This sample Vitamin A Deficiency and Its Prevention Research Paper is published for educational and informational purposes only. If you need help writing your assignment, please use our research paper writing service and buy a paper on any topic at affordable price. Also check our tips on how to write a research paper, see the lists of health research paper topics, and browse research paper examples.
Micronutrient Deficiencies
Micronutrient deficiencies – most notably vitamin A, zinc, iodine, and iron deficiencies – are a major public health problem globally, with low-income countries in Africa and Asia carrying the highest burden of disease. Young children and pregnant and breastfeeding women are the main groups affected, because their relative requirements for micronutrients are higher and thus the impact of deficiency is more severe than in other population subgroups. Micronutrient deficiencies have also been described as hidden malnutrition, as subnormal levels of certain micronutrients can increase general morbidity and mortality without any obvious clinical symptoms or signs to suggest deficiency. This (and that micronutrient deficiencies are mainly a public health problem in resource poor countries) explains why exact data on the prevalence and impact of micronutrient deficiencies are unavailable in many settings. However, it has been estimated that more than 2 billion people worldwide are affected and that many of them suffer from multiple micronutrient deficiencies (WHO/WFP/UNICEF, 2006).
Up to 250 million preschool-age children and most likely a substantial proportion of pregnant women are estimated to suffer from vitamin A deficiency (VAD) (WHO/FAO, 2004). Vitamin A (retinol) is essential for visual function, growth and development, maintenance of epithelial function, reproduction, and the immune system. Corneal scarring due to vitamin A deficiency is still the main cause of preventable childhood blindness in poor populations, mainly affecting children under 3 years of age. VAD also increases morbidity and mortality in children up to at least 6 years of age. School-age children may also be affected, but there is insufficient information on the prevalence and impact of VAD in older children.
Vitamin A Metabolism
Vitamin A is a fat-soluble vitamin (WHO/FAO, 2004). Vitamin A is either ingested as retinyl esters of fatty acids from animal sources or as provitamin A carotenoids from vegetable sources. In the digestive tract, they are freed from the food matrices that they are bound into. This is more effective for animal products than for vegetables and fruits. They are then solubilized for absorption, before passing into the intestinal mucosal cells. Products of fat digestion and bile secretion, such as cholesterol, phospholipids, bile salts, and so on, are important for the effective solubilization of retinol and carotenoids. Absorption is reduced if dietary fat intake is low (less than 5–10 g daily) or in the presence of digestive tract disorders, such as frequent gastroenteritis, which interfere with normal food absorption. Retinol and carotenoids are incorporated into chylomicrons together with other lipids and delivered into the bloodstream by way of the lymphatic system. Some carotenoids are converted into retinol in the intestinal mucosal cells. Ninety percent of retinol is stored in fat-storing liver cells until used, whereas carotenoids are stored in fatty tissues. If retinol stores are low, these carotenoids can be used after conversion to retinol in the liver. Retinol is transported from the liver to target tissues bound to retinol-binding protein (RBP), the mobilizing protein. The retinol–RBP complex (holo-RBP) connects in the blood with transthyretin, a larger hepatically synthesized protein, which prevents holo-RBP from being excreted by the kidneys. Disorders or dietary restrictions that impair hepatic synthesis of mobilizing and transporting proteins, as well as diseases that increase renal loss of larger proteins (e.g., fever) interfere with a normal vitamin A status.
The amount of provitamin A in a mixed diet that has the same vitamin A activity as 1mg of retinol is called its bioefficacy. The U.S. Institute of Medicine (IOM) estimated that 2 mg of b-carotene in oil or 12 mg of b-carotene in mixed foods has the same vitamin A activity as 1 mg of retinol (U.S. IOM, 2000). However, bioefficacy varies with the vegetable and fruit content of the diet and field studies suggest that in some populations the bioefficacy of b-carotene is even lower, requiring 21 mg of b-carotene to reach the same vitamin A activity as 1 mg of retinol (West et al., 2002).
Vitamin A Deficiency And The Eye
Ocular signs and symptoms, also known as xerophthalmia or clinical disease, are the only specific clinical indicators of VAD (WHO, 1996). However, their prevalence alone will greatly underestimate the overall prevalence of VAD, as nonspecific systemic effects of VAD frequently occur without the presence of xerophthalmia (sometimes called subclinical disease).
Night blindness (XN), due to lack of the visual pigment rhodopsin that contains a metabolite of vitamin A, is the most common and earliest ocular symptom of VAD, even though ocular signs of VAD such as Bitot’s spots may occur in the absence of XN. XN is defined as the inability to see well at low levels of light.
