one area to address and potentially improve resuscitation. Ultimately, a more complete understanding of the pathophysiology and prognostic determinants of SCA provides the best opportunity to achieve successful outcomes. With such knowledge, resuscitation care may be refined to best match treatment to the individual patient. Taken together, ongoing efforts to improve our understanding of SCA coupled with better and more complete dissemination and implementation of effective treatments will hopefully enable public health gains in resuscitation of SCA.
Challenges Of SCA Research
Research to improve prevention and outcome of SCA is imperative if we are to reduce the burden of SCA. However, SCA research, especially resuscitation research, can present particular challenges not typical of most medical research. Because SCA is unexpected, the victim is unconscious, and delays of even a few moments can adversely affect the chances of resuscitation, normal research procedures whereby potential study participants provide informed consent are not possible. In an effort to address this set of challenges, the federal government in the United States, for example, has developed regulations to guide researchers and oversight groups so that such research can proceed. However, the regulations have in some circumstances produced confusion among different stakeholders and in turn may have paradoxically hindered scientific progress (Nichol et al., 2006; Miros, 2007). Ongoing efforts hope to clarify the regulations so that future research may move forward while assuring the safety and protection of human subjects.
Conclusions
Sudden cardiac arrest is a substantial public health challenge. Successful advances targeting prevention in some Western societies may be countered as the prevalence of heart disease increases in other parts of the world. Efforts focused at prevention, diagnosis, and treatment of coronary artery disease and congestive heart failure remain a cornerstone of SCA preventive therapy. Refinement of established SCA risk factors as well as identification of novel predictors that are specific to SCA risk are an important public health priority. Improvements in resuscitation also offer a meaningful opportunity to reduce mortality from SCA. Public health gains through better resuscitation will require more complete implementation of established health service tenets (i.e., layperson CPR and hypothermia) as well as improvements in our understanding that enable innovative treatments. When combined, advances in prevention and resuscitation of SCA are a complementary and effective approach to reduce mortality from SCA and in turn improve public health.
Bibliography:
- AHA (2005a) 2005 AHA guidelines for CPR and ECC: Overview of CPR. Circulation 112(supplement I): IV-1–IV-18.
- AHA (2005b) 2005 AHA guidelines for CPR and ECC: Overview of CPR. Circulation 112(supplement I): IV-47–IV-50.
- Albert CM, Hennekens CH, O’Donnell CJ, et al. (1998) Fish consumption and risk of sudden cardiac death. Journal of the American Medical Association 279(1): 23–28.
- Albert CM, Manson JE, Cook NR, Ajani UA, Gaziano JM, and Hennekens CH (1999) Moderate alcohol consumption and the risk of sudden cardiac death among US male physicians. Circulation 100 (9): 944–950.
- Albert CM, Mittleman MA, Chae CU, Lee IM, Hennekens CH, and Manson JE (2000) Triggering of sudden death from cardiac causes by vigorous exertion. New England Journal of Medicine 343(19): 1355–1361.
- Albert CM, Ma J, Rifai N, Stampfer MJ, and Ridker PM (2002) Prospective study of C-reactive protein homocysteine, and plasma lipid levels as predictors of sudden cardiac death. Circulation 105(22): 2595–2599.
- Albert CM, Chae CU, Grodstein F, et al. (2003) Prospective study of sudden cardiac death among women in the United States. Circulation 107(16): 2096–2101.
- Ambrosio G and Tritto I (1999) Reperfusion injury: Experimental evidence and clinical implications. American Heart Journal 138: S69–S75.
- Arking DE, Chugh SS, Chakravarti A, and Spooner PM (2004) Genomics in sudden cardiac death. Circulation Research 94(6): 712–723.
- Arking DE, Pfeufer A, and Post W (2006) A common genetic variant in the NOS1 regulator NOS1AP modulates cardiac repolarization. Nature and Genetics 38(6): 644–651.
- Atwood C, Eisenberg MS, Herlitz J, and Rea TD (2005) Incidence of EMS-treated out-of-hospital cardiac arrest in Europe. Resuscitation 67(1): 75–80.
- Bailey JJ, Hodges M, and Church TR (2007) Decision to implant a cardioverter defibrillator after myocardial infarction: The role of ejection fraction v. other risk factor markers. Medical Decision Making 27(2): 151–160.
- Becker LB (2004) New concepts in reactive oxygen species and cardiovascular reperfusion physiology. Cardiovascular Research 61(3): 461–470.
