This sample Rubella Research Paper is published for educational and informational purposes only. If you need help writing your assignment, please use our research paper writing service and buy a paper on any topic at affordable price. Also check our tips on how to write a research paper, see the lists of health research paper topics, and browse research paper examples.
Introduction
Rubella was first described as a mild exanthemata’s illness of childhood early in the nineteenth century by German physicians, resulting in the name German measles. In 1941, Sir Norman Gregg, an Australian ophthalmologist, recognized that a number of children developed cataracts after an epidemic of rubella and proposed an association between maternal rubella infection and the development of cataracts, deafness, heart disease, and mental retardation in the infant (Gregg, 1941). In addition, Gregg is credited with introducing the concept of an intrauterine viral infection as having teratogenic potential. In 1962, rubella virus was isolated in cell culture (Parkman et al., 1962; Weller and Neva, 1962). This was the same year as the start of a worldwide pandemic that spread to the United States in 1964–1965 and resulted in more than 12 million cases of rubella. This was the last rubella epidemic to occur in the United States but it resulted in thousands of infections in pregnant women, causing 11 250 fetal deaths and 20 000 infants to be born with the congenital rubella syndrome. The financial cost of the epidemic was estimated at $1.5 billion. After the disastrous consequences of this epidemic, several attenuated rubella vaccines were developed and, in 1969, a national rubella vaccination program was begun. After introduction of the rubella vaccine, the incidence of rubella declined by more than 99% from the prevaccine era. In October 2004, 35 years after initiation of the vaccine program, an international panel of experts convened by the Centers for Disease Control and Prevention (CDC) concluded unanimously that rubella was no longer endemic in the United States (CDC, 2005).
Virology
Rubella virus is classified as a togavirus and is the only member of the genus Rubivirus. Unlike other togaviruses that cause eastern and western equine encephalitis and are classified as arthropod-borne viruses, rubella virus is known to infect only vertebrate hosts and man is the only known natural reservoir for rubella virus. Rubella virus is an enveloped RNA virus with a single antigenic type that does not cross react with other togaviruses. Rubella virus can be grown in several common laboratory cell lines. Sequencing of the approximately 10 000 nucleotide long, single-stranded rubella genome has been completed.
A variety of experimental animals can be infected with rubella virus although there are no reliable animal models of symptomatic rubella infection in humans or for congenital rubella syndrome.
Clinical Features
In susceptible populations, infection by rubella virus generally occurs in childhood or young adulthood and 20–50% of infections are subclinical. The incubation period ranges from 12–23 days. Among patients who develop symptoms, illness generally consists of nonspecific signs and symptoms including rash, postauricular or suboccipital lymphadenopathy, arthralgia, conjunctivitis, and low-grade fever. Rash is the most prominent feature of the illness and generally begins on the face as a maculopapular exanthem that spreads to coalesce before fading over several days. Transient arthralgia or arthritis is common, particularly among rubella-infected women. Encephalitis occurs at a rate of approximately 1 per 6000 cases. Thrombocytopenia occurs at a rate of approximately 1 per 3000 cases.
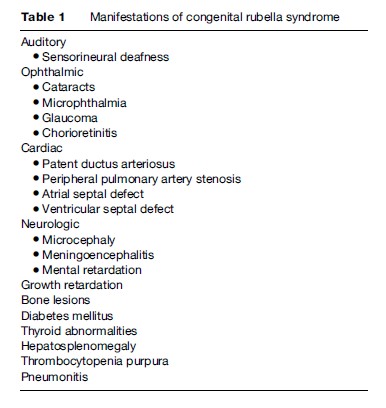
The most significant consequence of rubella infection occurs among pregnant women who are at increased risk of miscarriage, stillbirth, and fetal anomalies when rubella infection occurs early in gestation, particularly during the first trimester. Major anomalies associated with congenital rubella syndrome include auditory, ophthalmic, cardiac, and neurologic defects (Table 1). Up to 85% of infants born to mothers infected during the first 8 weeks of gestation develop anomalies. The risk of any anomaly decreases to approximately 50% following infection between the 9th and 12th week of gestation. After the 20th week of gestation, congenital defects due to maternal rubella infection are unusual.