Bitot’s spots (X1B) are cheesy or foamy patches of desquamated, keratinized epithelium on the conjunctiva. They are usually, but not always, found on the temporal conjunctiva, and are usually present in both rather than one eye only. Persistent, unresolved Bitot’s spots may be present in older children and adults with normal vitamin A status, but unlike Bitot’s spots in VAdeficient children these do not respond to vitamin A supplementation. Bitot’s spots may be preceded by conjunctival xerosis (X1A), a keratinizing metaplasia of the conjunctival epithelium, but this sign is rather subjective and unreliable.
Corneal xerosis (X2) is dryness of the inferior cornea ( peau d’orange appearance) which, if untreated, can go on to affect the whole cornea in some cases, leading to corneal ulceration (X3) and keratomalacia, a full-thickness necrosis of the cornea. If treated with oral vitamin A, X2 is fully reversible, whereas X3 is not. X3 is usually graded by the proportion of cornea affected, with X3A involving less than one-third of the cornea and X3B involving at least one-third. X3B often results in corneal perforation and irreversible blindness. X3s are rare even in a population with a VAD problem of public health importance, and occur most commonly in association with measles. The case-fatality ratio of affected children has been estimated at 50–90%. Corneal ulcers result in corneal scarring (XS) with the extent, type, and position of the scar determining the child’s remaining vision.
Ocular signs and symptoms are often associated with chronic VAD. They are important indicators of the prevalence of VAD in a population.
Vitamin A Deficiency And Morbidity/ Mortality
A series of randomized, controlled trials conducted in the 1980s and 1990s showed that improving the VA status of young children aged 6 months to 6 years decreased their mortality by an average of 20–30% (Beaton et al., 1993). Also, vitamin A supplementation decreased the incidence of severe infectious diseases and hospital admissions with the possible exception of respiratory tract infections. The converse is also true with infectious diseases – especially diarrheal disease and measles–associated with deterioration in VA status due to a higher demand and lower intake and/or absorption of VA. It is thought that protein-energy (macronutrient) malnutrition impairs VA status even further, possibly due to a shortage of transport proteins. However, because protein-energy malnutrition without vitamin A and other micronutrient deficiencies is rare, the importance of this has not been conclusively proven (WHO/FAO, 2004).
VAD or VA excess in the first trimester can lead to congenital anomalies. VAD may interfere with growth, but its impact is difficult to estimate, as VAD usually occurs together with other types of nutrient deficiencies. Combining VA with iron supplementation improves hemoglobin formation in anemic children. VAD may increase maternal third trimester and postpartum morbidity and mortality.
Indicators Of Vitamin A Deficiency
Technically, VAD has been defined as a concentration of retinol in liver stores of <0.07 mmol/g. However, since it is impractical and unethical to measure liver retinol concentrations directly, except in very exceptional circumstances, surrogate measures are used to determine the prevalence of VAD in a population (WHO, 1996). The most commonly used biological indicators are clinical/ functional or biochemical measures, as summarized in Table 1.
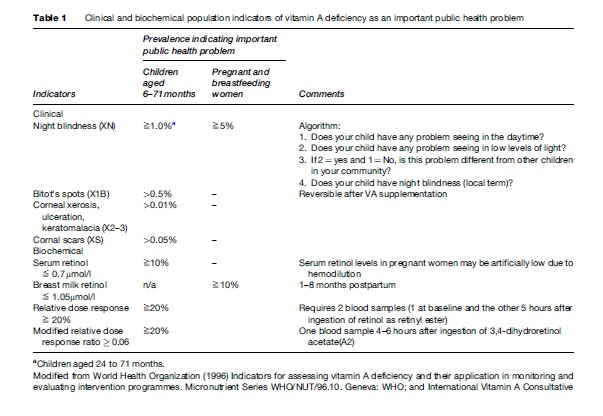
Information on night blindness is easily obtained either through proxy reports in children aged 24–71 months or directly from women who have had a live-born child within the past 3 years, using a standard algorithm (Table 1). The validity of night blindness reports varies in different populations, although it is believed to be valid in populations that use a specific, local term for night blindness (when assessing data, the specific local term for night blindness should be used). Night blindness usually responds to VA supplementation within 1 to 2 days. Ocular signs of VAD are uncommon in most populations and therefore require large sample sizes to establish their prevalence with reasonable precision.
