This sample Cryptosporidiosis, Giardiasis, and Other Intestinal Protozoan Diseases Research Paper is published for educational and informational purposes only. If you need help writing your assignment, please use our research paper writing service and buy a paper on any topic at affordable price. Also check our tips on how to write a research paper, see the lists of health research paper topics, and browse research paper examples.
Introduction
Diarrhea is a very common illness, especially in the developing world, and is frequently experienced by travelers. Cryptosporidium, Cyclospora, Isospora, Giardia, amoeba, and Sarcocystis are pathogenic protozoan parasites that can cause these gastrointestinal illnesses. Commensal parasites are also relatively common in developing countries and less frequently identified in the developed world. Worldwide, Giardia is the most common protozoan infection in the gastrointestinal tract of humans. It was probably first seen by Anton van Leuwenhoek in the late seventeenth century. In Tennessee, Giardia cysts have been identified in human feces from about 600 BC. Cryptosporidium and Giardia have also been reported from samples 500 to 3000 years old from the Andean regions in Peru, and from 4300to 1100-year-old samples from the coastal regions of Peru. Cryptosporidium became much more relevant to public health in the early 1980s with the emergence of the AIDS epidemic. Opportunistic and emerging parasitic infections also include Isospora, particularly in HIV and AIDS patients. Cyclospora has been observed in certain regions of the developing world; however, globalization of the food supply and increase in international travel have revealed that parasitic infections can also cause epidemics in the developed world.
Intestinal Coccidia
Cryptosporidiosis
Gastrointestinal cryptosporidiosis is characterized by profuse watery diarrhea, which is more severe in children, the elderly, and individuals with compromised immunity.
Although Cryptosporidium has been identified primarily in the gastrointestinal tract as a cause of diarrheal illness in humans, AIDS patients have also been reported with infections of the gallbladder and the respiratory tract. In immunocompetent individuals, diarrhea lasts, on average, 15 days; however, oocyst shedding in the feces can continue for more than 30 days. In immunocompromised patients, diarrhea is profuse and can last for months. Interferon gamma seems to play a role in controlling the infection.
The clinical presentation of cryptosporidiosis has been variable, and in the past, infection was considered to be caused by a unique species, Cryptosporidium parvum; this was later differentiated as genotype I (or human genotype) and II (or bovine genotype). Genotype I was later renamed C. hominis, and C. parvum (genotype II) is still considered the zoonotic group and consists of various genotypes.
Of the 15 species of Cryptosporidium described as infecting reptiles, fish, birds, and mammals, eight can also infect humans: C. hominis (of humans), C. parvum (of cattle), C. canis (of dogs), C. felis (of cats), C. meleagridis (of turkeys), C. muris (of rodents), C. suis (of pigs), and Cryptosporidium cervine genotype. Most of these are morphologically similar (Table 1); therefore, molecular assays are needed to differentiate between them.
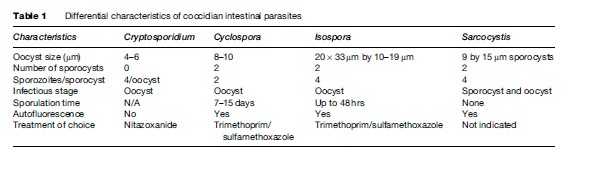
Oocysts are excreted in the feces of an infected individual or, in the case of immunocompromised persons, the sputum. These oocysts are already infectious and can infect susceptible individuals. When ingested or inhaled, the oocysts undergo a process of excystation, in which four sporozoites per oocyst are released. These sporozoites invade the epithelial cells of the gastrointestinal (preferentially ileum) or respiratory epithelial cells. Within the host cell, Cryptosporidium undergoes a series of asexual multiplication producing meronts: type I (8 merozoites) and type II (4 merozoites). Asexual multiplication can continue, or organisms can differentiate to the sexual forms of the parasite. The zygote is formed after the microgametocyte fertilizes the macrogametocyte. The zygote will mature and differentiate to either thin-walled oocysts (20%), which can continue the reinfection in the intestine, or thick-walled oocysts (80%), which are environmentally resistant, and therefore can survive in water or foods (Figure 1).
The localization of the Cryptosporidium parasitic vacuole is intracellular but extracytoplasmic. A feeding organelle is located between the parasite and the cytoplasmic membrane that allows selective transport of substances. This unique localization protects the parasite from the host’s cellular defense mechanisms and enables resistance to drug therapy. Cryptosporidium has three salvage enzymes for pyrimidines, which make it entirely dependent on the host. Two of these, uridine kinase-uracil phosphoribosyltransferase and thymidine kinase, are unique to C. parvum within the phylum Apicomplexa, and may account in part for the organism’s resistance to a broad range of drugs.
