This sample Perinatal Epidemiology Research Paper is published for educational and informational purposes only. If you need help writing your assignment, please use our research paper writing service and buy a paper on any topic at affordable price. Also check our tips on how to write a research paper, see the lists of health research paper topics, and browse research paper examples.
Introduction
It is well established that events during pregnancy and childbirth influence the health and development of the newborn baby. In recent years, we have come to realize that events occurring in pregnancy and early childhood may also affect health into adult life. Even events occurring before pregnancy and intergeneration ally can affect reproduction. Population-based studies of these relationships are called perinatal epidemiology, which has evolved into a major subspecialty of epidemiology. Events or exposures may affect both mother and child; those affecting the mother are labeled maternal, while the term perinatal refers to the child.
Perinatal surveillance has been established through a system of medical registration of births in a number of countries. In Norway, medical registration of births was established in 1967. The main reason was the thalidomide catastrophe in Europe some years earlier. The other Nordic countries followed suit: Denmark in 1968, Iceland in 1972, and Sweden in 1973. Finland established its registry somewhat later, in 1987. The registries have served several other purposes than monitoring the frequency and type of congenital malformations. The data, which contain civic and medical information on the child, parents, and family, have been extensively used for perinatal research, of which several examples will be given. In Norway, mother, father, and child are identified by unique individual registration numbers. This facilitates linkage of successive births to the same mother with the same father or different fathers and linkage across generations. The medical birth registries can also be linked to other health related registries, for example the cancer registries.
Traditionally, perinatal epidemiologists have focused on exposures and outcomes that occur in the perinatal period, which for research purposes covers the period from conception through birth and the first part of life. For perinatal surveillance and statistical comparisons of mortality and morbidity between regions and countries, a more specific definition is used.
Definitions And Definitional Pitfalls
According to Webster’s New World Medical Dictionary, the perinatal period ‘‘starts at the 20th to 28th week of gestation and ends 1 to 4 weeks after birth.’’ By international convention, strict limits are defined in the tenth revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10), stating that the perinatal period commences at 22 completed weeks (154 days) of gestation (the time when birth weight is normally 500 g), and ends 7 completed days after birth. The lower limit is arbitrarily chosen to represent the time when a fetus is potentially viable; before this time a terminated pregnancy would be labeled miscarriage or abortion, after this time it is a birth. However, the ICD-10 definition of birth does not take gestational age into account; it only distinguishes between live birth and fetal death (deadborn fetus).
The ICD-10 definition is not as straightforward as it looks at first glance. Gestational age is variously defined. According to ICD-10, it should be based on the date of the first day of the last normal menstrual period, or on the best clinical estimate if this date is not available (or uncertain). With the advent of second-trimester ultrasound dating, sonographic biometry has increasingly replaced menstrual dates in countries where ultrasound is an integral part of prenatal care. Seven completed days has three logical interpretations: 168 h from the hour of birth, the date of birth plus 6 completed days and the date of birth plus 7 completed days, which are all in use. Since the incidence of early neonatal death tapers off toward a minimum during the first week after birth, these different definitions do not seriously affect international comparisons.
In many countries, civil notification of stillbirth starts at 28 completed gestational weeks, which precludes the enumeration of stillbirths between 22 and 28 weeks. International perinatal comparisons are therefore most often restricted to births beyond 28 weeks. To avoid enigmatic definitions of gestational length, age is in some instances replaced by birthweight of 1000 g or more. The different limits for compulsory notification of stillbirths entails another source of error in comparisons, since births shortly past the time limit tend to be underreported. In a concerted European comparison of perinatal mortality, it was found that adjustment for different cut-off points for birthweight and gestational age would reduce the differences in perinatal mortality by between 14% and 40% in the different countries and regions (Graafmans et al., 2001).
