This sample Rabies Research Paper is published for educational and informational purposes only. If you need help writing your assignment, please use our research paper writing service and buy a paper on any topic at affordable price. Also check our tips on how to write a research paper, see the lists of health research paper topics, and browse research paper examples.
Introduction
Rabies is usually transmitted to humans by dog or bat bites, and is uniformly fatal. The virus, a single-stranded RNA Lyssavirus, invades nerves en route to the brain. The bite site and severity and the inoculum size are determinants of the infection risk and incubation period. Immediate wound cleansing and the prompt use of antirabies immunoglobulin and rabies vaccine are life-saving. Older vaccines are being replaced by safe, effective, and less costly tissue culture products. New reduced-dose intradermal administration schedules have made them more affordable (Wilde et al., 1999). Elimination of canine rabies in uncontrolled dog populations represents a major public health challenge.
Epidemiology
Extent Of The Disease And Routes Of Transmission
Rabies is generally transmitted through the bite of an infected mammal to another mammal. Rabies is believed to be capable of infecting all mammals. Both historically and currently, rabies was and is transmitted to humans primarily by canines, as well as by agriculturally important species such as cattle, horses, and sheep. Unless prevented by the measures described below, death inevitably results when the infection reaches and destroys its target, the central nervous system.
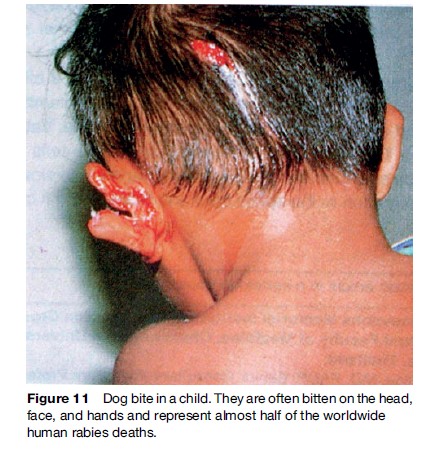
Rabies, and closely related Lyssaviruses, can be found throughout the world except in Greenland, Antarctica, and some isolated islands. Australia, previously considered rabies-free, harbors a Lyssavirus in fruitand insecteating bats, which causes a fatal rabies-like illness in humans (Warrilow, 2005) (Figure 1). Regions with large unsupervised dog populations present the greatest risk, since canines are the principal route of infection to humans. Vampire bat rabies in Central and South America is a hazard to humans and cattle. Worldwide rabies reporting is incomplete. The number of annual human deaths is unknown but is thought to be well over 50 000. Nearly 50% of these deaths are in children. They are less able to defend themselves against biting animals than adults, and are more likely to be bitten on high-risk body parts such as the face, head, and hands (WHO, 2005).
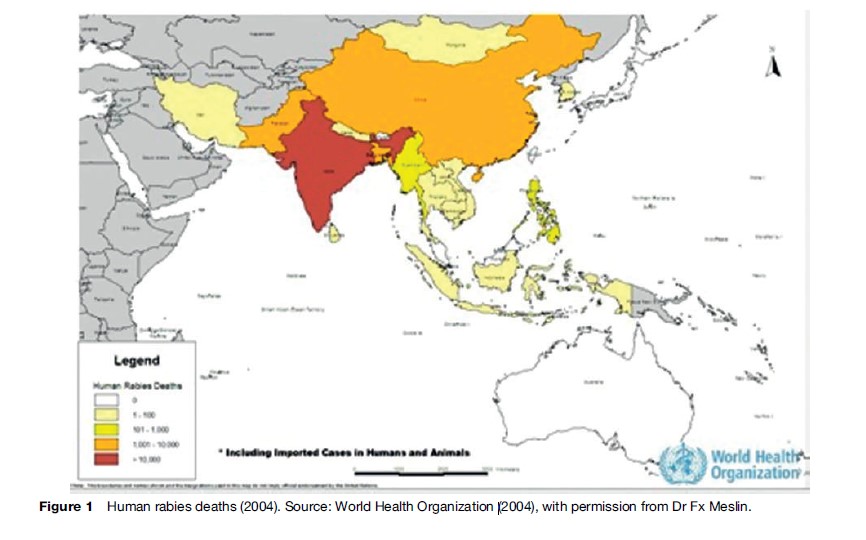
Rabies is responsible for more deaths than polio, yellow fever, Japanese encephalitis, SARS, or meningococcal meningitis. India alone had an estimated 30 000 annual rabies deaths during past decades. Pakistan estimates over 5000 (Wilde et al., 2005). Rabies is emerging again in China, which was virtually rabies-free during the reign of Mao Zedong. Japan, Taiwan, Malaysia, Singapore, and South Korea eliminated canine rabies decades ago. No other Asian countries have succeeded in doing so. Rabies can also be transmitted through the inhalation of bat secretions (e.g., in people exploring caves that house bats) and, rarely, through the transplantation of infected tissues. Rabies has also occurred in people who have bats in their homes without a history of bites.