Many drugs have been evaluated against Cryptosporidium but only a handful have had some effectiveness. In the United States, the only FDA-approved drug for treatment of cryptosporidiosis is nitazoxanide (approved for use in children). When nitazoxanide (500 mg) is given twice daily for 3 days to children without HIV infection, a 96% clinical recovery is achieved, and 93% of patients stop shedding Cryptosporidium oocysts. Paromomycin has also been evaluated with conflicting results, particularly in AIDS patients. In HIV-infected persons, highly active antiretroviral treatment (HAART) is the most effective means of resolving Cryptosporidium infection as it aids in the replenishment of CD4þ cells. Relapse of the infection may occur if treatment is discontinued.
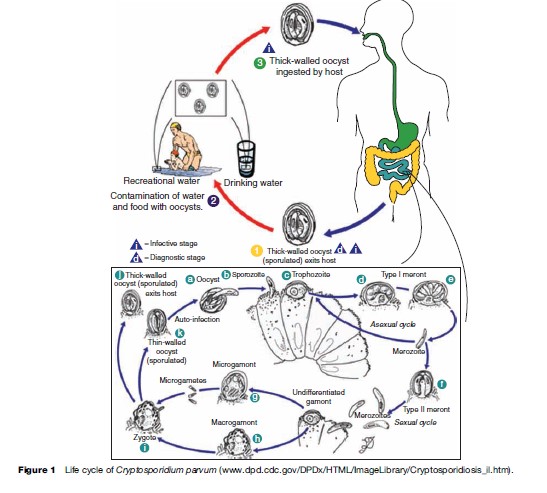
C. parvum oocysts measure 4 to 6 mm in diameter and can be identified by examining fecal or sputum samples using the modified acid-fast stain (Figure 2). Other antibody-based assays – including direct and indirect immunofluorescence, and enzyme immunoassays – are commercially available for use with clinical and environmental samples. Molecular assays are also used for diagnosis, genetic fingerprinting, and genotyping.
Although Cryptosporidium has been previously identified worldwide, the availability of molecular tools in recent years has enabled the determination of the prevalence of different species and genotypes in various parts of the world. Cryptosporidium has been isolated from various species of animals other than their natural host. Flies, rotifers, and free-living nematodes may serve as transport vectors of viable oocysts. Mussels, clams, and oysters can concentrate Cryptosporidium oocysts in their gills and hemolymph and have been isolated from shellfish worldwide. However, to date, outbreaks of cryptosporidiosis associated with consumption of shellfish have not been reported.
In parts of the developing world, where access to clean water and adequate sanitation are lacking, cryptosporidiosis is endemic, and young children are most severely affected. In the United States, investigations of foodborne outbreaks have implicated consumption of fresh vegetables and prepared foods (Table 2). Vegetables and produce eaten raw may be contaminated by irrigation water (containing Cryptosporidium oocysts) at the farm level, by water used to keep produce fresh and hydrated at the market level, or during food preparation at the consumer level. In other instances, manipulation of foods by food handlers with cryptosporidiosis or improper hygienic practices has initiated a foodborne outbreak.
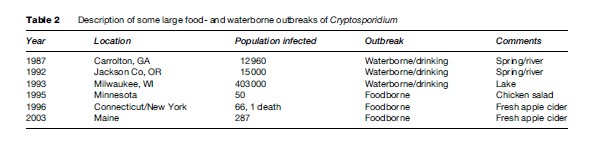
Waterborne transmission, either by drinking or recreational water, has proven to be the most frequent mode of transmission of this parasite. Cryptosporidium oocysts are highly resistant to chlorine at levels normally used to treat drinking water or chlorinated recreational water venues. In the United States, many Cryptosporidium waterborne outbreaks have been reported (see Table 2), and in recent years, swimming pools and other recreational water sites have been the source of infection. Outbreaks have occurred in swimming pools that were not properly maintained or treated regularly to inactivate potential contaminants. Much emphasis has been placed on preventing parasite transmission by way of tap or drinking water. The water industry has studied extensively procedures to adequately remove and inactivate Cryptosporidium oocysts. Regulations and methods to detect this parasite in water are available from the U.S. Environmental Protection Agency (see the section ‘Relevant Websites’).
Transmission may also occur via person-to-person contact (within households, among children in day care centers, between sexual partners) or by contact with animals excreting Cryptosporidium oocysts. Petting zoos have been implicated in Cryptosporidium outbreaks involving children who touched farm animals and did not properly clean their hands afterward.