Perinatal Mortality
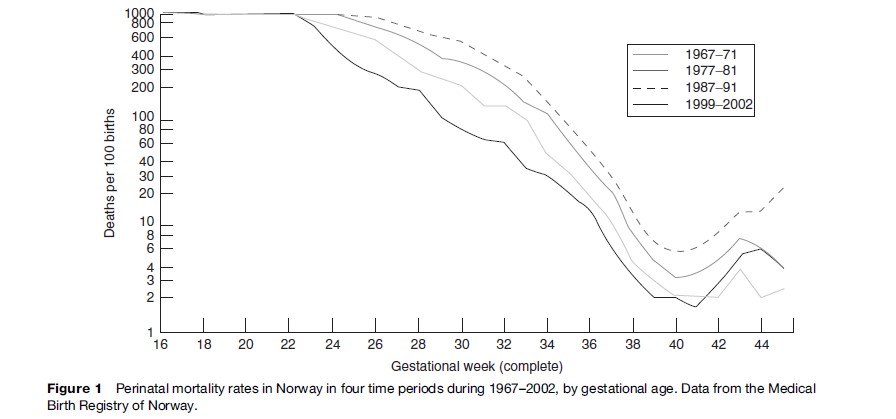
Perinatal mortality is defined as the number of fetal deaths past 22 (or 28) completed weeks of pregnancy plus the number of deaths among live-born children up to 7 completed days of life, per 1000 total births (live births and stillbirths). A joint interagency expert meeting on global indicators of sexual and reproductive health organized by WHO, UNICEF, and UNFPA in 2000 recommended perinatal mortality rate (PNMR) to be one of 17 chosen indicators. To avoid the problem of determining gestational age, the expert group advised that for comparative purposes 500 g and 1000 g should replace the time limits (World Health Organization, 2001). It goes without saying that PNMR will be higher when the 22-week limit or 500-g limit is used, because of the added number of stillbirths between 22 and 27 weeks. Figure 1 shows perinatal death rates in Norway in four time periods by gestational age. There has been substantial improvement in survival of extremely preterm births since 1967; in 1997–2004 there were 48% perinatal deaths among those born between 22 and 27 weeks, compared to 91% in 1967–76 (Skjærven, 2006). Norway was chosen for the example because there is compulsory notification of all births (stillbirths and live births) from 16 weeks onwards, which ensures nearly complete coverage. The remarkably enhanced survival over a span of 40 years is largely ascribed to better neonatal intensive care, which is of course related to those born alive. During the same period, the stillbirth rate in Norway past 28 weeks fell from 11.2 in 1967 to 2.9 per 1000 births in 2004. It is difficult to sort out the causes, but better overall maternal health status is thought to play an important part.
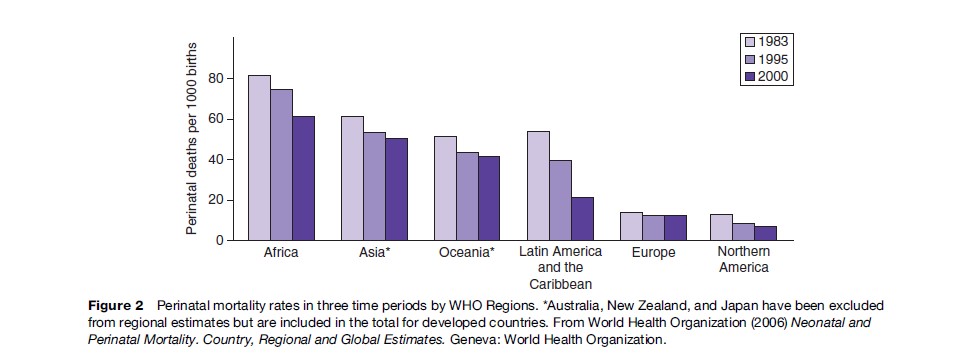
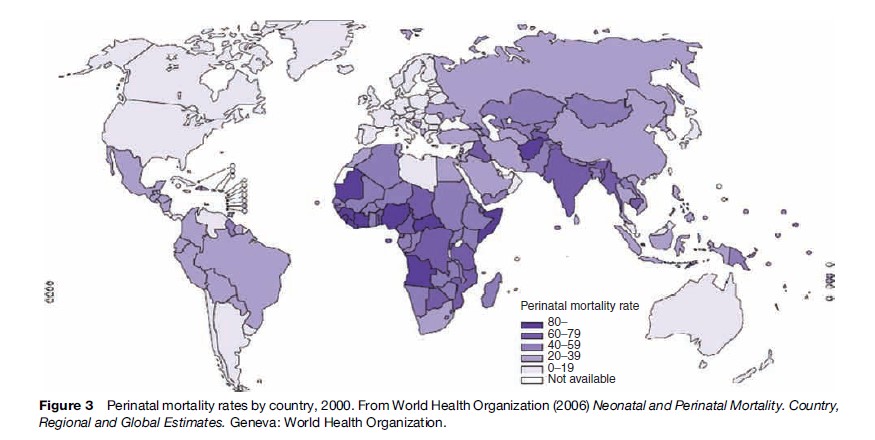
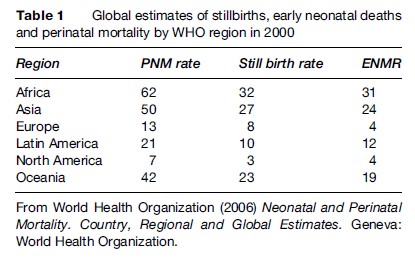
Using PNMR for global monitoring pinpoints the wide gap in reproductive health between rich and poor countries. Perinatal death is more common than maternal death, by a factor of about 100. Local registration will serve as an impetus to perinatal audit, which in turn may induce measures for improvement. Figure 2 shows perinatal mortality in the WHO regions of the world over a span of 18 years and Figure 3 gross country estimates in the year 2000. With a PNMR of 62 per 1000 births, Africa at present ranks higher than the Nordic countries did more than 100 years ago. However, a local population-based study in rural