New Lyssaviruses Are Being Discovered
There may still be undetected bat Lyssaviruses related to classical rabies virus in many parts of the world. Bats do not often interact with people, but transmission to humans and pets has been documented in The Americas, Europe, and Australia. Indigenous bat Lyssaviruses have now been identified in the Philippines, Thailand, Siberia, Central Asia, and Cambodia, as well as in Australia. The United Kingdom, previously considered rabies-free, recently experienced a human death from a European bat Lyssavirus.
Importance Of Animal Vector Control
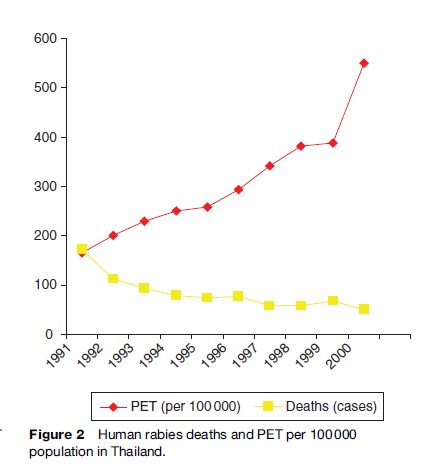
Dogs, cats, and other mammalian rabies vectors are occasionally transported from rabies-endemic regions to rabies-free ones. The recent introduction of rabies by Indonesian fisherman to previously rabies-free Flores Island resulted in an ongoing rabies outbreak with over 100 human deaths (Figure 2). Recreational hunters in the 1990s in the eastern United States unknowingly transported rabid raccoons from Florida to a hunting reserve, which led to a widely reported epidemic of rabies, with many animal and some human deaths that traveled up the East Coast of the United States. Thus, in order to eliminate rabies expansion through animal transport, animal control measures within countries and at national boundaries must be maintained.
Canines remain the most important animal vector for transmission of rabies to humans. We know virtually all that is needed to eliminate canine rabies, but cultural, political, and economic barriers have prevented implementation. Sustained vaccination of over 70% of the canine population can control rabies. In order to regularly vaccinate a large canine population, given their short life spans and rapid reproduction rates, canine numbers must be made manageable. This can only be done when societies and governments are motivated to enforce vaccination regulations and to reduce stray dog populations (Figure 3). It requires funding, legislation, and energetic enforcement. The World Health Organization (WHO) publishes detailed guidelines for human and veterinary professionals (WHO, 2005). Hindu and Buddhist countries have religious barriers to some control measures. These will have to be overcome by developing humane means of canine population control and education toward responsible pet ownership. Some efforts in this direction are now underway by the WHO, enlightened animal rights organizations, and governments.

Oral vaccination of foxes with bait containing vaccine has virtually abolished fox rabies in Europe. Efforts are being made in North America to apply this to foxes, skunks, and raccoons. There is as yet no strategy for controlling rabies in bats and most wildlife.