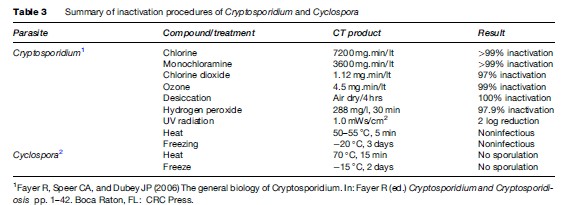
Cryptosporidiosis can be prevented by maintaining good hygienic practices, including washing hands after using toilets and before handling food. Special attention is needed for children and persons who contact animals. This is particularly important for food handlers. Hikers should avoid drinking river or lake water unless it is boiled, filtered (< 2 mm pore diameter), or, though more difficult, rendered safe by efficient chemical treatment. Water coagulation/flocculation, sedimentation, filtration, and disinfection can remove more than 99% of oocysts. UV, ozone, chlorine, and chlorine dioxide have been examined for inactivation efficiency in Cryptosporidium with variable results (Table 3).
Cyclosporiasis
Cyclospora cayetanensis was first reported in patients with acute diarrhea in 1979. At that time it was considered to be a coccidian-like or a cyanobacteria-like organism. In 1992, it was finally fully characterized and classified within the phylum Apicomplexa and coccidian. Unlike other coccidian parasites that infect humans, Cyclospora requires 7 to 15 days to fully sporulate and become infectious. Cyclosporiasis is characterized by persistent diarrhea, anorexia, nausea, abdominal pain, flatulence, and in some instances, fever.
As of today, Cyclospora cayetanensis is the only species described as infecting humans. Nonhuman primates also harbor other species of Cyclospora: C. cercopitheci, C. colobi, and C. papionis, which are morphologically similar to C. cayetanensis. These species seem to be also host-species specific. Molecular analysis of the 18S sRNA gene of C. cayetanensis and the three other Cyclospora species suggests that they are molecularly different, and that the genus Cyclospora is phylogenetically most closely related to the Eimeria species, although they are morphologically different. To date, none of the Cyclospora species have been propagated experimentally using animal models or human volunteers. Cyclospora oocysts measure 8–10 mm in diameter and stain variably using the modified acid-fast stain. Oocysts stain best when using a heated safranin stain protocol. Oocysts can be easily identified using phase contrast microscopy or by epifluorescence microscopy because the oocysts are autofluorescent. The last method is the most sensitive diagnostic assay in clinical specimens (see Figure 2).
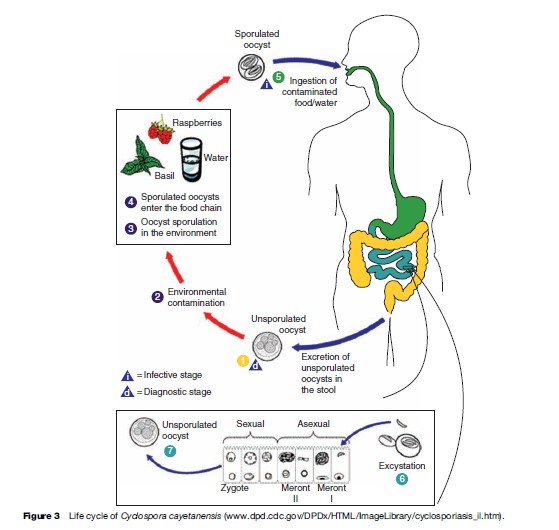
Unsporulated oocysts are excreted into the environment in the feces of infected individuals. After 7 to 15 days of incubation at 23 oC, the oocysts differentiate, and form two sporocysts per oocyst. Each of these sporocysts contains two sporozoites (see Figure 2).
Cyclospora infection is acquired when contaminated food or water containing oocysts are ingested. Oocysts excyst and sporozoites are released and infect the epithelial cells of the intestinal tract, particularly the terminal portion of the jejunum and ileum. Sporozoites multiply asexually producing type I and II meronts. Asexual multiplication may continue or sexual reproduction may initiate and produce oocysts that are excreted in the feces of infected individuals (Figure 3).
Trimethoprim/sulfamethoxazole (adults: 160–800 mg twice daily for 7 days; children: 5 mg/kg twice daily for 7 days) is effective for treatment of Cyclospora infection. Oocyst excretion and symptoms usually resolve 1 to 3 days post treatment. Ciprofloxacin has also been reported as an alternative treatment in sulfa-sensitive patients. Recurrence of infection in HIV-infected patients is more common and may require prolonged therapy.
The factors involved in parasite transmission have not been determined; however, food and waterborne transmission seem to be the main routes of transmission. In areas of endemnicity, Cyclospora presents a marked seasonality. The specific conditions that favor oocyst survival in these areas and seasons are unknown. For example, in Peru, cyclosporiasis is highly prevalent in areas near the Pacific Ocean coast (desert) during the warm season (December to May) when the ambient temperature is high and relative humidity is low. Cyclosporiasis is associated with ownership of domestic animals, especially birds, guinea pigs, and rabbits. In Nepal, the high incidence of Cyclospora in expatriates is consistent during the rainy season from April to June. In the moderate highlands of Guatemala, the prevalence of Cyclospora is between May and August and peaks in June when the seasonal rains are just beginning and the mean temperature is descending from its yearly high. Prevalence in these regions is high in children between 1.5 to 9 years old, suggesting that a specific immune response is developed and adults are less susceptible to this infection. Risk factors include drinking untreated water, ownership of fowl, and contact with soil.
