This sample Re-Emerging Diseases Research Paper is published for educational and informational purposes only. If you need help writing your assignment, please use our research paper writing service and buy a paper on any topic at affordable price. Also check our tips on how to write a research paper, see the lists of health research paper topics, and browse research paper examples.
Introduction And Definition Of Re-Emerging Infections
During the past 50 years remarkable gains have been achieved with the control of many infectious diseases. At the same time, new and previously unknown pathogens have emerged, and some, like HIV, have spread globally killing millions of individuals, disrupting societies, and reshaping the demographics of countries and regions. In addition, infectious diseases previously thought to be under control have re-emerged in many parts of the world. For purposes of this discussion, re-emerging infections are defined as those that have one or more of the following characteristics: increase in number of cases; expansion of current foci of infection or appearance in new geographic areas; appearance of infections in populations previously unaffected; and increase in severity of illness or mortality. This research paper explores the mechanisms through which infections re-emerge and the multiple factors in the world today that facilitate the reemergence of infections. Several specific infections are used as examples to illustrate key points. A discussion of the characteristics of infections that are most likely to reemerge in the future and a framework for preventing reemergence of infections, and thereby mitigating their consequences, concludes the paper.
Mechanisms For Re-Emergence
Multiple Factors Involved
Infectious diseases are dynamic; unless eradication of a microbe is achieved, which is rare, the interactions between microbes and humans undergo constant change and evolution. Microbes, with their rapid replication time, have the capacity to adapt to change much more rapidly than humans. At a fundamental level, infections that have been controlled re-emerge because: the microbe has changed, moved, or become more abundant; the host lacks or loses immunity (or capacity to respond to infection) or is not treated; or contacts between microbe and host increase. Although this may sound simple, multiple factors – biological, socioeconomic, demographic, and environmental – influence this dynamic relationship.
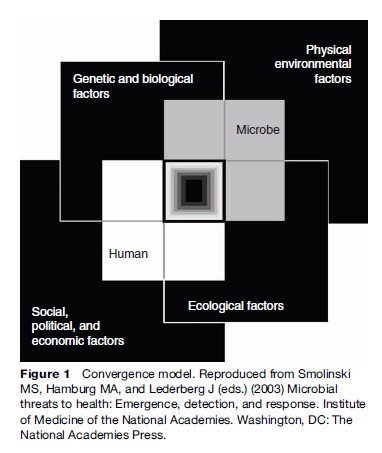
The convergence model (Figure 1) illustrates the broad context and interlocking domains of determinants in which infections emerge or re-emerge. Although the interactions between the human host and microbe are at the center of the process, other factors interact with each other and affect host, microbe, and their interactions. Disease emergence is often complex with multiple interacting factors involved. The model aptly depicts the central area of overlap as a black box, illustrating gaps in our understanding of many of the elements and how they interact.
For an infection to be defined as re-emerging, it must be recognized and characterized. It is important to acknowledge that many infections persist, reappear, and spread silently. Unless adequate clinical and laboratory facilities exist to accurately diagnose infections, they may go undetected or be categorized as a viral illness or flu. Microbes that cause infections that produce clinical signs and symptoms (such as fever and cough, fever and diarrhea, fever and muscle aches) similar to those of many common infections – such as influenza, tuberculosis (TB), salmonellosis, dengue fever, and malaria – may not be identified, especially in resource-poor settings where clinical laboratory support is absent or limited. Microbes that cause unusual clinical findings (e.g., vesicular skin eruption in monkeypox), high mortality (e.g., yellow fever), or produce large outbreaks (e.g., dengue fever and dengue hemorrhagic fever [DHF]) may be more likely to be identified.
Re-emerging infections are caused by all classes of pathogens (i.e., viruses, bacteria, fungi, helminths, protozoa) and involve pathogens with different modes of transmission (e.g., direct person-to-person transmission, airborne, vectorborne, food and waterborne) and different sources (e.g., another human, animal reservoir, soil and water). Typically multiple factors will have contributed to the re-emergence of a specific infection. These may vary by time or geographic region. Populations and regions vary in their vulnerability to the re-emergence of infections and capacity to intervene promptly, so to some extent re-emergence may be specific to time, place, and population. Because of the extensive linkages in the world today through trade and travel, re-emergence of an infection may have broader implications and pose greater risk to distant populations than it might have a few decades ago.
