This sample Tuberculosis Epidemiology Research Paper is published for educational and informational purposes only. If you need help writing your assignment, please use our research paper writing service and buy a paper on any topic at affordable price. Also check our tips on how to write a research paper, see the lists of health research paper topics, and browse research paper examples.
Introduction
Tuberculosis has killed more persons in the history of the world than any other infectious disease and is currently the second most common infectious disease killer, claiming 1.6 million deaths per year (nearly 4400 per day) (World Health Organization, 2007). It is estimated that in the past two centuries, TB has accounted for approximately one billion deaths (Ryan, 1992). The discovery of deformities consistent with tuberculosis disease in skeletons suggest that the disease was common in Egypt at least 4000 years ago (Nerlich et al., 1997). In 500 BC, Hippocrates described patients with ‘consumptive disease,’ a classic description that endures to this day, characterized by coughing bloody sputum, chest pain, and wasting. At the time, of course, there was no effective treatment and patients essentially withered away, consumed by their disease.
Tuberculosis grew in prevalence in Europe during the seventeenth century, as the growing population moved into larger cities, with concomitant crowded living conditions and poor sanitation and ventilation. By the first half of the nineteenth century, TB was responsible for one-quarter of all deaths on the continent as a result of industrialization and concentrated urban misery. As housing conditions improved toward the end of the nineteenth century, tuberculosis rates began to decline. However, as colonialism took Europeans to Africa and Asia, the disease accompanied the industrialization and urbanization processes in these regions. In India and China, TB rates reached epidemic levels at the turn of the twentieth century, though low population density limited the spread of TB in most of Africa. European immigration to the New World accompanied with early industrialization in Eastern urban centers brought high TB rates similar to the mainland European continent. Mortality rates similarly reflected epidemic proportions, with up to 600 deaths per 100 000 people (Stead et al., 1995).
TB declined in the industrialized world starting in the late nineteenth century aided by improvements in labor and housing conditions, and public health practices such as the advent of the Bacille Calmette–Gue´rin vaccine. It was not until widespread European immigration to South and sub-Saharan Africa at the turn of the twentieth century that TB became widespread there. The story was similar among the indigenous populations of what is now Alaska and Greenland, where TB took hold and ravaged the local population (with rates above 1000/100 000 persons) (Stead et al., 1995). In areas such as Indonesia and South East Asia, TB was introduced in the early twentieth century. It was not until the 1940s and the use of antibiotics – especially streptomycin and isoniazid – that the TB death rate began a steeper decline in Europe and North America (although the rate of decline in incidence did not change throughout that time). TB incidence rates in Western Europe and North America declined consistently through the twentieth century. In the late 1980s, however, a confluence of factors led to a reversal in the incidence rate in the United States (especially in New York City) (Frieden et al., 1995). This reversal was primarily due to the defunding of tuberculosis control programs, combined with a rise in HIV infection rates and outbreaks of multidrug-resistant tuberculosis (Binkin et al., 1999). Increasing economic inequality in the United States, with poverty, homelessness in urban settings, immigration from high-burden countries, and crowded housing also converged to cause a resurgence of TB.
A century ago, TB ravaged affluent and poor societies alike, although not equally. Today, however, rates of TB are telling indicators of a country’s wealth or poverty status and particularly of resource disparities within societies. Therefore tuberculosis, and the distribution of drug-resistant TB, is not only a matter of the transmission of TB infection, it is also a reflection of global resource distribution (Kim et al., 2005). In much of sub-Saharan Africa where there are few resources, TB rates are highest. In countries where TB therapy was available but improperly prescribed, or where there were frequent drug shortages, rates of multidrug-resistant TB have risen. In areas where TB programs were defunded and political support for TB control waned, there were both increases in TB and multidrug-resistant TB. Understanding social inequalities is therefore central to understanding the persistence and re-emergence of TB. The unprecedented scale of the current TB epidemic and the human rights aspects of the problem demand effective and urgent action. The next section summarizes the epidemiology of the current TB epidemic in terms of morbidity, mortality, and economic burden.