Northern Tanzania came up with an optimistically lower figure, 27 per 1000 births, for which no scientific explanation was found (Hinderaker et al., 2003). In developing countries, the ratio of stillbirths to first-week deaths is approximately 50/50. In Europe, stillbirths have the higher share, while in Northern America it is lower, the PNMR in both regions being around 10 per 1000 births (Table 1). In all likelihood, these latter differences are caused by registration shortfalls.
Causes Of Perinatal Death
To monitor perinatal mortality over time and make comparisons between countries and regions, one needs to know the causes of death. This implies a classification system that is clearly defined and fit for the purpose of introducing corrective measures. Various systems have been presented, with different emphasis on maternal and fetal or newborn conditions and complications. To determine the cause of death in a stillborn fetus as a rule requires an autopsy; this in practice is an exception rather than a standard procedure.
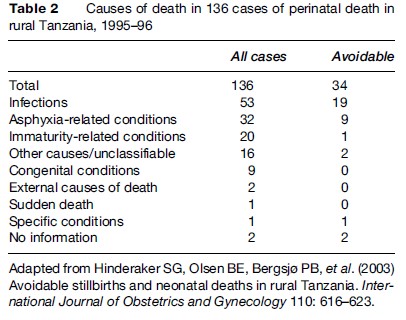
Table 2 is an example of causes of perinatal death in districts of rural Tanzania in 1995–96, grouped according to a classification system developed by the International Collaborative Effort (ICE) on Birth Weight, Plurality, Perinatal and Infant Mortality (Cole et al., 1989). The data derive from a prospective population-based study. Of the deaths recorded, 46% were stillbirths. Some of the neonates died more than 1 week after birth. Infection-related deaths dominated. Malaria was diagnosed in 20 out of 53 cases (Hinderaker et al., 2003).
Besides the direct cause of death, a number of other factors have been shown to influence the incidence of perinatal mortality. Gestational age and birth weight are obvious. Maternal age, parity, and a number of maternal conditions and diseases such as diabetes, the antiphospholipid syndrome, preeclampsia, and smoking are risk factors to varying degrees. In multiple pregnancies, the second in a set of twins carries the higher risk. More boys than girls die in the perinatal period. Isoimmunization due to blood group incompatibility has largely been eliminated following the introduction of anti-rhesus-gammaglobulin, but is still a risk factor. Intrapartum events (breech and transverse fetal presentation, mode of delivery, maternal hemorrhage) may threaten the baby’s life. In rich countries with decreasing PNMRs, congenital conditions take an increasing share of perinatal deaths.