Changes In The Pathogen
The types of changes in a pathogen that can contribute to the re-emergence of an infection include development of resistance to antimicrobial agents that were previously effective, acquisition of new virulence factors or emergence of strains that are more virulent or transmissible, and emergence and spread of strains against which available vaccines are ineffective. Examples of each follow.
Resistance, Virulence, And Transmissibility
Resistance of a microorganism to an antimicrobial drug refers to the capacity of an organism to survive in the presence of that drug in therapeutic concentrations. Microbes can also be characterized by their resistance to killing by physicochemical conditions (e.g., heat, cold, acid, other) that may be relevant for survival in the environment, but these attributes are not discussed in this section. Virulence is a quantitative measure of pathogenicity of an organism or its likelihood of producing disease. Transmissibility refers to the ease of spread of a microbe from one host to another. These are three separate attributes that are not necessarily linked. For example, the H5N1 virus, the influenza strain currently circulating in avian populations, has shown resistance to the adamantane group of antiviral drugs. It is highly pathogenic in chickens and humans (and some feline species) causing high mortality in those species. In contrast, some infected ducks excrete the virus without showing symptoms of infection. As of early 2007, it is poorly transmissible from human to human, but highly transmissible in chicken populations. In general, many of the viruses that cause the common cold are highly transmissible from person to person but cause only mild illness.
Resistance
Increasing resistance of microbes to antimicrobials is occurring globally and involves microbes that cause millions of human deaths annually, including Staphylococcus aureus, Streptococcus pneumoniae (the cause of pneumococcal pneumonia and meningitis), Mycobacterium tuberculosis (the cause of TB), malaria parasites, influenza viruses, and human immunodeficiency syndrome viruses (HIV), among others.
Increasing resistance among some bacteria was initially localized primarily to tertiary care hospitals (e.g., methicillin-resistant S. aureus) but has now moved into the community and around the world. Increasing resistance is found among antimicrobials used to treat all types of infections, including viral, bacterial, fungal, and parasitic infections. In some instances, alternative agents are available for treating resistant infections, but alternative drugs may be more toxic, less effective, unavailable because of limited supplies, or expensive. High cost alone can mean many populations will be unable to have access to effective treatment, rendering infections operationally untreatable. In addition, resistance of arthropod vectors (e.g., mosquitoes) to pesticides has complicated the control of vectorborne infections, like malaria and dengue.
Bacteria can become resistant through mutations or by acquiring genetic material from related or unrelated bacterial species through horizontal exchange. Genetic changes in bacteria can occur in the absence of antimicrobials, but the presence of antimicrobials puts selective pressure on microbial populations. Resistant organisms may be able to flourish when an antimicrobial agent kills off other organisms that may compete for resources and may become the predominant population. Resistance traits are transferred to the progeny and potentially to unrelated strains of bacteria. The broad and indiscriminate use of antimicrobials has contributed to the rising resistance, but even appropriate use of antimicrobial agents puts pressure on microbial populations. In addition, certain clones or strains of resistant bacteria may become widely disseminated. With penicillin-resistant S. pneumoniae, for example, only 10 clones were shown to be responsible for 85% of invasive disease by this organism in the United States in 1998 (Corso et al., 1998). Travel and social networks may be important in the spread of resistant (or virulent) clones.
- aureus resistant to methicillin has expanded in geographic range, but until recently only five major clones were responsible for most of the worldwide problem. The microbe spread initially in defined groups with close contact – for example, children in child care facilities, athletes, intravenous drug users, prison inmates, and military recruits – then the organism disseminated into the general community. This may have been facilitated by special virulence factors of the organism that favored its survival (Furuga et al., 2006).
Resistance as a single factor cannot fully explain the re-emergence of TB or malaria, for example, but presence of resistance has contributed to increasing morbidity and mortality in some populations and makes control more complicated and costly.
Virulence
A previously uncommon but more virulent strain of Clostridium difficile, a cause of colitis that can be severe, has emerged to cause multiple outbreaks with substantial mortality in hospitals in North America and Europe. It produces 16–23 times more toxin than other strains of C. difficile, which may account for its increased virulence. It is also resistant to fluoroquinolones, and their wide use may have favored its emergence (McDonald et al., 2005).