Biochemical measures of VA status require smaller sample sizes due to the higher prevalence of biochemical VAD (Table 1). There are several biochemical measures that are practicable in field settings (WHO, 1996), even though all of them are logistically more demanding and expensive than ocular indicators due to the need for appropriately collecting, transporting, and storing samples and for access to good laboratory facilities for analysis. There is no consistent correlation between the thresholds used for biochemical measures (Table 1) and adverse health effects, except for corneal disease in severely deficient children with serum retinol concentrations below 0.35 mmol/l.
The most commonly used biochemical measure for the assessment of VAD in populations is serum retinol, usually measured by high-pressure liquid chromatography (HPLC). However, serum retinol concentration cannot be used to diagnose VAD in individuals, because the serum retinol concentration is normally under effective homeostatic regulation and so only reflects body stores if these are very high or very low. Serum retinol is a negative acute phase reactant, so its concentration can be reduced substantially by acute infections.
The relative dose–response (RDR) test, and its logistically simpler variant the modified RDR (mRDR), are indirect measures of liver stores of vitamin A, which depend on the principle that a higher proportion of a large dose of retinol will be taken into the liver the lower the liver stores of vitamin A. In the mRDR, for example, a large dose of a variant of retinol found in fish (often known as A2), which is handled by the body in the same way as human retinol (A1), is given to the subject at time zero. A serum specimen is taken 4–6 hours later and the serum concentration of A2 compared with that of human retinol A1. Although much more time consuming than simply measuring the serum retinol concentration, the mRDR is increasingly used in research studies alongside serum retinol concentration because it is more stable in the presence of infections, and more closely reflects the individual’s underlying vitamin A status.
Demographic and ecological factors such as infant and childhood mortality, children’s anthropometric status, and breastfeeding and dietary patterns, have recently been proposed as a simple and cheap way to identify areas and populations at increased risk of VAD (WHO, 1996). Populations with under-5 mortality rates (U5MR) of more than 50 in 1000 have invariably been shown to have VAD of public health importance (IVACG, 2002). Furthermore, if the U5MR is between 20 and 50 in 1000, VAD is possible, but must be confirmed using biological indicators before interventions are introduced. A prevalence above the threshold for two biological measures, or for one biological measure and several demographic/ecological factors, is usually required to define a population as VA deficient.
Prevention Of Vitamin A Deficiency
Improving the vitamin A status of VA-deficient populations has far-reaching consequences. Not only will it usually lead to a reduction of the overall mortality by about 25%, deaths from measles by 50% and from diarrhea by 40% (Beaton et al., 1993), but reducing the severity of disease will also reduce the strain on inpatient and outpatient services and will improve the quality of life of children and their families (IVACG, 2002). Furthermore, improving the vitamin A status of women is likely to decrease the prevalence of genetic anomalies, and to reduce the prevalence of anemia (through vitamin A/iron/hemoglobin interactions), and improve maternal health and pregnancy outcomes.
In 2002, the UN Special Session on Children set a global target for the elimination of VAD as a problem of public health importance by the year 2010 (UN, 2002). This is to be achieved through three major strategies: (1) VA supplementation, (2) food fortification, and (3) improved dietary diversity and quantity of VA intake (including the promotion of breastfeeding).
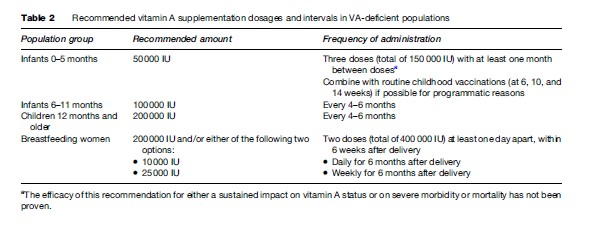
- VA supplementation: VA supplementation is the delivery of VA as capsules or tablets. It is highly effective both for preventing and for treating VAD. Prophylactic VA supplementation is one of the most cost-effective health interventions currently available to public health (World Bank, 1993). Recommended VA supplementation dosages and intervals are listed in Table 2. Supplementation should ideally be used alongside food fortification (where feasible) and strategies to increase the dietary intake of vitamin A. VA supplementation has been successfully added to childhood immunization programs (Expanded Program on Immunization (EPI), National Immunization Days). Prophylactic high-dose VA supplementation has been shown to be safe in all age and sex groups except pregnant women, and is recommended in children and breastfeeding women during the safe, nonfertile period up to 6 weeks after delivery. A lower dose regimen of not more than 10 000 IU daily or 25 000 IU weekly can be used for pregnant women or women in the fertile age group, as there is an increased risk of teratogenesis with higher doses especially early in the pregnancy (WHO, 1997). Long-term consumption of large amounts of VA can damage the liver, cause bone anomalies, joint pains, alopecia, headaches, vomiting, and skin desquamation. However, at recommended doses side effects are relatively uncommon and are mild (e.g., transient nausea in infants or children, bulging fontanel in infants).