Burden Of TB Morbidity And Mortality
Case Notifications
Currently, approximately one-third of the world’s population is infected with TB (World Health Organization, 2007). Among the 8.8 million estimated new cases, and 1.6 million estimated deaths, more than 95% are thought to occur in developing countries. This enormous disparity and imbalance of disease burden is due to the underfunding of public health services, the spread of HIV, and the emergence of drug-resistant tuberculosis. If tuberculosis control is not strengthened through improvements in both social conditions and health services, estimates are that approximately one billion people will be newly infected with the mycobacterium that causes TB, over 150 million people will develop TB disease, and 36 million people will die of TB between 2000 and 2020 (World Health Organization, 2002).
It is impossible to know the true incidence of TB, given that we do not know how many cases go undetected, which makes aggregate case notification data from countries very important. Case notification data reflect health service coverage and the efficiency of TB case-finding and reporting activities of a country’s programs. In countries with effective TB control programs, case notifications can closely approximate the true incidence. Often, poor program performance results in underdetection and underreporting of cases. Therefore in most of the developing world notifications represent only a fraction of the true incident cases as large sectors of the population have no access to effective treatment. Despite limitations, case notifications under stable program conditions in most countries provide useful data on the trend of TB incidence and rates stratified by age, sex, and some risk characteristics. The World Health Organization uses case notifications and modeling methods to estimate the global TB disease burden.
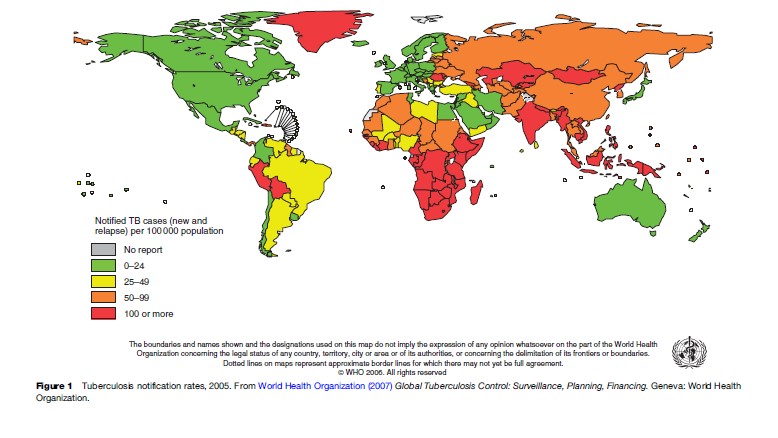
In 2005, 199 countries (99.9% of the world’s population) reported 5.1 million TB cases to WHO, of which 2.4 million of these were new sputum smear-positive cases. Of this total, 35% of these cases were diagnosed in the Southeast Asia region, 25% in the Western Pacific region, 23% in the African region, 4% in the American region, 5% in the Eastern Mediterranean region, and 7% in the European region (World Health Organization, 2007). Figure 1 shows the TB notification rates from 2005. A total of 26.5 million new and relapse cases were notified by DOTS programs between 1995 and 2005. Case notifications have been steadily falling for at least the last two decades in Southeast Asia and Western Pacific regions, in Western and Central Europe, and North and Latin America. Case reports have been increasing in Eastern Europe (the former Soviet Union) since 1990, and in sub-Saharan Africa since the mid-1980s, although the rate of increase has slowed in the past few years (Dye, 2006). The resurgence of TB in Eastern Europe is due to economic decline, poor TB control, and a poor general health infrastructure. The elevated incidence of drug resistance in this region (sometimes over 10% of new cases have multidrug-resistant TB) is probably a byproduct of these forces and not a cause.