An unusual example of a change in virulence has occurred in the attenuated live poliovirus vaccine. In rare instances (0.4–3 per million children vaccinated), the vaccine virus, which replicates in the gastrointestinal tract of the recipient, reverts to full neurovirulence. Not only can the vaccine-derived neurovirulent virus cause paralysis in the vaccine recipient, but vaccine-derived virus can also circulate in populations. Circulation of virulent vaccine-derived virus, in some instances with associated outbreaks of paralytic polio, has been documented in China, Egypt, Haiti, Madagascar, and the Philippines. Paralytic polio that has re-emerged in areas where circulation of wild poliovirus had ceased in some instances has been caused by vaccine-associated poliovirus (Kew et al., 2002). In other areas, wild poliovirus has been reintroduced from areas where polio persists in the human population. Geographic areas that appear to be at highest risk for circulation of vaccine-associated poliovirus are those with low vaccine coverage. Immunodeficient individuals (especially those with hypogammaglobulinemia) have been found to be long-term excreters of vaccine-derived poliovirus (in feces), one for at least 20 years (MacLennan et al., 2004). This remains a concern in making decisions about when it might be possible to stop using oral poliovirus and killed polio virus vaccines.
Strains, Serotypes, Serogroups, Subtypes
Microbes often exist as many different subtypes, serotypes, serogroups, or strains. This antigenic variation means persons who are immune (by natural infection or immunization) to one strain of an organism, may be susceptible to another. A previously uncommon serogroup of Neisseria meningitidis W135 (cause of meningococcal meningitis and sepsis) caused outbreaks among pilgrims to the Hajj in Saudi Arabia in 2000 and spread internationally. Because of previous outbreaks of meningococcal infections among the pilgrims (and subsequently their contacts in their countries of residence), Saudi Arabia started requiring all visitors to the Hajj to produce a certificate of vaccination against meningococcal disease upon entry into the country. Most visitors received a meningococcal vaccine active against serogroups A and C, the serogroups responsible for most epidemics of meningococcal disease in the past. It was in this context that meningococcal infections reemerged in the pilgrims in the spring of 2000, caused by serogroup W135. About 240 cases were reported in Saudi Arabia. In all, more than 400 cases in pilgrims and their contacts were identified in at least 14 different countries. Analysis of isolates from patients in France and the UK using multilocus sequence typing, DNA fingerprinting, and other techniques showed that they were indistinguishable from isolates from Saudi Arabia. Pilgrims who became infected could carry the W135 meningococcal clone and transmit infection, even if they did not develop symptoms. Of note, the vaccine used in the United States was a quadrivalent vaccine active against serogroups A, C, Y, and W135. Although the risk of disease was low in recipients of the quadrivalent vaccine, recipients could still become carriers of the outbreak strain as the vaccine does not prevent carriage (Dull et al., 2005). Saudi Arabia now requires pilgrims to the Hajj to have a vaccine active against W135. During February 2002, an epidemic caused by W135 began in Burkina Faso. By May more than 12 500 cases had been reported to the World Health Organization (WHO).
Dengue virus, a mosquito-transmitted flavivirus, is widespread in tropical and subtropical regions of the world and is expanding in geographic range and severity. Four different serotypes of dengue viruses cause disease in humans: DEN-1, DEN-2, DEN-3, and DEN-4. Infection with one dengue serotype is followed by only brief immunity to the other serotypes. Subsequently, if individuals are infected with a different dengue serotype, they are at increased risk (perhaps 100-fold greater) for severe disease, manifested as dengue hemorrhagic fever or dengue shock syndrome. In studies from Thailand, no cases of DHF were observed in patients with primary dengue infection, whereas 1.8–12.5% of those with a secondary infection developed DHF. The appearance of a new dengue serotype in a population that already has previously experienced high rates of infection with a different serotype may be followed by an outbreak of DHF. Infections that occurred a decade or more earlier may remain immunologically relevant and predisposed to severe illness. It is also becoming clear that dengue viruses vary in virulence, and introduction of a more virulent strain may be followed by particularly severe outbreaks.