Epidemiology
In the prevaccine era, rubella was endemic in the United States with epidemics occurring every 6–9 years. Rubella virus is transmitted through direct contact or exposure to an aerosol of nasopharyngeal secretions from a patient with rubella. Mucosal cells of the upper respiratory tract are the portal of viral entry and the nasopharyngeal lymphatic tissue is the site of initial viral replication. Regional spread and local viral replication account for the posterior cervical and occipital node enlargement. In the prevaccine era, the peak in disease activity occurred in late winter and early spring. Immunity following rubella virus infection is generally lifelong although reinfection has been documented and can result in congenital rubella syndrome. Rubella virus can be isolated from nasopharyngeal secretions from 7 days before to 14 days after the rash onset, although the period of maximal transmissibility begins a few days prior to 7 days after onset of the rash. Development of the rubella rash 14–23 days after exposure corresponds to the detection of rubella-specific antibody and has led to the proposal that the rash is a consequence of an immune-mediated process. Among children with congenital rubella, virus can be isolated from urine up to the first birthday and from the eye of children with congenital cataracts for several years.
Laboratory Testing
Postnatally acquired rubella can be diagnosed by a fourfold or greater rise in antibody titer between acute and convalescent serum specimens. The hemagglutination inhibition antibody assay was a common screening assay for immunity to rubella but has been replaced by more sensitive assays including enzyme immunoassay tests, latex agglutination assays, and immunofluorescent assays. Congenital rubella infection can be diagnosed by detection of rubella-specific immunoglobulin M (IgM) in a newborn infant. The presence of rising or stable rubellaspecific immunoglobulin G (IgG) over several months will also confirm congenital rubella infection. Rubella virus can be isolated from an infected person from throat or nasal aspirates, blood, urine, or cerebrospinal fluid. Molecular typing of rubella isolates may provide information regarding source of acquisition.
Vaccine Development
Several vaccine strains were developed using different cell lines soon after rubella virus was isolated in cell culture. However, since 1979, RA 27/3 (a strain isolated from an infected fetus and subsequently passaged in human diploid fibroblasts) has been the only rubella vaccine used in the United States and has been the most widely used vaccine throughout the world. Advantages of this strain include greater immunogenicity and a lower incidence of side effects than occurs with other vaccines. Nucleotide sequencing of the envelope genes of RA 27/3 have shown 31 amino acid changes compared with similar sequences in the wild-type rubella virus. Subcutaneous administration of RA 27/3 induces IgM and IgG antibodies as well as a cellular immune response.
Adverse events associated with administration of measles, mumps, and rubella (MMR) vaccine range from local reactions to rare systemic reactions. Approximately 5% of children will experience a temperature greater than 103 F 7–12 days after MMR vaccination. Transient lymphadenopathy or rashes may occur. Allergic reactions at the injection site include urticaria, pruritis, and purpura. Anaphylaxis after vaccination with MMR vaccines occurs at a rate of less than 1 case per million doses distributed. Thrombocytopenia following rubella or measles infection is considerably greater than the risk after MMR vaccination. Transient arthralgias or arthritis are rare after administration of RA 27/3 and occur more often among adult women than among children. Contraindications to rubella-containing vaccine use include pregnancy; severe illness; a history of reactions to vaccine components; a history of thrombocytopenia; recent administration of immune globulin and immunocompromised state due to diseases such as HIV, transplantation, or chemotherapy; or medications such as high-dose corticosteroids.
The major impetus for implementation of a rubella immunization program was prevention of the devastating consequences in women who are infected during the first 24 weeks of gestation. Unborn children constitute the group most likely to benefit from widespread use of the rubella vaccine. Initially, vaccination of susceptible women of childbearing age in the United States was not acceptable because data were not available on the potential risk of adverse effects of the vaccine strain on the fetus. An alternative approach was to focus the vaccination campaign on young children, because they represented the group most likely to spread the virus. Subsequently, additional efforts were directed at identification and immunization of susceptible post pubertal women as well as other groups of susceptible individuals, including military recruits and hospital personnel (CDC, 1969, 1978). In contrast, the United Kingdom initiated a policy of vaccinating 10to 14-year-old schoolgirls as well as susceptible women of childbearing age (Hinman et al., 1983; Tobin et al., 1985). This policy resulted in a reduction in cases of congenital rubella syndrome in the United Kingdom although rubella virus continued to circulate among adult males and unvaccinated children. After a rubella epidemic in 1986, this vaccination program was modified to vaccinate all children similar to the practice in the United States (Best et al., 1987; Reef et al., 2000; Vyse, 2002).