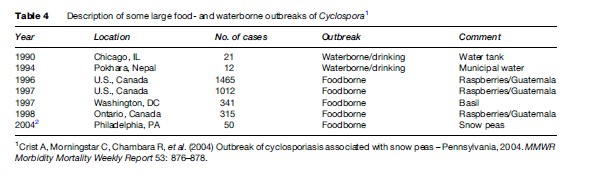
In the United States, cyclosporiasis has been associated with consumption of berries, basil, and lettuce imported from countries where Cyclospora is endemic (Table 4). Waterborne outbreaks have also been described. In 1990, staff from a Chicago hospital acquired Cyclospora. The epidemiological investigation revealed that tap water was the likely source of contamination. In Nepal, British soldiers acquired the infection by drinking municipal water that had acceptable levels of chlorine, indicating that Cyclospora oocysts are resistant to conventional chemical water treatment. Cyclospora can also be concentrated by shellfish and could be a potential source of contamination when these are ingested raw.
Cyclospora oocysts can be inactivated by heating and freezing temperatures (see Table 3). Appropriate water treatment and good farm practices may control the dissemination of this infection. Irrigation of produce with contaminated water containing Cyclospora oocysts is more likely the source of contamination; oocysts are highly resistant to environmental degradation. The time required for the oocysts to mature and become infectious makes person-to-person transmission unlikely.
Isosporiasis
Oocysts measure 20 33 mm by 10–19 mm. Oocysts stain well with the modified acid-fast stain. One or two sporonts may be observed in each oocyst. Each mature sporont has four sporozoites. This maturation process occurs in the environment and takes up to 48 hours (see Table 1). Isospora oocysts also autofluoresce; therefore this procedure is more sensitive than direct observation or staining because of the very thin oocyst wall (see Figure 2).
Development occurs in the epithelial cells of the distal duodenum and proximal jejunum. The asexual and sexual life cycle stages are similar to those seen in other coccidians. Chronic infections may persist for years. Symptoms include prolonged diarrhea (soft, foamy, and offensive smelling), weight loss, abdominal pain, and in some instances, fever. AIDS patients tend to have a more severe clinical presentation. AIDS patients not receiving the antibiotic trimethoprim/sulfamethoxazole (TMP-SMX) prophylaxis are more likely to present with isosporiasis and to experience relapses of infection. Extraintestinal isosporiasis (lymph nodes, liver, and spleen) has also been reported in AIDS patients.
The treatment of choice is TMP-SMX at 160–800 mg 4 times a day for 10 days and then 2 times a day for 3 weeks. Pyrimethamine and sulfadiazine are also effective, and other combinations of antimicrobials such as primaquine phosphate and nitrofurantoin or chloroquine phosphate have been used.
Isospora belli (row proposed to be in the genus Cystoisospora) is considered to be only infectious to humans and no other reservoirs have been identified. Transmission is waterborne and foodborne. Almost all reported cases have occurred in developing countries or among immigrants from or travelers to developing countries. Isospora is more frequently isolated in immunocompromised individuals. It has been reported in institutionalized care facilities where patients are housed for prolonged periods and with poor sanitary conditions. Prevention can be achieved by improvement in personal hygiene measures and sanitary conditions to eliminate fecal–oral transmission.
Sarcocystis
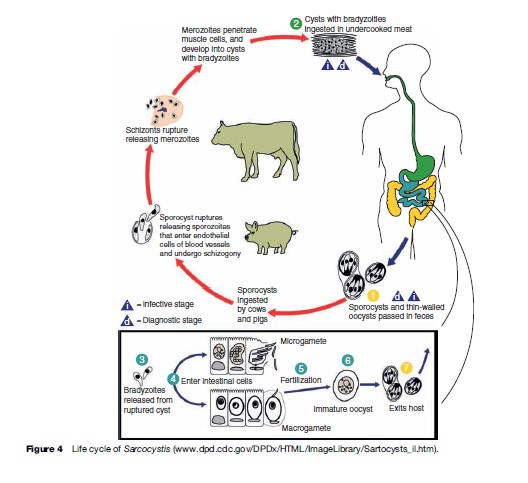
Parasites in the genus Sarcocystis are in the phylum Apicomplexa, class Sporozoea, subclass Coccidia, family Sarcocystidae. As such, they are most closely related to Toxoplasma, but have some affinity to other coccidia such as Cyclospora, Isospora, and Cryptosporidium. Until recently, a great deal of confusion existed concerning many very basic biological aspects of Sarcocystis, including, among others, the lifestyle, morphology, and natural host range. Their life cycle differs from most other coccidia in that they require two separate hosts for completion (Figure 4); there is an alteration of a sexual generation in the intestinal mucosa of the flesh-eating final or definitive host (usually a carnivore), with an asexual generation in the tissues of a prey or intermediate host (usually a herbivore). The transfer stage from final host to intermediate host is an environmentally hardy oocyst or sporocyst (Figure 5); each sporocyst contains four infective sporozoites. The sporocyst contains sporozoites that are infectious when passed and do not require further maturation in the environment. The transmitting stage that is infective to the final host is the merozoite, which is contained in cysts in the muscles or other tissues of the intermediate host (Figure 6).