Abundance
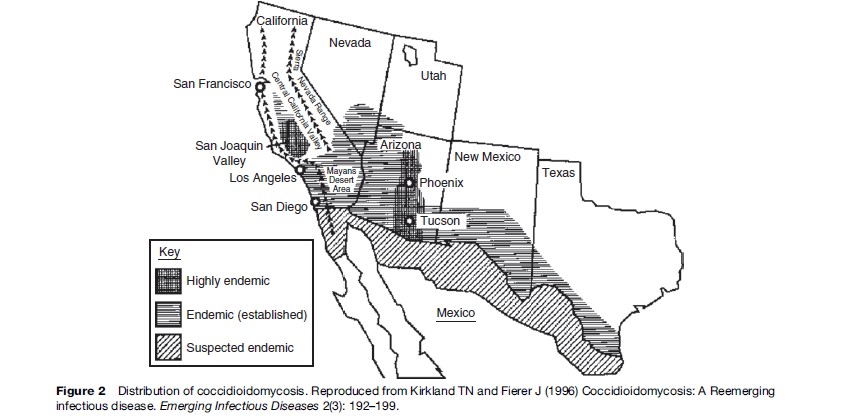
The presence (or absence) and abundance of many organisms is linked to the physicochemical environment, directly – for example, through temperature, rainfall, humidity, soil or water characteristics, pH, nutrients, and so on – or indirectly, for example, through the presence or abundance of arthropod vectors (e.g., mosquitoes), intermediate and reservoir hosts (such as rodents), and vegetation. Coccidioidomycosis, caused by a soil associated fungus, is found in arid and semiarid areas with alkaline soils in regions with hot summers and short, moist winters. Humans become infected by inhaling airborne arthroconidia. Growth of the fungi in soil is linked to temperature and rainfall. Outbreaks in the southwest United States have been correlated with the amount of rainfall over preceding months. The sequence of heavier than usual rainfall followed by prolonged drought in association with hot and dusty conditions favors dispersal of the fungus in a form that can be inhaled. But several other factors have also contributed to increasing cases of coccidioidomycosis in the U.S. southwest (Kirkland and Fierer, 1996). This area is one of the most rapidly growing parts of the United States; many new residents have moved from nonendemic areas, and thus lack immunity to the fungus. The area has become a popular place for retirement (Figure 2); persons more than 65 years of age have the highest incidence of infection of any age group. The HIV-infected population, another vulnerable group, has also grown in size. Land development, including construction, increases dust and potential exposures. Off-road vehicle use, which has become more common, also creates dust that can disperse the fungus. So although coccidioidomycosis is a ‘place’ disease that is influenced by geoclimatic conditions, the demographic shifts, human activities, and land development in the area have been important forces in the increase in cases. This disease would not have re-emerged if humans had not entered into and altered this environment.
An infection with a rodent reservoir host, such as hantavirus infection, can increase in response to geoclimatic conditions (Hjelle et al., 2000). The El Nino–Southern Oscillation (ENSO) has been associated with increased precipitation in the southwestern part of the United States. In this instance, the increased rainfall is associated with an expansion of the deer mouse population, presumably because of an expanded food supply for the mice, which are infected with the virus and excrete it in saliva, urine, and feces, typically without showing symptoms. Rodent populations can increase more than 10-fold in a year. More rodents mean more virus and more potential opportunities for human–rodent excreta contact and human infections.
Changes In The Human Host
Improved Sanitation And Increase In Clinically Apparent Hepatitis
As noted above, presence of antibodies because of past infection with one dengue serotype can predispose to more severe infection if exposure to a different serotype occurs. However, many infections are followed by long-lasting, sometimes lifelong, immunity. When infection with hepatitis A occurs in young children, infection is often mild or asymptomatic and often not diagnosed. With increasing age, severity of infection increases, and the case fatality rate may be 2% or higher in persons 65 years and older. Paradoxically, as availability of clean water and good sanitary facilities have improved in many countries, outbreaks of hepatitis A are now occurring, whereas they were unknown in the past when virtually everyone was infected and immune by age 5 years. By shifting upward the age at which individuals are infected, the virus causes acute, often severe clinical illness. Some countries that did not previously have visible hepatitis A outbreaks are now seeing large outbreaks in young adults.