Clinical Diagnosis Of Rabies
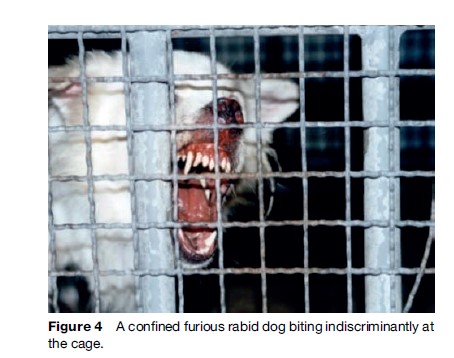
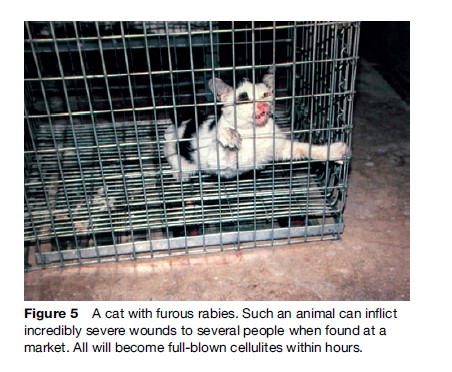
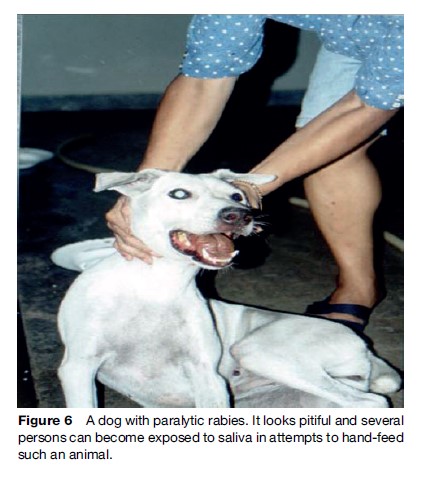
Rabies generally presents in one of two forms, ‘furious’ (Figures 4 and 5) or ‘paralytic’ (Figure 6). Diagnosis of canine and feline rabies is not difficult when it is of the furious form. Irritability, aggression, increased salivation, indiscriminate biting, and damaged and inflamed oral structures are obvious signs of the disease. The paralytic form of rabies (approximately 30% in dogs) presents diagnostic problems. The clinical picture is similar to other infections such as canine distemper. Euthanasia and histological examination of the animal’s brain is not always possible or available, and it is best to start postexposure prophylaxis (PEP) immediately in a possibly exposed human if rabies is suspected. A free interactive computer program can aid in the clinical diagnosis of canine rabies (www.soonak.com).
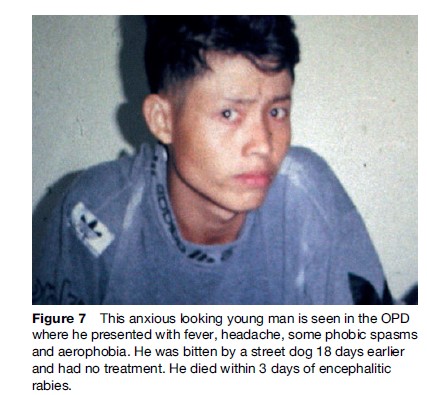
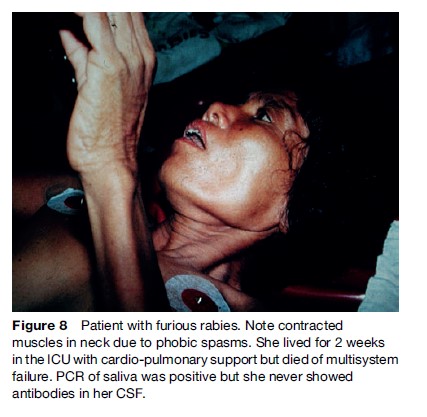
The ‘furious’ human form is characterized by a prodrome manifesting as a feeling of impending doom, pain, or abnormal sensation in or near the bite site (Hemachudha et al., 2002) (Figure 7). It is seen in 70% of cases. Other symptoms may be vague, such as anxiety, fever, headache, muscle aches, or even diarrhea. This is followed by the neurological phase, consisting of alternating intervals of agitation, aggression, and coherent calmness. Within a few days, coma ensues with respiratory failure, leading to rapid demise unless life is prolonged by intensive cardiopulmonary support. Several cases of humans surviving this stage have been reported, but they are the very rare exception, and there are only two longterm survivors known. Hydrophobic and aerophobic spasms of the neck and diaphragm may occur intermittently and may not appear together (Figures 8 and 9). Autonomic dysfunction may start early but usually becomes prominent in the neurological phase with excessive salivation, fluctuating blood pressure, cardiac arrythmia, pupillary dysfunction, and neurogenic pulmonary edema. Hallucinations and seizures are not usual in dog-related cases but are frequently seen in bat rabies (Hemachudha et al., 2002).