Perinatal Morbidity
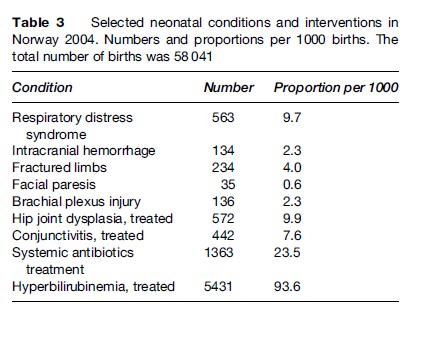
Perinatal epidemiologists also study diseases, injuries, and genetic aberrations diagnosed in utero, at birth, or in the early neonatal period, and their short or long-term consequences. They can be classified in much the same way as the causes of death in Table 2. The Medical Birth Registry of Norway routinely reports incidence rates of a number of specified neonatal conditions, shown in Table 3. The most common of these conditions was treated neonatal hyperbilirubinemia (or jaundice), which occurred in almost 10% of the newborns. Treatment for the majority of these was phototherapy. Systemic antibiotic treatment was given to 2.4%, while hip joint dysplasia and respiratory distress syndrome were diagnosed each in about 1% of the newborns (Skjærven, 2006).
Perinatal Audit
Perinatal audit is defined as ‘‘the systematic, critical analysis of the quality of perinatal care, including the procedures used for diagnosis and treatment, the use of resources and the resultant outcome and quality of life for women and their babies’’ (Dunn and McIlwaine, 1996). Peer review is another label. The definition covers a variety of quality assessment activities, with the implicit understanding that audit feedback should lead to changes to improve services. In this context, audit should be a formal procedure, with a multidisciplinary panel consisting of professionals of high standing, chosen among people of the same ranks and positions as those who cared for the cases to be audited, but preferably with no links to their departments. We have previously given a more detailed account of our experiences of perinatal audit through 20 years (Bergsjø et al., 2003) and will only give a few examples in this brief outline.
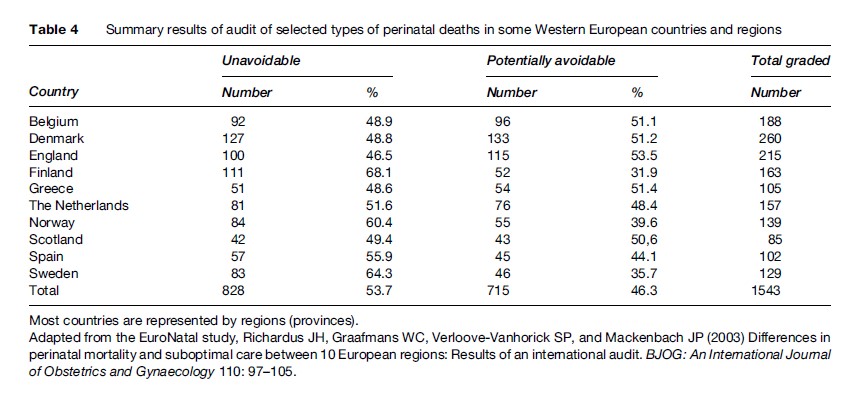
Audits of perinatal deaths have been used to advantage in both developing and developed countries. An example of the former is given in Table 2, which provides the added information that one of four cases was considered to be possibly avoidable. In a similar exercise covering ten European countries or regions, a multinational and multidisciplinary panel decided that between 35% and 54% of selected cases of perinatal death were potentially avoidable (Table 4). The cases had been selected among groups in which care and treatment were most likely to have a significant impact on the outcome, and the figures therefore only indicate relative differences in standards between countries and regions (Richardus et al., 2003). If all of the perinatal deaths within each birth population had been reviewed, the proportion of potentially avoidable cases would have been considerably lower.
Perinatal Risk Factors
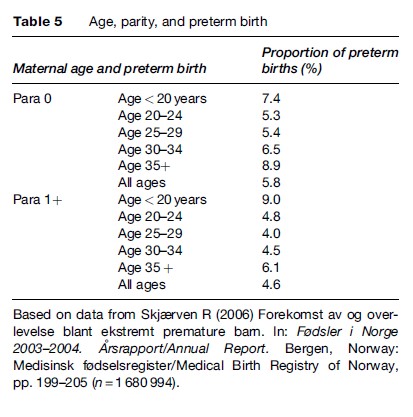
Birth registries serve as important surveillance systems allowing description of relevant factors associated with pregnancies and childbirth. For example, the frequencies of interventions such as cesarean section and adverse outcome of birth such as perinatal death. The associations between maternal age and parity and preterm birth are shown in Table 5. With the exception of teenaged mothers, the incidence of preterm birth is consistently lower among those who have given birth previously.