Estimated Incidence And Mortality
Global estimates of TB incidence are based on multiple inputs, including case notifications, such as population based surveys of TB prevalence, vital registration data, and independent assessments of surveillance system quality (Dye, 2006). In 2007, WHO estimated that prevalence and death rates of TB had been falling for several years, peaking sometime between 2000 and 2005. By 2005 the incidence rate was stable or in decline in all six WHO regions, a measure of the success of global DOTS programs. However, WHO also estimated that the global incidence (absolute number of new cases per year) of TB is growing at roughly 1% per year, due to the growing caseload in sub-Saharan Africa and countries of the former Soviet Union (Eastern Europe and Central Asia), and population growth (World Health Organization, 2007).
Worldwide, there were an estimated 8.8 million new cases of TB in 2005, with 3.9 million cases thought to be infectious (World Health Organization, 2007). The incidence and mortality rates vary widely by WHO region (Table 1). Developing countries show the brunt of the epidemic, precisely the places with fewer resources to combat the problem. It is estimated that 95% of the world’s cases and 98% of the TB deaths occur in the developing world (Raviglione and Uplekar, 2006). Figure 2 shows the estimated numbers of new TB cases charted by country. In 2005, almost 3 million new cases were diagnosed in South and Southeast Asia, which accounted for one-third of all cases worldwide. Nearly half of the world’s cases that arise every year occur in Asia. While there were fewer cases in Africa (2.5 million), the incidence per capita is nearly twice that of Asia, with 343 cases per 100 000 population. Similarly, most deaths from TB occurred in Asia (807 000), but the highest mortality per capita was in Africa (74 per 100 000).
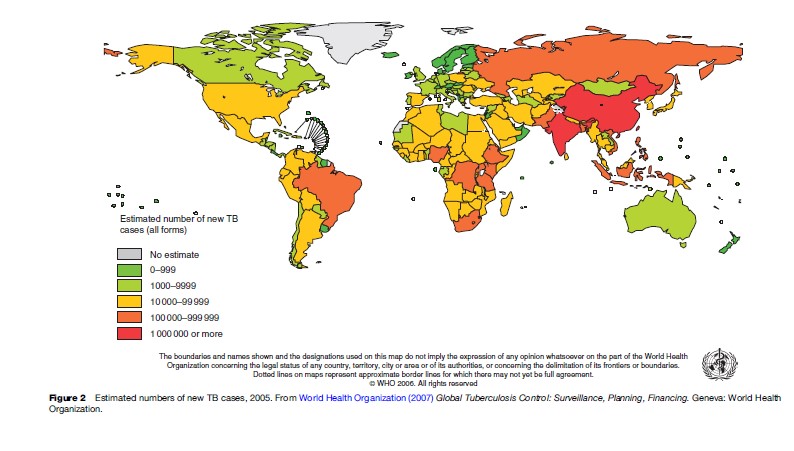
Twenty-two countries are labeled as high-burden countries and account for 80% of the world’s case burden. The five countries with the highest burden of TB were India (1.8 million cases), China (1.3 million cases), Indonesia (533 000 cases), Nigeria (372 000 cases), and Bangladesh (322 000 cases). The American (North and South) and European regions (WHO designation) account for 9% of the world’s total TB burden. However, in North America and many European countries, more than 50% of the TB cases reported are among persons who were not born in those countries (foreign born). Among the 15 countries with the highest estimated TB incidence rates, 12 are in Africa. This is largely explained by the relatively high rates of HIV infection. Swaziland, Djibouti, Namibia, Lesotho, Botswana, Kenya, Zimbabwe, Zambia, and South Africa all have estimated incidence rates above 600 per 100 000 (World Health Organization, 2007).
In most locales, men have greater incidence rates of TB than women, although in many of these places women are more likely to have poor access to health facilities and diagnostic services. The observed difference in incidence between men and women is due to an observed epidemiologic difference in exposure to infection (in the workplace, poor housing conditions, other congregate settings) and susceptibility to development of disease. Where the transmission of the TB bacillus is stable or increasing, the incidence rate is highest in young adults as most cases result from recent infection or reinfection. As transmission falls, the caseload shifts to older adults, as a higher proportion of cases can be attributable to reactivation of latent infection (Dye, 2006). In the established market economies where rates of TB are low, indigenous persons with TB tend to be older. Immigrants in these countries from high incidence areas tend to be younger, although this depends on the maturity of the epidemic in the host country.