- Becker LB, Han BH, Meyer PM, et al. (1993) Racial differences in the incidence of cardiac arrest and subsequent survival. New England Journal of Medicine 329(9): 600–606.
- Begley DA, Mohiddin SA, Tripodi D, Winkler JB, and Fananapazir L (2003) Efficacy of implantable cardioverter defibrillator therapy for primary and secondary prevention of sudden cardiac death in hypertrophic cardiomyopathy. Pacing and Clinical Electrophysiology 26(9): 1887–1896.
- Bernard SA, Gray TW, Buist MD, et al. (2002) Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. New England Journal of Medicine 346(8): 557–563.
- Biros M (2007) Struggling with the rule: The exception from informed consent in resuscitation research. Academic Emergency Medicine 14(4): 344–345.
- Bristow MR, Saxon LA, Boehmer J, et al. (2004) Cardiacresynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. New England Journal of Medicine 350(21): 2140–2150.
- Callaway CW and Menegazzi JJ (2005) Waveform analysis of VF to predict defibrillation. Current Opinions in Critical Care 11(3): 192–199.
- Carew HT, Zhang W, and Rea TD (2007) Chronic health conditions and survival after out-of-hospital ventricular fibrillation cardiac arrest. Heart 93(6): 728–731.
- Chen PS and Weiss JN (2005) Runway pacemakers in VF. Circulation 112: 148–150.
- Chugh SS, Jui J, Gunson K, et al. (2004) Current burden of sudden cardiac death: Multiple source surveillance versus retrospective death certificate-based review in a large US community. Journal of the American College of Cardiology 44(6): 1268–1275.
- Clarke S, Schellenbaum G, and Rea TD (2005) Socioeconomic status and outcome of out-of-hospital cardiac arrest. Academic Emergency Medicine 12(10): 941–947.
- Cobb LA, Fahrenbruch CE, Walsh TR, et al. (1999) Influence of cardiopulmonary resuscitation prior to defibrillation in patients with out-of-hospital VF. Journal of the American Medical Association 281(13): 1182–1188.
- Cobb LA, Fahrenbruch CE, Olsufka M, and Copass MK (2002) Changing incidence of out-of-hospital ventricular fibrillation, 1980–2000. Journal of the American Medical Association 288(23): 3008–3013.
- Corrado D, Leoni L, Link MS, et al. (2003) Implantable cardioverterdefibrillator therapy for prevention of sudden death in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation 108(25): 3084–3091.
- Culley L, Rea TD, Murray JA, et al. (2004) Public access defibrillation in out-of-hospital cardiac arrest: A community based study. Circulation 109(15): 1859–1863.
- Cupples LA, Gagnon DR, and Kannel WB (1992) Longand short-term risk of sudden coronary death. Circulation 85(1 Suppl): 111–118.
- De Vreede-Swagemakers JJ, Gorgels AP, Dubois-Arbouw WI, et al. (1997) Out-of-hospital cardiac arrest in the 1990s: A populationbased study in the Maastricht Area on incidence characteristics and survival. Journal of the American College of Cardiology 30(6): 1500–1505.
- Fox CS, Evans JC, Larson MG, Kannel WB, and Levy D (2004) Temporal trends in coronary heart disease mortality and sudden cardiac death From 1950 to 1999: The Framingham Heart Study. Circulation 110(5): 522–527.
- Fox CS, Evans JC, Larson MG, et al. (2005) A comparison of death certificate out-of-hospital coronary heart disease death with physician-adjudicated sudden cardiac death. The American Journal of Cardiology 95(7): 856–859.
- Friedlander Y, Siscovick DS, and Weinmann S (1998) Family history as a risk factor for primary cardiac arrest. Circulation 97: 155–160.
- Gillum R (1989) Sudden coronary death in the United States: 1980–1985. Circulation 79(4): 756–765.
- Gilmore C, Rea TD, and Eisenberg MS (2006) Three-phase model of cardiac arrest: Time-dependent benefit of bystander CPR. American Journal of Cardiology 98(4): 497–499.
- Goldberg R (1989) Declining out-of-hospital sudden coronary death rates. Additional pieces of the epidemiologic puzzle. Circulation 79(6): 1369–1373.
- Goldberger Z and Lampert R (2006) Implantable cardioverterdefibrillators: Expanding indications and technologies. Journal of the American Medical Association 295(7): 809–818.
- Goldenberg I, Jonas M, Tenenbaum A, et al. (2003) Current smoking, smoking cessation, and the risk of sudden cardiac death in patients with coronary artery disease. Archives of Internal Medicine 163(19): 2301–2305.