Another example is the association between the length of maternal education and preterm birth in different time periods, as shown in Table 6. Mothers with a low education level had increased risk of preterm birth in all three time periods, 1967–76, 1977–86, and 1987–98.
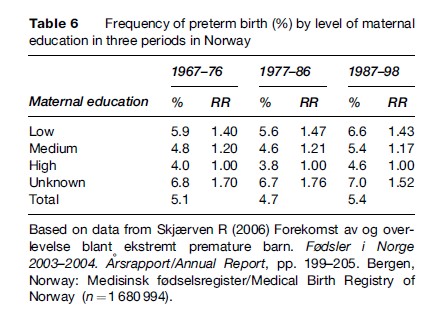
Table 7 shows the association between the risk of preterm birth by sex of the fetus, previous preterm birth, and previous fetal death. It can be seen that male fetuses have 20% increased risk of being born preterm (6.5% vs. 5.4%). A previous preterm birth increases the incidence from 5.2% to 11.4% (RR = 2.19) and a previous fetal death increases the risk from 5.6 to 8.1 (RR = 1.45).
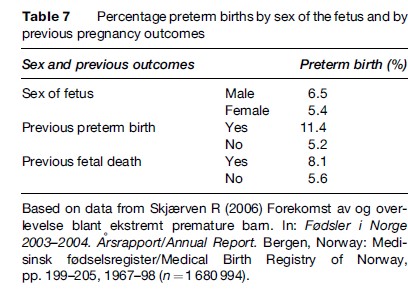
Time trends of preterm births of less than 37 completed weeks’ gestational age in Norway 1967–98 are shown in Table 8. It appears that little change in the frequency has occurred over this time period, in spite of considerable changes in habits of living (diet and smoking), in vitro fertilization, ultrasound dating of pregnancies, and a more interventional obstetric practice. Perinatal survival has improved considerably for these tiny babies, as shown in Figure 1. The causation of preterm birth is mainly unknown, although a significant part of the causation is linked to genes from both parents. The genetic and environmental components of the causation call for innovative research in order for decision makers to develop effective preventive strategies.
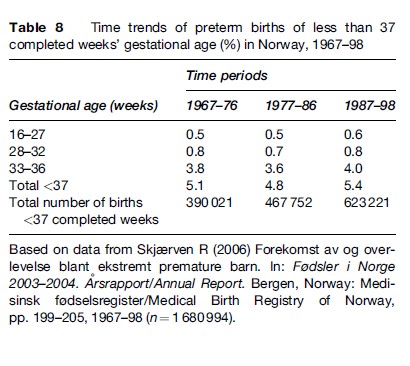
Analytical Studies
In searching for risk factors, two major approaches are employed within perinatal epidemiology. The individuals may be defined according to a specific outcome variable, for example preterm birth. Risk factors can be related to this outcome by the case–control approach. Is the risk factor more prevalent among the cases than among the controls? Control cases can be matched to the cases for one or more variables, such as age and parity. Alternatively, the total cohort can be used for the comparisons, in which case it is called a case–cohort study. The other approach involves identification of individuals according to the presence of the risk factor under study and following the individuals over time and recording the outcome(s) in question. This is called a prospective, or cohort, study. Recently, huge cohorts of pregnancies have been established allowing cohort analyses, or so-called nested case–control studies, which mean case–control studies within the cohort material.
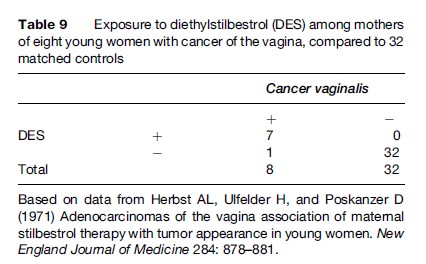
During the 1960s, diethylstilbestrol (DES) was widely used to prevent abortion or preterm birth (on scanty evidence, later shown to be invalid). An investigation that started in Boston in 1971 (Herbst et al., 1977) is a classical example of a case–control study in perinatal epidemiology. Three physicians at the Vincent Memorial Hospital reported a striking association between maternal use of DES during the first trimester of pregnancy and the development 15–20 years later of vaginal cancer in daughters born of these mothers. Seven young women were diagnosed with this cancer that was rarely seen in young women. Because of this clustering of cases (seven within 4 years), it was decided to conduct a case–control study comparing these seven cases plus an additional one from another hospital and their families with an appropriate comparison group (controls) in order to identify factors that might explain the appearance of the cancers. Four matched controls were selected among young women who did not have vaginal cancer (matched for age and place of birth). Thus the study consisted of eight cases and 32 controls. As shown in Table 9, seven of the eight women with vaginal cancer had mothers who had been exposed to DES in their pregnancies compared to none of the 32 controls. No other differences between cases and controls were found. This was such a strong observation that causality was suspected. Thus a previously unknown cause of a disease was disclosed.