The ongoing global TB epidemic is of great concern for many reasons: (1) increasing global social and economic inequality, with a widening income gap between the rich and poor in various countries; (2) historical neglect of TB control programs; (3) changing global demographics (increasing world population and age structure); (4) worsening drug resistance in many areas; and (5) impact of the HIV pandemic (Maher et al., 2006).
TB/HIV
One of the primary causes of the resurgence in TB worldwide is the increase in HIV infection, even in rural areas. Among the 1.6 million estimated annual deaths, 195 000 are estimated to also be infected with HIV (World Health Organization, 2007). HIV is the strongest risk factor for persons infected with Mycobacterium tuberculosis to acquire TB disease – either drug-susceptible or drug-resistant TB – following infection. The HIV virus suppresses the body’s immune system, which promotes progression of either recently acquired or latent tuberculosis infection to TB disease. Persons infected with HIV who become infected with M. tuberculosis have a very high risk of developing TB disease within 2 years. The HIV epidemic has therefore telescoped the TB epidemic of both susceptible and drug-resistant strains, shortening the time to generate the epidemic from years to months (Maher et al., 2006). As a result, the global HIV epidemic has increased the burden of TB in many countries. Between 1990 and 1999, the incidence of TB in sub-Saharan Africa increased by over 250%, with an estimated one-third of all new TB cases occurring among people who were HIV infected. This happened despite many countries having a well-functioning national TB control program. In areas of high tuberculosis prevalence, therefore, TB is one of the most common opportunistic infections. TB may also accelerate the course of HIV infection, increasing HIV viral load in some patients, and is thought to be the primary cause of death in up to 40% of those who are HIV infected (de Jong et al., 2004).
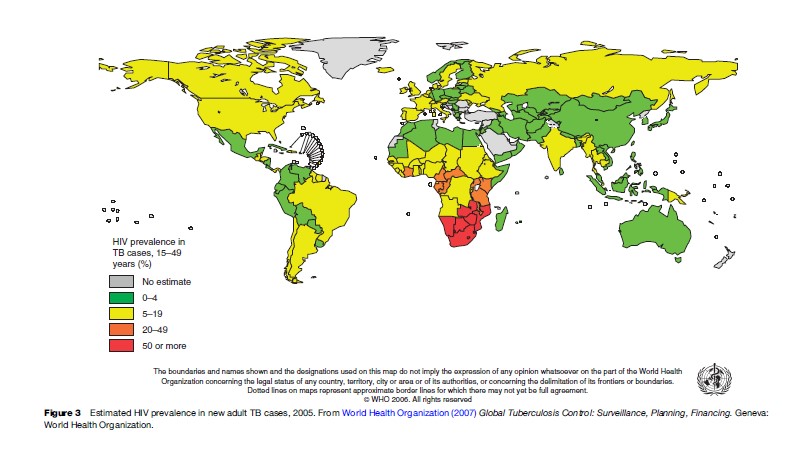
The proportion of adults with TB who were infected with HIV varies greatly by region, from roughly 30% in Africa to less than 2% in the Western Pacific. In high TB burden countries such as China, Indonesia, Bangladesh, and Pakistan, HIV infection rates in TB cases have remained below 1%. Where HIV infection rates are high in the general population (generalized epidemic), they are generally also high in persons with TB. Estimates for high TB burden southern African countries such as South Africa, Botswana, Zimbabwe, and Zambia exceed 50%. HIV has probably had a smaller effect on the prevalence of TB than on the incidence of TB, as HIV significantly reduces the life expectancy of persons with TB, and in the general population as well (Dye, 2006). Due to HIV and TB mortality, life expectancy in many southern African countries has dropped below 45 years.