- Hallstrom A, Boutin P, Cobb L, and Johnson E (1993) Socioeconomic status and prediction of VF survival. American Journal of Public Health 83(2): 245–248.
- Hallstrom AP, Cobb LA, and Yu BH (1996) Influence of comorbidity on the outcome of patients treated for out-of-hospital VF. Circulation 93(11): 2019–2022.
- Hallstrom AP, Ornato JP, Weisfeldt M, et al. (2004) Public-access defibrillation and survival after out-of-hospital cardiac arrest. New England Journal of Medicine 351(7): 637–646.
- Hemingway H, Malik M, and Marmot M (2001) Social and psychosocial influences on sudden cardiac death, ventricular arrhythmia and cardiac autonomic function. European Heart Journal 22(13): 1082–1101.
- Hennessy S, Bilker WB, Knauss JS, et al. (2002) Cardiac arrest and ventricular arrhythmia in patients taking antipsychotic drugs: Cohort study using administrative data. British Medical Journal 325(7372): 1070.
- Herlitz J, Hartford M, Karlson BW, Dellborg M, Kallstrom G, and Karlsson T (2000) Experiences from treatment of out-of-hospital cardiac arrest during 17 years in Goteborg. European Heart Journal 21(15): 1251–1258.
- Huikuri HV, Castellanos A, and Myerburg RJ (2000) Sudden death due to cardiac arrhythmias. New England Journal of Medicine 345(20): 1473–1482.
- Hunt SA, Abraham WT, Chin MH, et al. (2005) AHA/ACC 2005 guideline for the diagnosis and management of chronic heart failure in the adult. Circulation 112: e154–e235.
- Hypothermia After Cardiac Arrest Study Group (2002) Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. New England Journal of Medicine 346(8): 549–556.
- Iribarren C, Crow RS, Hannan PJ, et al. (1998) Validation of death certificate diagnosis of out-of-hospital sudden cardiac death. American Journal of Cardiology 82(1): 50–53.
- Januzzi JL Jr., Stern TA, Pasternak RC, and DeSanctis RW (2000) The influence of anxiety and depression on outcomes of patients with coronary artery disease. Archives of Internal Medicine 160(13): 1913–1921.
- Josephson M and Wellens HJJ (2004) Implantable defibrillators and sudden cardiac death. Circulation 109(22): 2685–2691.
- Jouven X, Desnos M, Guerot C, and Ducimetiere P (1999) Predicting sudden death in the population: The Paris Prospective Study I. Circulation 99: 1978–1983.
- Kannel WB and Schatzkin A (1985) Sudden death: Lessons from subsets in population studies. Journal of the American College of Cardiologists 5(supplement 6): 141B–149B.
- Kannel WB, Wilson PW, D’Agostino RB, and Cobb J (1998) Sudden coronary death in women. American Heart Journal 136(2): 205–212.
- Kron J, Herre J, Renfroe EG, et al. (2001) Leadand device-related complications in the Antiarrhythmics Versus Implantable Defibrillators Trial. American Heart Journal 141(1): 92–98.
- Kuisma M, Repo J, and Alaspaa A (2001) The incidence of out-of-hospital ventricular fibrillation in Helsinki, Finland, from 1994 to 1999. Lancet 358(9280): 473–474.
- Laurent I, Adrie C, Vinsonneau C, et al. (2005) High-volume hemofiltration after out-of-hospital cardiac arrest: A randomized study. Journal of the American College of Cardiologists 46(3): 432–437.
- Leaf A, Kang JX, Xiao YF, and Billman GE (2003) Clinical prevention of sudden cardiac death by n-3 polyunsaturated fatty acids and mechanism of prevention of arrhythmias by n-3 fish oils. Circulation 107(21): 2646–2652.
- Leeder S, Raymond S, and Greenberg H (2004) A Race Against Time: The Challenge of Cardiovascular Disease in Developing Economies. The Earth Institute, Columbia University. http://www.earthinstitute. columbia.edu (accessed October 2007).
- Lemaitre RN, Sis covick DS, Raghunathan TE, Weinmann S, Arbogast P, and Lin DY (1999) Leisure-time physical activity and the risk of primary cardiac arrest. Archives of Internal Medicine 159(7): 686–690.
- Leor J, Poole WK, and Kloner RA (1996) Sudden cardiac death triggered by an earthquake. New England Journal of Medicine 334(7): 413–419.