- Food fortification: Commonly used food products, such as sugar, wheat flour, monosodium glutamate (MSG) (a food flavoring that is widely used especially in Asia), and margarine, can be enriched with vitamin A. A successful fortification program requires, among other things, a suitable food ‘vehicle’ that is centrally processed in a limited number of factories and then widely consumed (even by the poor) on a regular basis in amounts that do not vary too much between individuals (WHO, 1996).
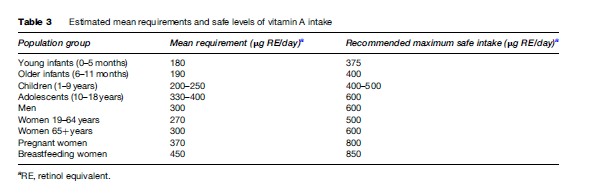
- Increased dietary intake from natural (i.e., unfortified) VA-rich foods: The aim of this strategy is to achieve a continually adequate intake of VA according to recommended dietary intake (Table 3). Approaches to achieve this require intersectoral action aimed at increasing the production of affordable VA-rich foods and encouraging their consumption. Such approaches may also reduce poverty by providing a new source of income, other dietary improvements, and an opportunity for other health promotion and education. However, these approaches require time and sustained efforts to achieve measurable effects. Partly for these reasons, there are few dietary improvement (often called dietary diversification) programs whose cost-effectiveness has been rigorously evaluated.
VA supplementation, with its ability to achieve an immediate impact, coupled with the fact that it can be delivered through one sector (health) and can be coupled to other public health interventions such as mass immunization days, has been by far the most widely implemented intervention for the prevention of VAD in low and middle-income countries. Promoting exclusive breastfeeding for the first 6 months of life is an important dietary measure to improve VA status in infants.
Other public health measures such as immunization against measles, improvements in water supply and hygiene to prevent diarrheal disease, and regular deworming will also have direct and indirect effects on the prevalence and consequences of VAD in a population.
Vitamin A supplementation appears to be proportionately as effective for mortality prevention among HIVinfected infants and children as for uninfected, and should form an adjunct to highly active antiretroviral therapy (HAART). In other words, all HIV-infected infants and children in VAD populations should be supplemented with 4–6 monthly high doses of vitamin A, alongside their uninfected peers. However, although initial observational studies showed a strong association between VAD and both HIV disease progression in adults of both sexes and of mother-to-child transmission (MTCT) of HIV, these associations are not likely to be causal (or may be due to reverse causality), as more recent randomized, controlled trials of vitamin A supplementation to slow progression or MTCT have been disappointing.
Emergencies such as wars and natural disasters often precipitate or greatly exacerbate an epidemic of VAD due to interrupted food supplies and an increase in infectious diseases (WHO/WFP/UNICEF, 2006). Distribution of appropriately fortified foods (e.g., VA-enriched dried skimmed milk) is recommended, but women and young children need additional vitamin and mineral supplements, including vitamin A.
Treatment Of Vitamin A Deficiency
Children with xerophthalmia, measles, or severe proteinenergy malnutrition should be treated with high-dose vitamin A (WHO/UNICEF/IVACG, 1997). Children should receive 50 000 IU if they are under 6 months, 100 000 IU if they are 6–11 months, and 200 000 IU if they are 12 months or order. For xerophthalmia, this dosage is recommended three times (on days 1, 2, and 14–30), for measles twice (on successive days), and for severe protein-energy malnutrition once. Although extremely uncommon except in famine situations, adolescents or adults with severe xerophthalmia (i.e., X2 or X3) should receive three doses of vitamin A (days 1, 2, and 14–30). This even applies to women, in whom the increased risk of teratogenesis in the fetus if the woman is in the early stages of pregnancy is far outweighed by the substantial risk of blindness in the mother.