In some areas with both high TB and HIV prevalence, the symptoms of TB disease are often equated with HIV-positive status by lay people. Persons exhibiting TB symptoms are therefore often stigmatized as HIV-positive, which causes them to modify their social and health-seeking behavior, and ultimately hinders TB control efforts at a clinical level. The stigma surrounding both diseases promotes shame and silence, which further exacerbates the spread of both diseases. Approaching TB control with a human rights lens therefore becomes imperative (World Health Organization, 2001).
Although HIV has had a more profound impact on the epidemiology of TB worldwide than any other risk factor, other important risk factors should not be ignored (Maher et al., 2006). More attention needs to be paid to the increased risk of developing TB from smoking, as well as from air pollution and other respiratory diseases. Chronic diseases such as diabetes also play a large role, as does the prevalence of undernutrition.
Drug-Resistant Tuberculosis
The emerging worldwide epidemic of drug-resistant tuberculosis is a byproduct of ineffective or poorly organized systems for TB control. It is therefore entirely a human-made problem. Modulation of gene expression, or dissemination of genes conferring drug resistance through transformation of stretches of DNA, do not occur in the TB bacteria as with other pathogens. Conversely, the natural selection of rare drug-resistant strains (for isoniazid–rifampin resistance, the mutation frequency is 1:1014) inside the human body occurs when inconsistent or interrupted treatment (health services and/or patient factors) is given, when the wrong drugs are prescribed for the wrong amount of time, when drugs of inferior quality are given, or when there is an interrupted supply of drugs. Strains are identified as MDR TB (multidrug-resistant TB) if they are resistant to at least isoniazid and rifampin, the two most important drug used to treat the disease. When susceptibility to these two core drugs is lost, more toxic and more expensive drugs must be used to treat the disease. While drug-susceptible TB is generally treatable, the regimen for MDR TB can take up to 2 years to complete and costs a TB program over 100 times more than treating a drug-susceptible case. Before the advent of a worldwide concerted effort to treat MDR TB, treatment success rates were 50% for new cases and 30% for retreatment cases in those who adhered to observed therapy instituted by a program (Espinal et al., 2000).
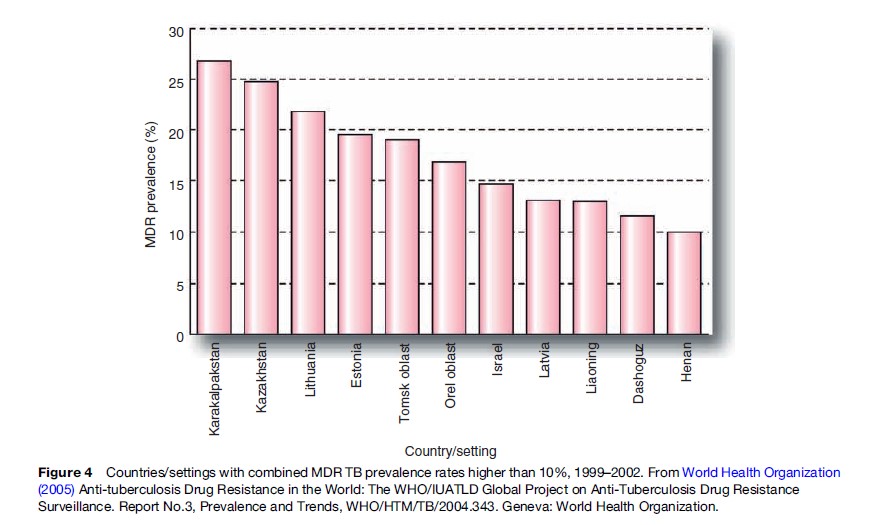
In 1994, the World Health Organization and International Union Against Tuberculosis and Lung Disease launched the Global Project on Antituberculosis Drug Resistance Surveillance. The project’s goal is to measure the prevalence and monitor the trend of antituberculosis drug resistance worldwide using standardized methodology (World Health Organization, 2003). As of 2007, three reports have been published, with the further report due out in late 2007. Over 90 countries have contributed data to the reports, although there is a great deal of missing data from sub-Saharan Africa. According to the last report, the prevalence of MDR TB varies greatly by region. Tuberculosis patients in Eastern Europe and Central Asia are ten times more likely to have MDR TB than patients in the rest of the world. China, Ecuador, Peru, Israel, and South Africa also have problems with increasing MDR TB rates, with the latter country burdened by more than 6000 cases per year. The highest rates of MDR TB occur in Estonia, Kazakhstan, Karakalpakstan (Uzbekistan), Tomsk Oblast in Russia, Lithuania, and Israel. Overall, MDR TB among isolates from new cases ranges from 0% to 14%, with a median of 1%. The median prevalence among previously treated cases was 7% (World Health Organization, 2005). Recent WHO modeling of the incidence of MDR TB estimated that 424 203 (95% CI 376 019–620 061) new cases occurred in 2004. China, India, and the Russian Federation accounted for 62% of the estimated global burden of MDR TB, roughly 261 362 cases (Zignol et al., 2006). Figure 4 shows the countries or settings with the highest rates of MDR TB among new and previously treated cases of TB between 1999 and 2002.