At least two species, Sarcocystis hominis (or bovihominis) and Sarcocystis suihominis, use man as the final host, and sporogonic development occurs in the lamina propria of the intestinal mucosa. For S. hominis and S. suihominis, the intermediate hosts are cattle and swine, respectively. For a yet undetermined number of species, not all of which have been identified, man serves as an accidental intermediate host, and asexual reproduction occurs first in the vascular endothelial cells and then in skeletal or cardiac muscle fibers. These are collectively referred to as Sarcocystis ‘lindemanni ’ and neither the final host(s) nor the natural intermediate host(s) is known.
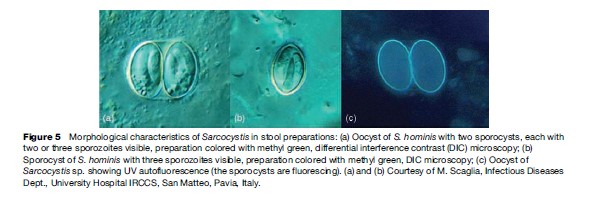
The diagnosis is by finding oocysts or free sporocysts in the feces. Concentration methods routinely used for fecal examination aid in finding parasites, which can be scanty in number. The sporocysts (see Figure 5) are broadly ovoid and measure about 9 by 15 mm for S. hominis and a bit smaller for S. suihominis, 10 by 13 mm. The sporozoites are visible through the thin, colorless, transparent sporocyst wall. Detection of human infection with cyst stages (merozoites enveloped in a protective wall) would be through routine histologic examination of muscle biopsy.
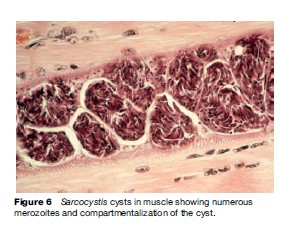
The sporogonic stages in the human intestine are generally reported to be only slightly pathogenic, with mild, transient fever and diarrhea being reported. Other studies report more severe acute diarrhea and vomiting with chills and perspiration lasting up to 24 hours. Experimental infections in man and other hosts (primates) have demonstrated that the prepatent period is on the order of 10 to 15 days for S. suihominis and 18 to 39 days for S. hominis, and that sporocyst shedding may last from 9 to 30 days, and up to 6 months in some cases. In man, no inflammation or other evidence of pathogenicity has been ascribed to the cyst stages in muscle. However, observations on experimental infection in animals indicate that the early, proliferative stage can result in massive destruction of the vascular endothelium. Cyst stages in the muscles of intermediate hosts persist for months if not years.
The known prevalence of Sarcocystis in the human population is spotty; muscle sarcocystosis has been reported in people in South-East Asia, India, Africa, Europe, and North and South America, and in some areas, prevalence rates of 10 to 30% have been observed. However, intestinal Sarcocystis has only been reported from portions of South-East Asia. The prevalence of infection in animals has been poorly documented in most geographical areas as well. However, in some regions where specifically looked for, such as southern Germany, over 99% of examined cattle were found to harbor Sarcocystis. Human infection is best prevented by not eating uncooked meat. Bovine and swine infections are prevented by protecting animal food and water from human fecal contamination.
Several drugs, including sulfadiazine, tinidazole, and acetylspiramycin may be effective in treating the intestinal infection, but because Sarcocystis infections in humans are subclinical or produce mild transient symptoms, specific therapy is not indicated.