- Lloyd-Jones DM, Martin DO, Larson MG, and Levy D (1998) Accuracy of death certificates for coding coronary heart disease as the cause of death. Annals of Internal Medicine 129(12): 1020–1026.
- Lynd LD and O’Brien BJ (2003) Cost-effectiveness of the implantable cardioverter defibrillator: A review of current evidence. Journal of Cardiovascular Electrophysiology 14(supplement 9): S99–S103.
- Maron BJ, Estes NA 3rd, Maron MS, Almquist AK, Link MS, and Udelson JE (2003) Primary prevention of sudden death as a novel treatment strategy in hypertrophic cardiomyopathy. Circulation 107(23): 2872–2875.
- Mosca L, Banka CL, Benjamin EJ, et al. (2007) Evidence-based guidelines for cardiovascular disease prevention in women: 2007 update. Circulation 115: 1481–1501.
- Mozaffarian D (2007) JELIS, fish oil, and cardiac events. Lancet 369(9567): 1062–1063.
- Myerburg RJ and Castellanos A (1997) Cardiac arrest and sudden cardiac death. In: Zipes DP (ed.) Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine, pp. 742–743. New York: WB Saunders Publishing Co.
- Narang R, Cleland JG, Erhardt L, et al. (1996) Mode of death in chronic heart failure: A request and proposition for more accurate classification. European Heart Journal 17(9): 1390–1403.
- Nash MP, Mourad A, Clayton RH, et al. (2006) Evidence for multiple mechanisms in human ventricular fibrillation. Circulation 114(6): 536–542.
- Nichol G, Powell J, van Ottingham L, et al. (2006) Consent in resuscitation trials: Benefit or harm for patients and society? Resuscitation 70(3): 360–368.
- Page RL, Joglar JA, Kowal RC, et al. (2000) Use of automated external defibrillators by a US airline. New England Journal of Medicine 343(17): 1210–1216.
- Peberdy MA, Kaye W, Ornato JP, et al. (2003) Cardiopulmonary resuscitation of adults in the hospital: A report of 14720 cardiac arrests from the National Registry of Cardiopulmonary Resuscitation. Resuscitation 58(3): 297–308.
- Pratt CM and Moye LA (1995) The Cardiac Arrhythmia Suppression Trial: Casting suppression in a different light. Circulation 91(1): 245–247.
- Priori SG, Aliot E, Blomstrom-Lindqvist C, et al. (2002) Task force on sudden cardiac death European Society of Cardiology: Summary of Recommendations. Europace 4(1): 3–18.
- Rea TD and Paredes VL (2004) Quality of life and prognosis among survivors of out-of-hospital cardiac arrest. Current Opinions on Critical Care 10(3): 218–223.
- Rea TD, Crouthamel M, Eisenberg MS, Becker LJ, and Lima AR (2003a) Temporal patterns in long-term survival after resuscitation from outof-hospital cardiac arrest. Circulation 108: 1196–2001.
- Rea TD, Eisenberg MS, Becker LJ, et al. (2003b) Emergency medical services and mortality from heart disease: A community study. Annals of Emergency Medicine 41(4): 494–499.
- Rea TD, Eisenberg MS, Sinibaldi G, and White RD (2004a) Incidence of EMS-treated out-of-hospital cardiac arrest in the United States. Resuscitation 63(1): 17–24.
- Rea TD, Eisenberg MS, Sinibaldi G, and White RD (2004b) Incidence of out-of-hospital cardiac arrest. The American Journal of Cardiology 93(12): 1455–1460.
- Rea TD, Helbock M, Perry S, et al. (2006) Increasing use of cardiopulmonary resuscitation during out-of-hospital ventricular fibrillation arrest: Survival implications of guideline changes. Circulation 114(25): 2760–2765.
- Rho RW and Page RL (2007) The automated external defibrillator. Journal of Cardiovascular Electrophysiology 18: 1–4.
- Rosamond W, Flegal K, Friday G, et al. (2007) Heart disease and stroke statistics – 2007 Update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 115(5): e69–e171.
- Sacher F, Probst V, Iesaka Y, et al. (2005) Multicenter study of prophylactic ICD implantation in Brugada syndrome. Heart Rhythm 5 (supplement 1): S40.
- Shah M, Akar FG, and Tomaselli GF (2005) Molecular basis of arrhythmias. Circulation 112(16): 2517–2529.
- Siscovick DS and Podrid PJ (2007) Overview of Sudden Cardiac Death. In: Rose BD (ed.). Waltham, MA: UpToDate.