In response to the MDR TB problem, WHO and partners formulated the DOTS-Plus Strategy in 1998, which provides for the treatment of patients with MDR TB using more effective second-line medications. Given the expense of these drugs, the Stop TB Partnership set up the Green Light Committee to allow access to concessionally priced second-line medications through a bulk-purchasing mechanism for those programs that could demonstrate an effective DOTS strategy. Beginning at five pilot projects, the DOTS-Plus strategy grew to include over 50 sites. Treatment success rates only approach 65–70% in the best of these programs (Leimane et al., 2005). International guidelines for the treatment of drug-resistant TB have been published (World Health Organization, 2006). The problem of default from therapy, however, continues to plague the treatment of MDR TB patients. DOTS-Plus and the treatment of MDR TB patients has now been rolled into the global WHO Stop TB Strategy.
In addition, HIV may also be contributing to increases in MDR TB prevalence among TB patients. HIV infection has been associated with many outbreaks of MDR TB (mainly due to a lack of infection control), as well as acquired rifamycin resistance. In the continent with the highest rates of HIV infection, the only country with trend data on anti-TB drug resistance is Botswana, a country with one of the highest TB incidence rates worldwide at approximately 600 TB cases per 100 000 population. Three national drug resistance surveys conducted from 1995 to 2002 reported statistically significant increases in resistance to any anti-TB drug, to isoniazid, and to streptomycin among new TB cases without a history of prior TB treatment (Nelson et al., 2005). The prevalence of MDR-TB among new cases increased from 0.2% to 0.8%, although this was not statistically significant. Given the challenges in Botswana and other sub-Saharan African countries, such as relatively high treatment default rates and uncontrolled use of second-line drugs for MDR TB, the possibility exists for further increases in MDR TB.
To make matters worse, extensively drug-resistant tuberculosis (XDR TB) was described in 2005–2006 following a joint survey by WHO and CDC (Centers for Disease Control and Prevention, 2006a). XDR TB is currently defined as MDR TB that is also resistant to any fluoroquinolone and one of the three second-line injectable drugs (amikacin, kanamycin, or capreomycin) (Centers for Disease Control and Prevention, 2006b). The most recent reports have noted XDR TB in all areas of the world, but most commonly in Asia and Eastern Europe. The definition was created to put a name to a face of a problem that had been described by clinicians who reported encountering patients with difficult-to-treat highly resistant TB. Even the best estimates of successful treatment are that only 25–35% of XDR TB cases will be cured (Holtz et al., 2005). In 2006, investigators from South Africa reported the occurrence of highly resistant TB in HIV-infected persons who were enrolled in antiretroviral treatment programs, but whose case-fatality was alarmingly high (98%) despite adequate response to drugs (Gandhi et al., 2006). A large proportion of the cases had never had TB before, and several were health-care workers, raising the question about nosocomial spread and insufficient infection control practices in that region. The specter of virtually untreatable strains of TB being propagated throughout sub-Saharan Africa threatens to undo decades of TB control work in the region where TB is already on the rise.