Most studies that use data from the medical birth registries use a cohort design where both exposure and outcome variables are collected from the registry. A recent example concerns fetal and infant survival following preeclampsia. The study showed that fetal and infant survival in preeclamptic pregnancies have improved over the past 35 years in Norway. However, the selective risk of neonatal death following a preeclamptic pregnancy has not changed over time (Basso et al., 2006).
Some researchers combine data from a medical birth registry with data from other registries. Fossa˚ et al. linked data from the patient registry of the Norwegian Radium Hospital and from the Norwegian Cancer Registry with data from the Birth Registry of Norway. They examined the parenthood in successive births after adult cancer. Female cancer survivors delivered more preterm births and low-birth-weight infants than women in a matching noncancer population. Male cancer survivors, on the other hand, did not differ from noncancer fathers in the outcome of successive births (Fossa˚ et al., 2005).
Recurrence Risk
Sibling studies became possible because the Norwegian Medical Birth Registry employs the unique individual identification system, whereby it is possible to link successive births to the same mothers. Bakketeig (1977) presented the first data showing the strong tendency to repeat low birth weight, preterm birth, and small for gestational age (SGA) birth. Table 10 shows the relative prediction of adverse outcomes among second births based on the outcome of the mothers’ first birth. For example, the prediction of low birth weight (LBW) is 5.6 times as strong if the elder sibling was also LBW as opposed to having appropriate birth weight. The risk of preterm birth was 4.0 times higher if the mother’s first birth was also a preterm delivery, compared to a nonpreterm birth. A similar risk was shown for a SGA second birth where the first birth was also SGA compared to not being SGA. This tendency to repeat was later confirmed in much larger data sets (Bakketeig et al., 1979). Mothers somehow appeared to be programmed to produce births of a certain gestational age and size. If they departed from this pattern the offspring was at a greater risk of perinatal death (Bakketeig and Hoffman, 1983).
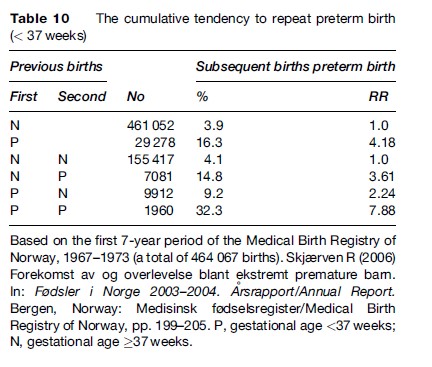
The tendency to report birth outcomes was found to be cumulative. As shown in Table 10, two preterm births increased the risk of a subsequent preterm birth nearly eightfold compared to the two first births not being preterm.
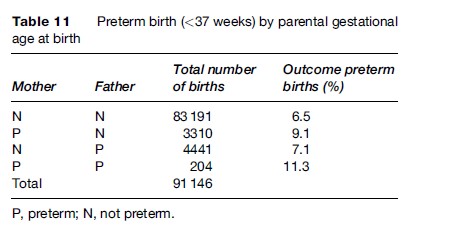
The risk of preterm birth is dependent on whether the mother or the father, or both parents, were born preterm, as shown in Table 11.
Skjærven et al. (1988) examined whether the birth weight and perinatal mortality of second birth were conditional on the weight of the first birth. They showed that a woman’s successive offspring tend to have similar birth weights. Mean birth weights among second births differ by as much as 1000 g depending on the weight of the first birth. Also, the survival of the second baby at a given weight is strongly affected by its weight relative to the first baby’s weight. A baby may be average size compared to the population norm, but small compared to its elder siblings. Such a baby has increased mortality that goes with being relatively small. For example, an infant weighing 3250 g is relatively large if the mother’s previous baby weighed 2250 g, but relatively small if the previous birth weighed 4250 g. In the first case, the perinatal mortality risk of the 3250-g baby is 2.2 per 1000 while in the second case the baby with the same weight has perinatal mortality of 9.0 per 1000 (or four times higher).