One-third of human cases present as the paralytic form resembling Guillain-Barre´ syndrome (GBS), due to a different host response to the infection (not to differences in the infecting virus). It is difficult to diagnose without experience and sophisticated laboratory help. Nerve electrophysiological studies are identical to those seen with GBS, and thus efforts to identify the virus or its RNA in sputum, urine, tissues, or cerebrospinal fluid may be required to confirm the diagnosis. Phobic spasms can be seen in only half of paralytic cases. Survival time is shorter in the furious form than in the paralytic form (mean of 5 versus 11 days). Rabies can also present in atypical ways, particularly when associated with bat exposure. Rabies must be considered in any patient presenting with unclear encephalopathy. Use of illicit or ‘recreational’ drugs, medications, or alcohol may mislead clinicians. Rapid deterioration to coma suggests rabies. Constant rigidity of muscles is the hallmark of tetanus. Acute hepatic porphyria can be excluded by appropriate tests. A history of recent animal encounters (which may be absent in cryptic bat cases), fever, muscular paralysis with preserved consciousness and intact sensory function, urinary incontinence, percussion myoedema, inspiratory spasms, and respiratory failure suggest paralytic rabies (Hemachudha et al., 2002, 2005; Jackson, 2006).
Rabies awareness is inadequate in non-endemic countries. This became evident from recent transplantation related cases in Germany and the United States. One case was diagnosed as drug abuse-related psychosis and the other as drug intoxication and a possible subarachnoid hemorrhage. The history that the former person had just returned from India having experienced a dog bite and that the latter had been bitten by a bat were not elicited or were disregarded. They were used as tissue donors for 10 recipients, of which 7 died of rabies. Interestingly, one of the survivors was a liver transplant recipient who had been previously vaccinated against rabies and had an anamnestic rabies-neutralizing antibody response.
Laboratory Diagnosis
The most secure method of diagnosis is by examination of brain tissue. Antemortem laboratory diagnosis of rabies in animals is not recommended since distribution of virus in organs may be variable, and shedding in saliva, urine, and spinal fluid is intermittent. Postmortem brain examination needs to demonstrate rabies antigen by direct fluorescent antibody (DFA) test, immunohistochemistry, or molecular methods. The DFA test is the gold standard; indeed, there were no false-negative results in a prospective study of 8987 brain impression smears (Tepsumethanon et al., 1997). Brain tissue, dried on filter paper, can be kept at room temperature for many days for rabies virus RNA detection. These techniques are available in many tertiary care centers and referral laboratories. To be of clinical value, results must be rapidly available, sensitive, and specific, since they will contribute to evidence-based postexposure prophylaxis decisions for exposed patients. In contrast to the above-mentioned methods, detection of classical Negri bodies in histopathological specimens is neither sensitive nor specific. Knowledge of the genetic sequences of rabies virus is useful for epidemiological surveillance and the study of transmission dynamics, as there are strains of rabies that circulate predominantly in foxes, bats, and other species.
Rapid antemortem laboratory diagnosis in humans is important for formulating ethical and rational management decisions (Hemachudha and Rupprecht, 2004). Reverse transcription polymerase chain reaction (RT-PCR) and other diagnostic molecular techniques can be performed, with results known within a day. Saliva, cerebrospinal fluid, urine, hair follicles, and tears should be used simultaneously owing to the intermittency of virus secretion. Negative results require repeat testing when there is a clinical suspicion of rabies. Brain imaging studies such as computerized tomography (CT) or magnetic resonance imaging (MRI) are most useful in excluding other diseases. MRI findings are usually localized to the brainstem, hippocampus, and hypothalamic regions, but are not sufficiently unique to make a specific diagnosis of rabies, they may be normal early after onset of clinical symptoms.
Brain necropsy can be done via the superior orbital fissure using a kidney or liver biopsy needle. It is invaluable when a complete necropsy is impossible.