Flagellated Protozoa
Giardia
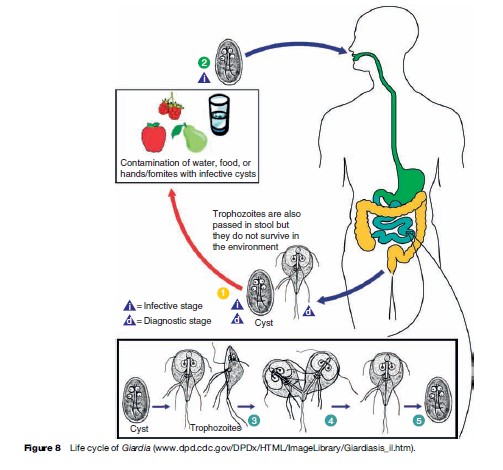
Giardia intestinalis (also known as G. lamblia) is a flagellated protozoa that inhabits the small intestine of man and other animals, including monkeys, rodents, dogs, cats, horses, goats, cattle, birds, reptiles, and fish. Like many other protozoa, G. intestinalis has a trophozoite and a cyst stage. The trophozoite is oblong, pear-, or kidney-shaped, rounded anteriorly, and pointed posteriorly. The trophozoite is flattened laterally, being convex dorsally and concave ventrally; much of the ventral surface comprises the sucking disk that the organism uses to attach firmly to the intestinal mucosa. The trophozoite is microscopic in size, averaging 10 to 20 mm long by 5 to 15 mm in breadth; a prominent pair of nuclei on each side of the organism near the anterior end gives a facelike appearance (Figures 7 and 8). There are four pairs of flagella, one pair arising near the anterior and posterior end, respectively, and two pairs arising near mid-body. Rapid movement of the flagella allows the trophozoite to move from place to place. Trophozoites divide by a complicated process of longitudinal binary fission that results in two daughter trophozoites (see Figure 8). Transmission from one host to another is accomplished by viable cysts (see Figure 8). As trophozoites transit down the colon, they prepare for encystation by retracting their flagella. The cytoplasm becomes condensed, and a thin, tough hyaline membrane (cyst wall) is secreted. The cysts are oval in shape and measure 8 to 12 mm in length by 7 to 10 mm in breadth. Mature cysts have four nuclei located at one end of the cyst. As the cyst matures, internal structures and the sucking disk are doubled. When excystation occurs in a new host, division results in two identical trophozoites, which grow flagella and initiate infection.
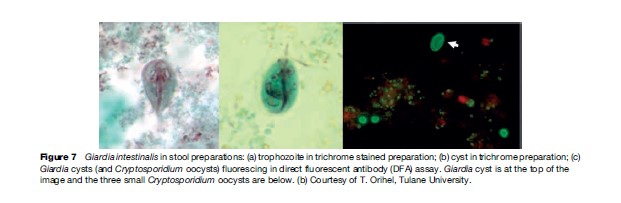
Diagnosis of infection is typically by microscopic detection of cysts in freshly collected stool (or trophozoites in diarrheic stools). Organisms can occasionally be seen in direct exams, but a concentration procedure is recommended. Because of their distinctive shape, appearance of the nuclei, and other features, the diagnosis can often be made on wet, unstained samples. However, staining may enhance detection and confirmation of infection. In addition to direct or stained specimens, commercial direct fluorescent antibody (DFA) assays are available and often used as the gold standard for diagnosis (see Figure 7). Enzyme-linked immunosorbent assay (ELISA) formatted tests are commercially available and are extremely useful for screening large numbers of samples. Although stool samples are the normally preferred specimens on which diagnosis is based, occasionally, a duodenal aspirate may be required. In other situations, a ‘string test’ may be used in which the patient is asked to swallow a gelatin capsule containing a string. The string is retrieved and examined for attached organisms.
Infection with Giardia in an appreciable number of cases results in irritation of the duodenum with excess secretion of mucus and dehydration, accompanied by epigastric pain, flatulence, and chronic diarrhea with steatorrheic-type stool containing a large amount of mucus and fat but typically with no blood. It is recognized that giardiasis can cause stunting and interference with growth, particularly in children in developing countries where repeated infections are the norm.
Metronidazole or tinidazole is the recommended drug of choice for treating giardiasis. Nitazoxanide, furazolidone, and paromomycin are alternatives. Paromomycin is not absorbed from the gastrointestinal tract and is often used during pregnancy but it is less efficacious than the other agents.
Transmission of Giardia is by viable cysts that are swallowed (see Figure 8). Contaminated food and water are the most common source of exposure (Table 5) although intimate contact with an infected individual may represent a common mechanism. Giardiasis is typically more common in children than in adults, especially in a crowded setting such as day care centers. However, in the United States and other developed countries, outbreaks of giardiasis are also observed in adults. These are often linked to contaminated food or drink, or are associated with recreational water venues. High frequencies of infection have been reported among hikers and campers who drank from remote mountain streams. These areas were often felt to be not at risk for human contamination and suggested that the role that beaver and other infected wild animals could play a role in human infection. Cats and dogs are also recognized to harbor Giardia. Despite the morphologic similarity of the organisms infecting humans and animals, molecular analysis has shown distinct clades or assemblages that seem to suggest some degree of host specificity, with certain assemblages being more restricted in their host preference than others. Further differences in virulence between isolates have also been proposed, but evidence to date has been inconsistent.
Giardia cysts have a relatively high resistance to routine water treatment procedures, including chlorination, which has led to numerous waterborne outbreaks. Surface water can be widely contaminated, and as a result, giardiasis is one of the most common intestinal parasitic infections, even in the United States. This implies that to provide potable water, surface water should be treated by flocculation, sedimentation, filtration, and finally, chlorination. Use of chlorine alone at levels normally used in municipal treatment facilities does not rapidly inactivate cysts, especially at lower water temperatures, so other measures must be in place. Purifying water for use when camping or traveling overseas can include boiling, filtration through filters with pore size of less than 1 mm, or treatment with chlorine or iodine preparations (some recommend iodine preparations to be more effective than chlorine preparations).