- Siscovick DS, Weiss NS, Fletcher RH, and Lasky T (1984) The incidence of primary cardiac arrest during vigorous exercise. New England Journal of Medicine 311(14): 874–877.
- Siscovick DS, Weiss NS, and Fox N (1986) Moderate alcohol consumption and primary cardiac arrest. American Journal of Epidemiology 123(3): 499–503.
- Siscovick DS, Raghunathan TE, Psaty BM, et al. (1994) Diuretic therapy for hypertension and the risk of primary cardiac arrest. New England Journal of Medicine 330(26): 1852–1857.
- Siscovick DS, Raghunathan TE, King I, et al. (1995) Dietary intake and cell membrane levels of long-chain n-3 polyunsaturated fatty acids and the risk of primary cardiac arrest. Journal of the American Medical Association 274(17): 1363–1367.
- Siscovick DS, Lemaitre RN, and Mozaffarian D (2003) The fish story: a diet-heart hypothesis with clinical implications: N-3 polyunsaturated fatty acids, myocardial vulnerability, and sudden death. Circulation 107(21): 2632–2634.
- Smith SC, Allen J, Blair SN, et al. (2006) AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update. Circulation 113: 2363–2372.
- Sotoodehnia N, Siscovick DS, Vata M, et al. (2006) Beta-2 adrenergic receptor genetic variants and risk of sudden cardiac death. Circulation 18 113(15): 1842–1848.
- Spaulding CM, Joly LM, Rosenberg A, et al. (1997) Immediate coronary angiography in survivors of out-of-hospital cardiac arrest. New England Journal of Medicine 336(23): 1629–1633.
- Stecker EC, Vickers C, Waltz J, et al. (2006) Population-based analysis of sudden cardiac death with and without left ventricular systolic dysfunction: Two-year findings from the Oregon Sudden Unexpected Death Study. Journal of the American College of Cardiology 47(6): 1161–1166.
- Thomas SP, Thiagalingam A, Wallace E, Kovoor P, and Ross DL (2005) Organization of myocardial activation during VF after myocardial infarction. Evidence for sustained high-frequency sources. Circulation 112: 157–163.
- Valenzuela TD, Roe DJ, Cretin S, Spaite DW, and Larsen MP (1997) Estimating effectiveness of cardiac arrest interventions: A logistic regression survival model. Circulation 96(10): 3308–3313.
- Valenzuela TD, Roe DJ, Nichol G, Clark LL, Spaite DW, and Hardman RG (2000) Outcomes of rapid defibrillation by security officers after cardiac arrest in casinos. New England Journal of Medicine 343(17): 1206–1209.
- Wannamethee G and Shaper A (1992) Alcohol and sudden cardiac death. British Heart Journal 68(5): 443–448.
- Weiss JN, Chen PS, Qu Z, Karagueuzian HS, and Garfinkel A (2000) Ventricular fibrillation : How do we stop the waves from breaking? Circulation Research 87(12): 1103–1107.
- Weiss JN, Qu Z, Chen PS, et al. (2005) The dynamics of cardiac fibrillation. Circulation 112(8): 1232–1240.
- Whang W, Albert CM, Sears SF Jr, et al. (2005) Depression as a predictor for appropriate shocks among patients with implantable cardioverter-defibrillators: Results from the Triggers of Ventricular Arrhythmias (TOVA) study. Journal of the American College of Cardiologists 45(7): 1090–1095.
- White RD, Bunch TJ, and Hankins DG (2005) Evolution of a community-wide early defibrillation programme experience over 13 years using police/fire personnel and paramedics as responders. Resuscitation 65(3): 279–283.
- Yach D, Hawkes C, Gould CL, and Hofman KJ (2004) The global burden of chronic diseases: Overcoming impediments to prevention and control. Journal of the American Medical Association 291(21): 2616–2622.
- Zheng ZJ, Croft B, Giles WH, et al. (2001) Sudden cardiac death in the United States, 1989 to 1998. Circulation 104(18): 2158–2163.
- Zipes DP (2005) Epidemiology and mechanisms of sudden cardiac death. Canadian Journal of Cardiology 21(supplement A): 37–40.
- Zipes DP, Camm AJ, Borggrefe M, et al. (2006) ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Circulation 114(10): e385–e484.
See also:
Free research papers are not written to satisfy your specific instructions. You can use our professional writing services to buy a custom research paper on any topic and get your high quality paper at affordable price.