Postexposure Prophylaxis (PEP)
The principles of postexposure prophylaxis (PEP) are (1) to cleanse the bite wound and (2) to provide the bite victim with antibodies to the rabies virus. Antirabies antibody prevents the virus from infecting cells and prevents death if virus is present before nerve cell invasion occurs.
PEP requires immediate cleansing of bite wounds with flowing water and soap and later with antiseptic agents. Washing with water may decrease the size of the virus (inoculum), while soap and antiseptic agents may denature the virus, thus preventing infection. This is followed by risk evaluation and the administration of a course of tissue culture vaccine (see Table 1). It takes 7–10 days for a significant level of vaccine-induced natural antibody to form. Unfortunately, this may leave sufficient time for the virus to invade peripheral nerve cells. Once inside nerve cells, it cannot be reached by antirabies antibody and can travel through the nerve cell centrally to the brain. Passive immunity must therefore be provided as soon as possible after the bite by aggressively injecting antirabies immunoglobulin (RIG) into and around the bite wounds to neutralize virus (WHO, 2005). Immunoglobulin, if injected intramuscularly at a distant site from the wound, is nearly useless. This was first reported in 1963 and was recently confirmed in our laboratory. A vaccine series is then started to induce active immunity with host antibody production. Consultation with an expert is mandatory when unusual problems are encountered. Various RIG preparations are available, as discussed below. PEP is costly and often substandard in many endemic regions of the world, and thus prevention through vector control is both more cost-effective (WHO, 2005) and efficacious.
Delay in starting PEP must be avoided at all cost. Rabies incubation periods may be as short as a few days or as long as many years, depending on the site of the bite, host factors, and size of the inoculum (Hemachudha and Rupprecht, 2004). Table 1 summarizes the approach to rabies-exposed patients. It is best to initiate PEP unless immediate laboratory studies of the responsible animal exclude rabies. If the animal is observed and later found free of the virus, the vaccine series can be discontinued. There are no contraindications to rabies PEP (WHO, 2005). An infected wound can be injected safely with RIG as long as antibiotics are also used to treat bacterial infection. A vaccine history in the responsible dog is not an absolute justification for not providing PEP to a bite victim, unless the vaccination has been thoroughly documented and more than one annual dose had been administered.
Table 2 lists current WHO-recognized vaccines, which are tissue and avian culture products, as well as some older preparations derived from animal neuronal tissues, which had the potential to induce autoimmune neurological syndromes. Additional modern vaccines are now appearing from India, China, and South America. WHO has approved four post exposure vaccine schedules using tissue culture vaccines (WHO, 2005). These are:
- The ‘gold standard’ Essen Regimen, which consists of one intramuscular full-dose injection on days 0, 3, 7, 14, and 28 after the exposure;
- The Zagreb regimen, which consists of two full-dose intramuscular injections on day 0 and one dose each on days 7 and 21;
- The Thai Red Cross Intradermal Regimen, which consists of two injections of 0.1 mL of any WHOrecognized tissue culture vaccine at two different lymphatic drainage sites on days 0, 3, 7, and 28; and
- The Oxford Intradermal Regimen, which consists of one injection of 0.1 mL of any WHO-recognized tissue culture vaccine at eight different body sites on day 0, at four sites on day 7, and at one site on days 28 and 90.
Intramuscular injections of vaccine must be administered in the deltoid or lateral thigh regions, avoiding fat. Intradermal vaccines are injected into arms or legs and, in the case of the Oxford Regimen, into the abdominal and intrascapular regions. The appearance of a split-skin ‘bubble’ at the injection site demonstrates successful intradermal and not subdermal administration (as in tuberculin testing). Reduced-dose intradermal PEP (schedules 3 and 4) significantly decrease the cost of vaccination and are used at rabies control clinics of several developing countries. Many studies have shown equivalent immunogenicity and efficacy (WHO, 2005). WHO-recognized tissue culture vaccines have excellent safety records. Adverse reactions with tissue culture vaccines are minor and equivalent to those seen with Expanded Program for Immunization (EPI) vaccines such as those against polio, mumps, and measles. Transient erythema, discomfort, and itching at injection sites as well as mild regional lymphadenopathy are reported with injections. Mild transient fever, headache, and malaise are also seen. Human diploid cell rabies vaccine (HDCV) and, very rarely, other tissue culture vaccines may cause mild serum-sickness-like reactions in individuals who have had a prior rabies series and are later given frequent boosters. These are not due to the viral components of the vaccine (Fischbein et al., 1993).