Giardiasis can occur year-round in all settings, temperate as well as tropical. However, there is strong evidence that some seasonality occurs in temperate regions, such as the United States, with increased incidence in the summer months, peaking in early fall. Increased recreational water activity during summer months has been postulated to be a reason for this increase in incidence. Giardiasis can also be a common cause of traveler’s diarrhea during or shortly after return from a trip abroad.
Dientamoeba
Dientamoeba fragilis is a relatively small amoeba-like organism varying from 3 to 20 mm in diameter. Only a trophozoite stage is recognized in man, which may have one to four nuclei; with two being the usual number (Figure 9 (a)). The mode of transmission is unknown, but there is some evidence that much like Histomonas meleagridis of turkeys, D. fragilis may be carried inside the egg of some common nematode parasite. The presence of small structures thought to be D. fragilis has been reported in pinworm eggs (Enterobius vermicularis). It has been noted that D. fragilis and pinworm infection occur together more frequently than would be expected, and limited evidence indicates that experimental infection with pinworm eggs also resulted in infection with D. fragilis.
Dientamoeba fragilis has not been reported to invade tissues, but there is some evidence its presence may, on occasion, produce irritation of the intestinal mucosa with excess secretion of mucus and hypermotility of the bowel leading to a mucous diarrhea. Standard antiamoebic therapy including iodoquinol, paromomycin, or tetracycline appears to be satisfactory for D. fragilis.
Nonpathogenic Amoebas
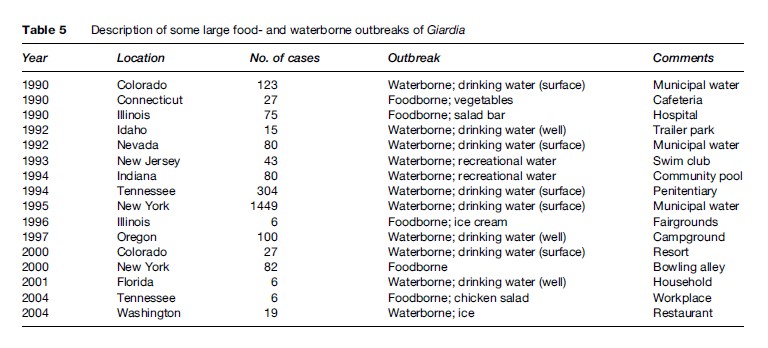
A number of protozoa in the amoeba group infect the human alimentary canal but are not believed to cause significant disease and are often referred to as the nonpathogenic amoebas. These include Entamoeba coli, Entamoeba hartmanii, Entamoeba polecki, Entamoeba gingivalis, Endolimax nana, and Iodamoeba butschlii. The finding of one of these organisms in a stool sample is significant for several reasons; it indicates exposure to fecal contamination with inherent risk of exposure to other pathogenic organisms. As well, the various stages found in submitted samples may provide diagnostic challenges for the microscopist to distinguish between one of these organisms and Entamoeba histolytica, a tissue-invasive intestinal protozoa.
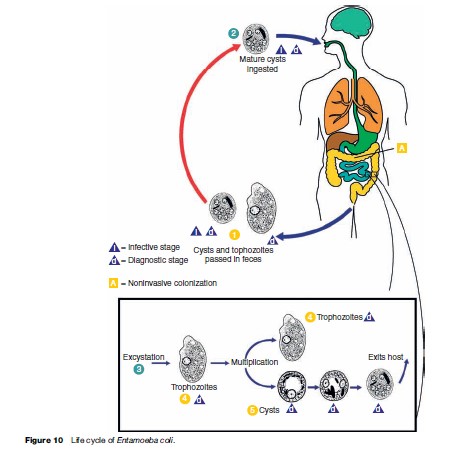
These organisms share some features of morphology and life cycle (Figure 10) in common, yet differ in others. All have a trophozoite stage that exhibits amoeboid (pseudopod) movement, and, with the exception of E. gingivalis, all form cyst stages in preparation for evacuation into the environment. Although trophozoites can be observed in stool preparations (Figure 11), especially in diarrheic samples, it is the cyst stage that is moderately durable in the environment and can withstand moderate putrefaction and desiccation. The cyst stage is when trophozoites transmitted through fecal contamination of food or drink (and possibly on contaminated objects), reach the mouth, are swallowed, and initiate infection. No cyst stage has been identified for E. gingivalis and transmission is presumed to occur via droplet spray or other exchange of oral secretions from the mouth of an infected individual to another during close contact. Although most commonly detected in the gingiva and tonsillar crypts, E. gingivalis has also been recovered from vaginal and cervical smears in women with intrauterine devices.