In the United States the incidence of reported cases of rubella fell sharply following initiation of rubella immunization of young children in 1969 (Figure 1). From the estimated 2 million cases a year in the prevaccine era, fewer than 1000 cases were reported in 1983. The incidence of rubella continued to fall during the 1980s and 1990s although clusters of disease occurred among groups of susceptible individuals, including people with religious or philosophic exemption to immunization. Although rubella had been a disease of childhood, the proportion of remaining cases among people 20 years of age or more increased to 79% in 1998. Sustained implementation of the rubella vaccination program resulted in a marked decrease in incidence among all age groups. Since the mid-1990s, most reported cases of rubella in the United States occurred among foreign-born young adults (particularly from Latin America) who were born in countries without routine rubella immunization programs (Danovaro-Holliday et al., 2000; Reef et al., 2002). As shown in Figure 1, outbreaks of rubella usually are followed by an increase in newborns with congenital rubella syndrome. Each year from 1992 through 1999, an average of 6 or more cases per year of congenital rubella syndrome were reported (Reef et al., 2002).
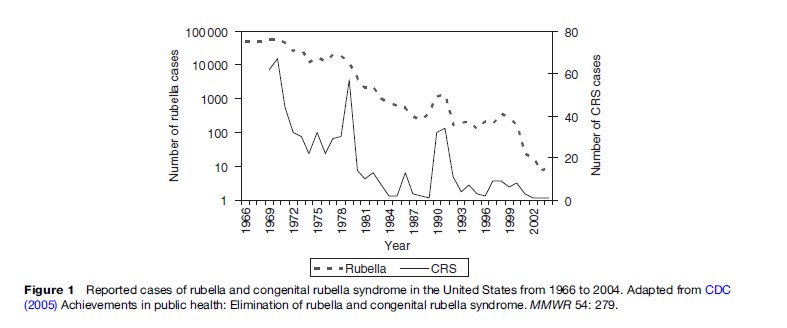
As of December 2002, more than 90% of children in the United States had received a first dose of rubella-containing vaccine by 19–35 months of age and more than 90% had received two doses by school entry. The epidemiology of rubella following vaccine introduction mimicked the remarkable success of the measles immunization program (Meissner, 2004). After 2001, fewer than 25 rubella cases occurred each year, during a time of careful surveillance. Four cases of congenital rubella syndrome occurred during the same time period, and the mothers of three of the children were born outside the United States. The low number of cases of rubella and congenital rubella syndrome and long periods without reported cases justifies the conclusion that rubella is no longer endemic within the United States (CDC, 2005).
The absence of endemic rubella is not equivalent to the absence of rubella cases. Travelers and immigrants from areas of the world where rubella is endemic will continue to spread the virus. If immunization practices are relaxed, pockets of susceptible persons will accumulate and the risk of rubella transmission will return. In 2003, member countries of the Pan American Health Organization established a goal of eliminating rubella and congenital rubella syndrome from the Western Hemisphere by 2010. As of 2004, 43 of the 44 countries and territories in the Western Hemisphere had initiated routine rubella vaccination programs that target young children combined with catch-up mass vaccination campaigns to reach older children, adolescents, and adults.
Current Status Of Rubella Worldwide
The global impact of congenital rubella syndrome is estimated at 100 000 infants per year born with symptomatic intrauterine rubella infection. However, global efforts to control rubella have begun. In 2003, 25% of the world population lived in a country with a national rubella vaccination program. The efforts for control of rubella through Latin America, particularly Mexico, as well as the highest recorded immunization rates in the United States, have resulted in the lowest incidence of reported rubella in the history of the United States. While rubella remains endemic on other continents, more than half the member countries of the World Health Organization include routine rubella immunization as part of their childhood vaccination series, raising the exciting possibility of global eradication of rubella at a future date (Robertson et al., 2003). Until this time is reached, efforts must include continued surveillance for rubella and congenital rubella syndrome, rapid response to outbreaks, and increased international efforts to support improved global rubella control.