Economic Burden Of TB
Tuberculosis remains a prominent disease not only because of the number of people affected, or the human rights concerns about the neglected global response despite its treatability, but also because it strikes down adults in the prime of their productive lives. More than 80% of the burden of TB, as measured by disability- adjusted life years lost, is due to premature death rather than illness or disability (World Health Organization, 2000). Tuberculosis therefore extracts an enormous economic burden on developing countries. These costs fall into two categories: (1) Direct costs to the health services and to the patient and patient’s family and (2) indirect costs to society, community, and the patient’s family through lost production (World Health Organization, 2000). Tuberculosis patients and their families pay the cost of TB through their suffering, pain, and grief. A protracted illness can also cause psychological and social stress and costs. There is a great deal of stigma that remains surrounding TB, as well as with TB/HIV, and many patients may be rejected by society, their job, or even their own family. In some societies, TB survivors are seen as unmarriageable or unfit for work. Discrimination in the health sector and in society can lead to mental health stress and reduction in quality of life.
The largest indirect cost to the patient is lost income through debilitation and sickness. Most patients are too sick to work for 3–4 months during their illness, and up to 8–10 months if they have MDR TB, resulting in the loss of a significant portion of their annual income. If the patient dies, there is the further loss of 15–20 years of income depending on the age of the patient. The substantial nontreatment costs borne by patients and families of patients are often greater than the costs of treatment by the health sector. Households develop strategies for dealing with actual losses and potential losses. Selling assets is a common strategy, which is not advantageous in the long term for patients or families (Maher et al., 2006). Reducing food intake, withholding school fees, or delaying marriages of children can all have important long-term consequences for families.
Conclusion
Tuberculosis is an age-old disease than continues to afflict the poor and disadvantaged populations around the globe with alarmingly high mortality rates. Due to poor attention to global TB control, the annual incidence of TB continues to rise at 1% per year, although recent gains have been made in fighting the disease where it kills the most people, such as in India and China. HIV and drug-resistant TB present challenges to the global public health community that will not be easily overcome. TB will continue to be with us for our lifetime. With the advent of more rapid diagnostic techniques and more effective drugs against drug-susceptible TB and drug-resistant TB, we will hopefully see an interruption in the transmission of TB and a reversal in the global incidence in the twenty-first century to correct the political, social, and public health missteps of the twentieth century.
Bibliography:
- Binkin N, Vernon AA, Simone PM, et al. (1999) Tuberculosis prevention and control activities in the United States: An overview of the organization of tuberculosis services. International Journal of Tuberculosis and Lung Disease 3(8): 663–674.
- Centers for Disease Control and Prevention (2006a) Emergence of Mycobacterium tuberculosis with extensive resistance to second-line drugs – Worldwide, 2000–2004. Morbid Mortal Weekly Report 55(11): 301–305.
- Centers for Disease Control and Prevention (2006b) Notice to readers: Revised definition of XDR-TB. Morbid Mortal Weekly Report 55(43): 1176.
- Corbett EL, Watt CJ, Walker N, et al. (2003) The growing burden of tuberculosis: Global trends and interactions with the HIV epidemic. Archives of Internal Medicine 163: 1009–1021.
- De Jong BC, Israelski DM, Corbett EL, and Small PM (2004) Clinical management of TB in the context of HIV infection. Annual Review of Medicine 55: 283–301.
- Dye C (2006) Global epidemiology of tuberculosis. Lancet 367: 938–940.
- Espinal MA, Kim SJ, Suarez PG, et al. (2000) Standard short-course chemotherapy for drug-resistant tuberculosis: Treatment outcomes in 6 countries. Journal of the American Medical Association 283(19): 2537–2545.
- Frieden TR, Fujiwara PI, Washko RM, and Hamburg MA (1995) Tuberculosis in New York City – Turning the tide. New England Journal of Medicine 333(4): 229–233.