Focusing on mothers with two births Vatten and Skjærven (2003) showed that women who changed partners between their pregnancies had an increased risk of preterm birth, birth with low birth weight, and increased risk of infant mortality, compared with women with the same partner in their pregnancies.
Mjelve et al. (1999) studied mothers’ two first singleton births. They showed that siblings’ gestational ages were significantly correlated (r ¼ 0.26) and that the risk of having a preterm birth was nearly ten times higher among mothers whose firstborn had been delivered before 32 weeks’ gestation compared to mothers whose first birth had been at 40 weeks’ gestation. They also found that the perinatal mortality in preterm second births was significantly higher among mothers whose first infant was born at term compared with mothers whose first born child was delivered at 32–37 weeks. They concluded that since perinatal mortality among preterm births is dependent on gestational age in the mother’s previous birth, a common threshold of 37 weeks’ gestation for defining preterm birth as a risk factor for perinatal death may not be appropriate for all births.
Generational Studies
Skjærven et al. (1997) focused on whether a baby’s survival is related to its mother’s birth weight. They linked births during 1981–94 to data on all mothers born from 1967 onwards, thereby forming 105 014 mother–offspring units. The mother’s birth weight was strongly associated with the weight of her baby. Mortality among small babies was much higher where the mothers were born large. For example, babies weighing between 2500 and 3000 g had a threefold higher perinatal mortality if their mothers’ birth weight was 4000 g or higher, compared to those whose mothers had been small at birth (between 2500 and 3000 g).
Magnus et al. (1993) examined the correlation of gestational age and birth weight across generations. Based on linkage of 1967–69 births and 1986–89 births, they obtained 11 072 pairs of mother–firstborn offspring. A low correlation coefficient (0.086) was found for gestational age across generations, whereas the correlation between maternal and offspring birth weight was 0.242. Mothers with birth weight below 2500 g had a significantly increased risk (OR ¼ 3.03, 95% CI 1.79–5.11) of having a low weight baby compared with mothers with birth weight above 4000 g. On the other hand, if the mother was born before 37 weeks’ gestation, the risk of having a preterm birth was not significantly increased (OR ¼ 1.48, 95% CI 0.96–2.21) compared with mothers born at term. The authors concluded that in contrast to birth weight, variation in human gestational age does not appear to be influenced by genetic factors to any large degree.
Record Linkage To Registries Outside Medical Birth Registries
In a study focusing on preeclampsia and subsequent breast cancer Vatten et al. (2002) showed that women with preeclampsia and/or hypertension in their first pregnancy had a 19% (95 CI, 9%–29%) lower risk of breast cancer compared to other parous women. This was based on data from the Medical Birth Registry of Norway linked to the Cancer Registry of Norway. The results indicate that pathophysiology surrounding preeclampsia and gestational hypertension plays a role in breast cancer etiology.
Maternal And Child Cohorts
Large cohorts of pregnancies have been established for research purposes in Denmark and Norway. Pregnant women have been included from early on in their pregnancies. Information has been collected as well as biological material (blood, urine) from the women and partly from their partners (Norway). These cohorts are important additional sources for further studies on maternal and child health. To exemplify, one can establish case–control (or case–cohort) studies within the cohort on its scientific strength. We may want to test a specific hypothesis on causation of a certain type of cancer. Each of these cohorts contains a variety of information on, for example, lifestyle of the mother when the patient was a fetus or baby, in addition to biological material collected during pregnancy, which would allow for testing of a specific hypothesis on causation.
Bibliography:
- Bakketeig LS (1977) The risk of repeated preterm or low weight delivery. In: Reed DM and Stanley FJ (eds.) The Epidemiology of Prematurity. Baltimore, MD: Urban and Schwarzenberg.
- Bakketeig LS, Hoffman HJ, and Harly EE (1979) The tendency to repeat gestational age and birth weight in successive births. American Journal of Obstetrics and Gynecology 135: 1086–1103.