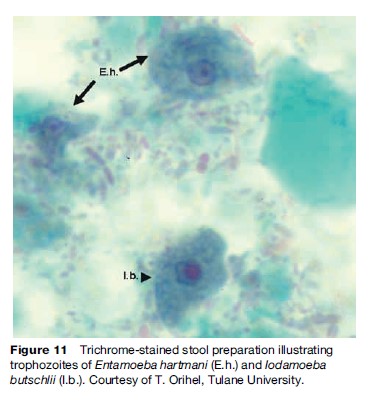
The mature cyst of E. coli is remarkably variable in size and measures 10 to 30 mm in diameter, and contains eight nuclei (Figure 9(b) and 9(c)). Immature cysts may contain two or four nuclei and can be confused with the pathogenic E. histolytica. Mature cysts of E. hartmani measure 5 to 10 mm, contain four nuclei (Figure 9(d)), and can best be distinguished from E. histolytica by size (E. histolytica cysts are from 10 to 20 mm, or about twice the size), but because of similar number of nuclei and inaccurate measurements, the two are often confused. E. poleki, cosmopolitan parasites of pigs and monkeys, is rarely diagnosed in humans, but most closely resembles E. coli, although the cysts tend to be smaller; measuring 5 to 11 mm in diameter. E. nana is a relatively small amoeba that typically produces ovoid cysts measuring 5 to 14 mm in diameter (Figure 9(e)). They can be confused with cysts of E. histolytica and E. hartmani because of cyst size and the presence of four nuclei, but the large, distinct nuclear karyosome readily distinguish this amoeba from other species of Entamoeba. I. butschlii is a smallto mediumsized amoeba that generally is easily distinguished from other amoebas, especially in the cyst stage. The nucleus has a large, prominent karyosome in both the trophozoite and cyst stage; the cyst has only a single nucleus. The cyst measures 6 to 15 mm in longest diameter and is likely to be pyriform or ovoid rather than spheroid. However, the most conspicuous feature of the cyst is the large glycogen vacuole (Figure 9(f )).
All of these amoebas are cosmopolitan in distribution, with infections tending to be more common in developing countries and in communities with poor hygiene and sanitation. Infection rates of 30 to 50% are common, and can approach 100%. Animal reservoir hosts are not thought to play important roles in human infection, although monkeys and dogs have been found naturally infected with an amoeba very similar to E. coli. Many monkeys also harbor an amoeba indistinguishable from E. nana. I. butschlii is a natural parasite of primates and the species I. suis of hogs is believed to be the same species.
Bibliography:
- Crist A, Morningstar C, Chambara R, et al. (2004) Outbreak of cyclosporiasis associated with snow peas – Pennsylvania, 2004. MMWR Morbidity Mortality Weekly Report 53: 876–878.
- Fayer R, Speer CA, and Dubey JP (2006) The general biology of Cryptosporidium. In: Fayer R (ed.) Cryptosporidium and Cryptosporidiosis, pp. 1–42. Boca Raton, FL: CRC Press.
- Herwaldt BL (2000) Cyclospora cayetanensis: A review, focusing on the outbreaks of cyclosporiasis in the 1990s. Clinical Infectious Diseases 31: 1040–1057.
- Sathyanarayanan L and Ortega Y (2006) Effects of temperature and different food matrices on Cyclospora cayetanensis oocyst sporulation. Journal of Parasitology 92: 218–222.
- Beaver PC, Jung RC, and Cupp EW (1984) Clinical Parasitology, 9th edn. Philadelphia, PA: Lea and Febiger.
- Dillingham R, Pape JW, Herwaldt BL, and Guerrant RL (2006) Cyclospora, isospora, and sarcocystis infections. In: Guerrant RL, Walker DH and Weller PF (eds.) Tropical Infectious Diseases: Principles, Pathogens, and Practice, 2nd edn., pp. 984–1002. Philadelphia, PA: Elsevier.
- Garcia LS (ed.) (2001) Diagnostic Medical Parasitology, 4th edn. Washington, DC: American Society for Microbiology.
- Gorbach SL, Bartlett JG, and Blacklow NR (eds.) (2004) Infectious Diseases, 3rd edn. Philadephia, PA: Lippincott, Williams and Wilkins.
- Hill DR and Nash TE (2006) Intestinal flagellate and ciliate infections. In: Guerrant RL, Walker DH, and Weller PF (eds.) Tropical Infectious Diseases: Principles, Pathogens, and Practice, 2nd edn., pp. 984–1002. Philadelphia, PA: Elsevier.
See also:
Free research papers are not written to satisfy your specific instructions. You can use our professional writing services to buy a custom research paper on any topic and get your high quality paper at affordable price.