Vaccines alone will protect the vast majority of exposed patients, but it is not possible to predict which victim will die if not given passive immunization with RIG. The provision of passive immunity to protect severely exposed patients during the first critical days can be life-saving. Patients with facial, head, and hand bites are at the highest risk of death, and they represent a high-priority group if RIG immunoglobulin is in short supply (Figure 10). The original unpurified equine or sheep serum-derived antisera had a deservedly bad reputation for serum sickness and anaphylaxis. Second-generation, highly purified equine antirabies immunoglobulins (ERIG) contain whole IgG molecules. They have an acceptable safety margin, causing only 1–7% serum sickness reactions, depending on the product and batch.
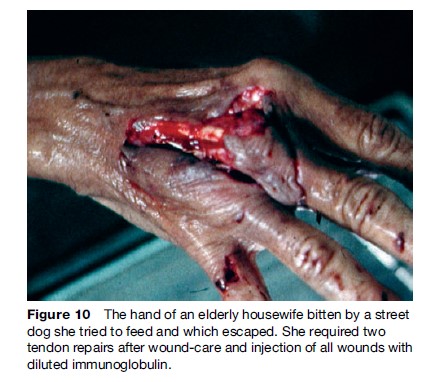
An effort was then made to reduce the serum sickness rate by further purification and splitting the antibody using pepsin digestion. This reduced the serum sickness rate only slightly. We have, however, become aware of several cases of treatment failure where split equine or human IgG products were used. Human rabies immunoglobulin (HRIG) appears to be as effective as the whole IgG ERIG and has virtually no adverse reactions. It is, however, in extremely short supply and is very expensive, and therefore is not available where it is needed the most, as in poorer rabies-endemic countries. A new equine product has been chromatography-purified, pepsin digested and heat-inactivated. It is safe, but its efficacy is controversial. Several new manufacturers of ERIG have emerged in Thailand, India, South America, and China. Some products are undergoing WHO preapproval studies and may appear on the international market in the near future. In our view, it is unfortunate that most of these are pepsin-digested split IgG products. Monoclonal rabies antibody technology has emerged and monoclonal antibodies have been found to be safe and highly effective in animal experiments, and are now undergoing human studies. It will take time for such products to become commercially available. HRIG and ERIG must remain on the WHO essential drug list until we have a safe, effective and affordable monoclonal product commercially available (Goudsmit et al., 2006).
Pre-Exposure Vaccination (PREP)
Human and equine rabies immunoglobulins are not available in many rabies-endemic regions, and PEP is often not carried out to WHO standards. Pre-exposure prophylaxis (PREP) is therefore recommended for travelers, workers in certain occupations who are likely to come in contact with infected animals, and laboratory workers exposed to Lyssaviruses. One study from Thailand showed that 9% of tourists had unanticipated or unwanted canine contact, suggesting that vaccination of tourists to endemic regions should be more widely considered. Recent studies have demonstrated that immunity following WHO-recommended tissue culture vaccine injections is very long-lasting. One study showed that neutralizing antibodies can be detected as long as two decades after completing a PREP or PEP series. Booster injections then result in an accelerated antibody response. WHO recommends one intramuscular or intradermal booster injection on days 0 and 3 in an individual who has experienced a possible rabies exposure after having had a reliable history of PREP or PEP with a WHO-recognized tissue culture rabies vaccine (WHO, 2005). An alternate method is to administer intradermal injections of 0.1 mL vaccine at four sites (deltoid and lateral thigh) at one sitting. This saves clinic costs and travel time but has not yet been WHO-approved. Laboratory scientists at high risk of rabies, such as those working with potentially rabid animals or live virus, are still advised to have either periodic antibody titer determinations or a booster every 5 years. Some diplomatic missions, nongovernmental organizations, military, and UN teams recommend PREP for their staff when transferred to rabies-endemic countries.