Conclusion
VAD is one of the most prevalent – and yet most easily and cost-effectively prevented – public health problems in low-income countries and a substantial burden of disease and mortality in children in many middle-income countries as well. It is a scandal that this remains the case more than a decade after it was conclusively shown in meta-analyses of a substantial number of large-scale, well-conducted randomized, controlled trials that vitamin A supplementation of young children in VAD populations is one of the most cost-effective interventions available to public health. Although it is very unlikely that the United Nations target of the elimination of VAD as a problem of public health importance by 2010 will be reached by that date, all public health practitioners and policy makers should be committed to ensuring that this target is achieved within the decade after that.
Bibliography:
- Beaton GH, Martorell R, L’Abbe´ KA, Edmonston B, McCabe G, Ross AC, and Harvey B (1993) Effectiveness of vitamin A supplementation in the control of young child morbidity and mortality in developing countries. Geneva, Switzerland: ACC/SCN of the United Nations, ACC/SCN state-of-the-art series, Nutrition Policy Discussion Paper, No 13.
- International Vitamin A Consultative Group (IVACG) (2002) The Annecy Accords to Assess and Control Vitamin A Deficiency. Summary of Recommendations and Clarifications. Washington DC: IVACG.
- United Nations (2002) A World Fit for Children. Resolution Adopted by the General Assembly 27th Special Session, (A/Res/S-27/2). New York: United Nations.
- S. Institute of Medicine, Food and Nutrition Board, Standing Committee on the Scientific Evaluation of Dietary Reference Intakes (2000) Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington, DC: National Academy Press.
- West CE, Eilander A, and van Lieshout M (2002) Consequences of revised estimates of carotenoid bioefficacy for dietary control of vitamin A deficiency in developing countries. The Journal of Nutrition 132(supplement 9): 2920S–2926S.
- World Bank (1993) Investing in Health: The 1993 World Development Report. New York: Oxford University Press and World Bank.
- World Health Organization (WHO) (1996) Indicators for Assessing Vitamin A Deficiency and their Application in Monitoring and Evaluating Intervention Programmes. Micronutrient Series WHO/ NUT/96.10. Geneva, Switzerland: WHO.
- World Health Organization (1997) Safe Vitamin A Dosage During Pregnancy and Lactation. Geneva, Switzerland: WHO.
- World Health Organization and Food and Agriculture Organization of the United Nations (FAO) (2004) Vitamin and Mineral Requirements in Human Nutrition: Report of a joint FAO/WHO expert consultation, Bangkok, Thailand, 1998. Geneva, Switzerland: WHO and FAO.
- World Health Organization, UNICEF, IVACG Task Force (1997) Vitamin A supplements. A guide to their use in the treatment and prevention of vitamin A deficiency and xerophthalmia. Geneva, Switzerland: WHO.
- World Health Organization, World Food Programme, UNICEF (2006) Preventing and controlling micronutrient deficiencies in populations affected by an emergency. Joint statement by the World Health Organization, the World Food Programme, and the United Nations Children’s Fund. Geneva, Switzerland: WHO.
- Christian P (2002) Maternal Night Blindness: A New Indicator of Vitamin A Deficiency. Washington DC: International Vitamin A Consultative Group (IVACG).
- Ross AC (1992) Vitamin A status: Relationship to immunity and the antibody response. Proceedings of the Society for Experimental Biology and Medicine 200: 303–320.
- Sommer A (2005) Innocenti Micronutrient Research Report #1. Report of First Meeting on Micronutrients and Health. Florence, Italy: UNICEF Innocenti Research Center.
- Sommer A and Davidson FR (eds.) (2002) Assessment and control of vitamin A deficiency: The Annecy Accords. The Journal of Nutrition 132(supplement 9): 2843S–2990S.
- Sommer A and West KP (1996) Vitamin A Deficiency: Health, Survival and Vision. New York: Oxford University Press.
- http://www.a2zproject.org – A2Z, The USAID Micronutrient and Child Blindness Project.
- http://www.micronutrientforum.org – Micronutrient Forum, ILSI Research Foundation (Previously International Vitamin A Consultative Group (IVACG) and International Nutritional Nutritional Anemia Consultative Group (INACG)).
- http://www.micronutrient.org – The Micronutrient Initiative.
- http://www.sightandlife.org – Sight and Life.
- http://www.unicef.org – United Nations Children’s Fund (UNICEF).
- http://www.who.int – World Health Organization (WHO).
See also:
Free research papers are not written to satisfy your specific instructions. You can use our professional writing services to buy a custom research paper on any topic and get your high quality paper at affordable price.