- Bakketeig LS and Hoffman HJ (1983) The tendency to repeat gestational age and birth weight in successive birth related to perinatal survival. Acta Obstetricia et Gynecologica Scandinavica 62: 85–92.
- Basso O, Rasmussen S, Weinberg CR, Wilcox AJ, Irgens LM, and Skjærven R (2006) Trends in fetal and infant survival following preeclampsia. Journal of the American Medical Association 296: 1357–1362.
- Bergsjø P, Bakketeig LS, and Langhoff-Roos J (2003) The development of perinatal audit: Twenty years’ experience. Acta Obstetricia et Gynecologica Scandinavica 82: 780–788.
- Cole S, Hartford RB, Bergsjø P, and McCarthy B (1989) International Collaborative Effort (ICE) on Birth Weight, Plurality, Perinatal, and Infant Mortality: III. A method of grouping underlying causes of infant death to aid international comparisons. Acta Obstetricia et Gynecologica Scandinavica 68: 113–117.
- Dunn PM and McIlwaine G (eds.) (1996) Perinatal audit. Prenatal and Neonatal Medicine 1: 160–194.
- Fossa˚ SD, Magelssen H, Melve K, Jacobsen AB, Langmark F, and Skjærven R (2005) Parenthood in survivors after adulthood cancer and perinatal health in their offspring. A preliminary report. Journal of the National Cancer Institute Monographs 34: 77–82.
- Graafmans WC, Richardus J-H, Macforlane A, et al. (2001) Comparability of published perinatal mortality rates in Western Europe: the quantitative impact of differences in gestational age are birthweight criteria. BJOG: An International Journal of Obstetrics and Gynecology 108: 1237–1245.
- Herbst AL, Ulfelder H, and Poskanzer D (1971) Adenocarcinomas of the vagina association of maternal stilbestrol therapy with tumor appearance in young women. New England Journal of Medicine 284: 878–881.
- Hinderaker SG, Olsen BE, Bergsjø PB, et al. (2003) Avoidable stillbirths and neonatal deaths in rural Tanzania. International Journal of Obstetrics and Gynecology 110: 616–623.
- Magnus P, Bakketeig LS, and Skjærven R (1993) Correlations of birth weight and gestational age across generations. Annals of Human Biology 20: 231–238.
- Mjelve KK, Skjærven R, Gjessing HK, and yen N (1999) Recurrence of gestational age in sibships: Implication of perinatal mortality. American Journal of Epidemiology 150: 756–762.
- Richardus JH, Graafmans WC, Verloove-Vanhorick SP, and Mackenbach JP (2003) Differences in perinatal mortality and suboptimal care between 10 European regions: Results of an international audit. BJOG: An International Journal of Obstetrics and Gynaecology 110: 97–105.
- Skjærven R (2006) Forekomst av og overlevelse blant ekstremt premature barn. Fødsler i Norge 2003–2004. A˚ rsrapport/Annual Report, pp. 199–205. Bergen, Norway: Medisinsk fødselsregister/ Medical Birth Registry of Norway.
- Skjærven R, Wilcox AJ, and Russel D (1988) Birthweight and perinatal mortality of second births conditional on weight of the first. International Journal of Epidemiology 17: 830–838.
- Skjærven R, Wilcox AJ, yen N, and Magnus P (1997) Mothers’ birth weight and survival of their offspring: Population-based study. British Medical Journal 314: 1376–1380.
- Vatten LJ and Skjærven (2003) Effects on pregnancy outcome of changing partner between first two births: Prospective population study. British Medical Journal 327: 1138.
- Vatten LJ, Romundstad PR, Triohopulos D, and Skjærven R (2002) Preeclampsia in pregnancy and subsequent risk of breast cancer. British Journal of Cancer 87(9): 971–973.
- World Health Organization (2001) Reproductive Health Indicators for Global Monitoring. Report of the second Interagency meeting. WHO, Geneva 17–19 July 2000. WHO/RHR/01.19. Geneva, Switzerland: World Health Organization.
- World Health Organization (2006) Neonatal and Perinatal Mortality. Country, Regional and Global Estimates. Geneva, Switzerland: World Health Organization.
See also:
Free research papers are not written to satisfy your specific instructions. You can use our professional writing services to buy a custom research paper on any topic and get your high quality paper at affordable price.