Many countries are failing to control canine rabies. Since children represent half of rabies deaths (WHO, 2005), this has led to suggestions to include rabies vaccine as part of the childhood EPI in high-risk regions. Cost– benefit considerations and priority of funds for other vaccinations have, however, prevented implementation (Figure 11).
Management Of Human Rabies
Treatment of human rabies was the subject of a Canadian and U.S. CDC-sponsored conference in Toronto in 2002. The expert consensus was that only comfort care should be provided, given the uniformly fatal prognosis ( Jackson et al., 2003). Intensive curative efforts should be reserved for a time when promising new technologies become available. We have as yet no known proven antiviral agents against Lyssaviruses. However, the survival of a 15-yearold girl, bitten by a bat who had not received PEP, has created hope. Treatment consisted of intensive care and induced deep coma with ketamine and benzodiazepine to lessen excitotoxicity as well as ribavarine. This patient was unusual in that she had neutralizing antibodies on admission in both serum and spinal fluid but no demonstrable viable virus or viral RNA. Her case was similar to the other survivor, a 6-year-old (reported in 1972), who had a bat bite and early antibodies in serum and spinal fluid. He was treated with supportive care only and made a full recovery. His virus could not be isolated, either. These bat-derived agents could have been of less virulence, or both subjects may have managed to mount an unusually rapid and aggressive immune response that controlled their disease. By way of example, we recently treated a rabies patient who received the coma induction regimen with ketamine and ribavarine, never developed neutralizing antibodies, and died of multisystem failure on the eighth hospital day. Ample virus was identified throughout his hospital course. Approximately 25% of our dogrelated human patients developed serum-neutralizing antibodies regardless of the form of rabies. However, none had neutralizing antibodies in CSF. Similar comainduction regimens have been applied to 11 other patients in the United States, Europe, Asia, and Canada without success.
Bibliography:
- Fischbein DB, Yenner KM, Dreesen DW, et al. (1993) Risk factors for systemic hypersensitivity reactions after booster vaccinations with human diploid cell rabies vaccines, a nationwide prospective study. Vaccine 14: 1390–1394.
- Goudsmit J, Marissen WE, Weldon WC, et al. (2006) Comparison of an anti-rabies human monoclonal antibody combination with human polyclonal anti-rabies immune globulin. Journal of Infectious Diseases 15(193): 796–801.
- Hemachudha T and Rupprecht C (2004) Rabies. In: Roos K (ed.) Principles of Neurological Infectious Diseases, pp. 151–174. New York: McGraw Hill
- Hemachudha T, Wacharapluesadee S, Lumlertdaecha B, et al. (2002) Human rabies: A disease of complex neuropathogenetic mechanisms and diagnostic challenges. Lancet Neurology 1(2): 101–109.
- Hemachudha T, Wacharapluesadee S, Mitrabhakdi E, Wilde H, Morimoto K, and Lewis RA (2005) Pathophysiology of human paralytic rabies. Journal of NeuroVirology 11: 93–100.
- Jackson AC (2006) Rabies: New insights into pathogenesis and treatment. Current Opinion in Neurology 19(3): 267–270.
- Jackson AC, Warrell MJ, and Rupprecht CE (2003) Management of rabies in humans. Clinical Infectious Diseases 36(1): 60–63.
- Tepsumethanon V, Lumlertdacha B, Mitmoonpitak C, et al. (1997) Fluorescent antibody test for rabies: Prospective study of 8987 brains. Clinical Infectious Diseases 25: 1459–1461.
- Warrilow D (2005) Australian bat lyssavirus: A recently discovered new rhabdovirus. Current Topics in Microbiology and Immunology 292: 25–44.
- WHO (2005) WHO Expert Consultation on Rabies, First Report. Technical Report 931. Geneva, Switzerland: WHO.
- Wilde H, Khawplod P, Khamoltham T, et al. (2005) Rabies control in South and Southeast Asia. Vaccine 23: 2284–2289.
- Wilde H, Tipkong P, and Khawplod P (1999) Economic issues in postexposure rabies treatment. Journal of Travel Medicine 4: 238–242.
See also:
Free research papers are not written to satisfy your specific instructions. You can use our professional writing services to buy a custom research paper on any topic and get your high quality paper at affordable price.