Figure 1 Reported cases of rubella and congenital rubella syndrome in the United States from 1966 to 2004. Adapted from CDC (2005) Achievements in public health: Elimination of rubella and congenital rubella syndrome. MMWR 54: 279.
Bibliography:
- Best JM, Welch JM, Baker DA, and Banatevala JE (1987) Maternal rubella at St. Thomas’ Hospital in 1978 and 1986: Support for augmenting the rubella vaccination program. The Lancet 2: 88–90.
- Centers for Disease Control and Prevention (CDC) (1969) Prelicensing statement on rubella virus vaccine: Recommendation of the Public Health Service Advisory committee on Immunization Practices. MMWR Morbidity and Mortality Weekly Report 18: 21–22.
- CDC (1978) Recommendation of the Immunization Practices Advisory Committee. MMWR Morbidity and Mortality Weekly Report 27: 451–459.
- CDC (2005) Achievements in public health: Elimination of rubella and congenital rubella syndrome. MMWR Morbidity and Mortality Weekly Report 54: 279.
- Danovaro-Holliday MC, LeBaron CW, Allensworth C, et al. (2000) A large rubella outbreak with spread from the workplace to the community. Journal of the American Medical Association 284: 2733–2739.
- Gregg NM (1941) Congenital cataract following German measles in the mother. Transactions of the American Ophthalmological Society 3: 35–46.
- Hinman AR, Orenstein WA, Bart KL, and Preblud SR (1983) Rational strategy for rubella vaccination. The Lancet 1: 39–43.
- Meissner HC, Strebel PM, and Orenstein WA (2004) Measles vaccines and the potential for worldwide eradication of measles. Pediatrics 114: 1065–1069.
- Parkman PD, Beuscher EL, and Artenstein MS (1962) Recovery of rubella virus from army recruits. Proceedings of the Society for Experimental Biology and Medicine 111: 225–230.
- Reef SE, Plotkin S, Cordero JF, et al. (2000) Preparing for the elimination of congenital rubella syndrome (CRS): Summary of a workshop on CRS elimination in the United States. Clinical Infectious Diseases 31: 85–95.
- Reef SE, Frey TK, Theall K, et al. (2002) The changing epidemiology of rubella in the 1990s. Journal of the American Medical Association 287: 464–472.
- Robertson SE, Featherstone DA, Gacic-Dobo M, and Hersh BS (2003) Rubella and congenital rubella syndrome: global update. Revista Panamericana de Salud Publica 14: 306–315.
- Tobin JO, Sheppard S, Smithells RW, Milton A, Noach N, and Reid D (1985) Rubella in the United Kingdom, 1970–1983. Review of Infectious Diseases 7: S47–S52.
- Vyse AJ, Gay NJ, White JM, et al. (2002) Evolution of surveillance of measles, mumps and rubella in England and Wales: Providing the platform for evidence based vaccination policy. Epidemiologic Reviews 24: 125–136.
- Weller TH and Neva FA (1962) Propagation in tissue culture of cytopathic agents from patients with rubella-like illness. Proceedings of the Society for Experimental Biology and Medicine 111: 215–225.
- American Academy of Pediatrics (2006) Rubella. In: Pickering LK, Baker CJ, Long SS, and McMillan JA (eds.) Red Book: 2006 Report of the Committee on Infectious Disease, 27th edn., pp. 574–579. Elk Grove Village, IL: American Academy of Pediatrics.
- Chantler J, Wolinshky JS, and Tingle A (2001) Rubella virus. In: Knipe DM and Howley PM (eds.) Field’s Virology, 4th edn., pp. 963–990. Philadelphia, PA: Lippincott, Williams and Wilkins.
- Plotkin SA (2004) Rubella vaccine. In: Plotkin SA and Orenstein WA (eds.) Vaccines, 4th edn., pp. 409–433. Philadelphia, PA: WB Saunders.
- Reef SE and Cochi SL (2006) The evidence for the elimination of rubella and congenital rubella syndrome in the United States: A public health achievement. Clinical Infectious Diseases 43: S1–S168.
See also:
Free research papers are not written to satisfy your specific instructions. You can use our professional writing services to buy a custom research paper on any topic and get your high quality paper at affordable price.