- Gandhi NR, Moll A, Sturm AW, et al. (2006) Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet 368 (9547): 1554–1556.
- Holtz TH, Riekstina V, Zarovska E, Laserson KF, Wells CD, and Leimane V (2005) XDR-TB: Extreme drug-resistance and treatment outcome under DOTS-Plus, Latvia, 2000–2002. International Journal of Tuberculosis and Lung Disease 9(supplement 1): S258.
- Kim JY, Shakow A, Mate K, Vanderwarker C, Gupta R, and Farmer P (2005) Limited good and limited vision: Multidrug-resistant tuberculosis and global health policy. Social Science and Medicine 61: 847–859.
- Leimane V, Riekstina V, Holtz TH, et al. (2005) Clinical outcome of individualised treatment of multidrug-resistant tuberculosis in Latvia: A retrospective cohort study. Lancet 365: 318–326.
- Maher D and Raviglione MC (2006) Tuberculosis – a WHO perspective. In: Schlossberg D (ed.) Tuberculosis and Nontuberculous Mycobacterial Infections, 5th edn., pp. 133–146. New York: McGraw-Hill Medical.
- Nelson LJ, Talbot EA, Mwasekaga MJ, et al. (2005) Antituberculosis drug resistance and anonymous HIV surveillance in tuberculosis patients in Botswana, 2002. Lancet 366: 488–490.
- Nerlich AG, Haas CJ, Zink A, Szeimies U, and Hagedorn HG (1997) Molecular evidence for tuberculosis in an ancient Egyptian mummy. Lancet 350: 1404.
- Raviglione MC and Uplekar MW (2006) WHO’s new stop TB strategy. Lancet 367: 952–955.
- Ryan F (1992) Tuberculosis: The Greatest Story Never Told. Worcestershire, UK: Swift Publishers.
- Stead WW, Eisenach KD, Cave MD, et al. (1995) When did Mycobaterium tuberculosis infection first occur in the New World? An important question with public health implications. American Journal of Critical Care and Medicine 151: 1267–1268.
- World Health Organization (2000) The Economic Impacts of Tuberculosis. The Stop TB Initiative 2000 Series, (WHO/CDS/STB/2000.5) Geneva, Switzerland: World Health Organization.
- World Health Organization (2001) Guidelines for Social Mobilization: A Human Rights Approach to Tuberculosis, WHO/CDS/STB/2001.9. Geneva, Switzerland: World Health Organization.
- World Health Organization (2002) Global tuberculosis control: Surveillance, planning, financing. Geneva, Switzerland: World Health Organization.
- World Health Organization (2003) Guidelines for Surveillance of Drug Resistance in Tuberculosis, WHO/CDS/CSR/RMD/2003.3. Geneva, Switzerland: World Health Organization.
- World Health Organization (2005) Anti-tuberculosis Drug Resistance in the World: The WHO/IUATLD Global Project on Anti-Tuberculosis Drug Resistance Surveillance. Report No.3, Prevalence and Trends, WHO/HTM/TB/2004.343. Geneva, Switzerland: World Health Organization.
- World Health Organization (2006) Guidelines for the Programmatic Management of Drug-Resistant Tuberculosis. WHO/HTM/TB/2006.361. Geneva, Switzerland: World Health Organization.
- World Health Organization (2007) Global Tuberculosis Control: Surveillance, Planning, Financing. Geneva, Switzerland: World Health Organization.
- Zignol M, Hosseini MS, Wright A, et al. (2006) Global incidence of multidrug-resistant tuberculosis. Journal of Infectious Diseases 194: 479–485.
- http://www.who.int/tb/hiv/en/ – World Health Organization TB/HIV website.
- http://www.who.int/tb/en/ – World Health Organization tuberculosis website.
- http://www.who.int/tb/xdr/ – World Health Organization, XDR-TB: Extensively drug-resistant tuberculosis.
See also:
Free research papers are not written to satisfy your specific instructions. You can use our professional writing services to buy a custom research paper on any topic and get your high quality paper at affordable price.