Outbreaks Of Vaccine-Preventable Infections
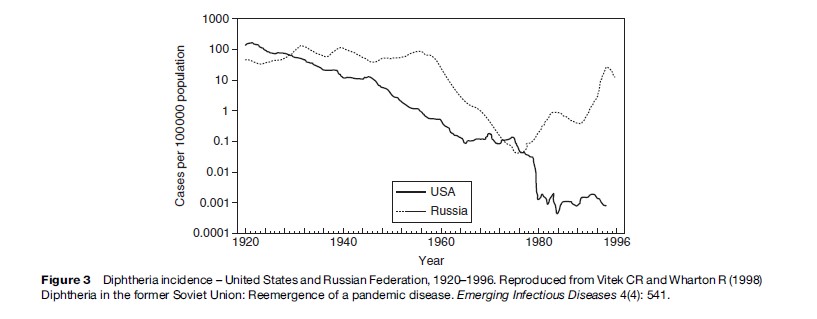
Infections that have been prevented by immunization programs can re-emerge if immunization programs and other supports fail. After having been largely controlled for several decades, diphtheria re-emerged in the Russian Federation in 1990 and spread to all of the newly independent states and Baltic states of the former USSR (Dittmann et al., 2000) (Figure 3). Between 1990 and 1998, more than 157 000 cases and 5000 deaths were reported by countries of the former Soviet Union. Diphtheria, an acute bacterial infection caused by the toxin-producing bacterium Corynebacterium diphtheriae, which spreads from person to person through close contact, has a case fatality rate of 5–10%. Infection can be prevented by immunization with diphtheria toxoid; when infection occurs, mortality can be reduced by treating with diphtheria antitoxin. Universal childhood immunization was introduced in the 1940s and 1950s; developing countries also achieved high levels of immunization after the Expanded Program on Immunization (EPI) in the 1970s. Even before 1990, serologic studies had shown a substantial percentage of adults lacked immunity to diphtheria. Adults in the former Soviet Union who were in the 40–49-year-old age group in the 1990s had the lowest levels of immunity. They had lived during a time when diphtheria was largely controlled by immunization, so had not been exposed to natural infection. Most had never received any doses of vaccine since childhood, as adult booster doses were not recommended at that time. Toxigenic strains of diphtheria from Afghanistan were introduced into a refugee population. Infection spread first to large urban centers and then along major transportation routes to other cities and towns, and finally to rural areas. The spread was facilitated by the presence of large numbers of displaced persons. The proportion of cases in persons more than 15 years old was 64–82% in some areas. Relatively few cases occurred in adults aged 50 years or more, presumably because of past exposure to diphtheria, which was still occurring when they were children. The highest incidence and death rates were in individuals between the ages of 40 and 49 years. At the outset of the epidemic, the case fatality rate exceeded 20%, probably due to delayed treatment and lack of diphtheria antitoxin.
Multiple factors contributed to the massive outbreak as analyzed by Dittmann and colleagues (2000). Among those identified were: less intensive immunization, use of lower potency vaccine, antivaccine campaigns in some areas, deterioration of the health-care infrastructure, delay in outbreak control, and lack of adequate supplies for prevention and treatment in many areas. Other countries were also affected by the outbreak as cases were imported into several European countries and the United States. Most countries today do not routinely give diphtheria toxoid to adults; in many countries 30–60% of adults may be susceptible to diphtheria, which is a phenomenon created by the vaccine era and vaccine policy of giving vaccine primarily to young children.
Increased Risk Of Infection Because Of Altered Immune Response
Tuberculosis
The appearance of and spread of infection with HIV has had a profound effect on the burden from many other infectious diseases, most notably TB. The interaction between HIV/AIDS and TB is bidirectional, with each making the other worse. Infection with M. tuberculosis upregulates HIV replication, increases viral load, accelerates the decline of CD4 count, leads to more rapid progression of HIV, and increases the risk of death. The impact of HIV on TB is to increase the risk of primary infection, increase risk of rapid progression, increase likelihood of reactivation in those with latent infection, and increase the risk of reinfection. About one-third of humans are infected with M. tuberculosis, though infection is latent and asymptomatic in the majority. In most individuals the likelihood that TB infection will progress to active disease is only 5–10% over a lifetime. In persons co-infected with HIV and TB the risk of developing active TB may be as high as 10% per year. In areas where the incidence of TB was already high, the spread of HIV infection has led to an increase in incident cases of TB. Although the spread of HIV infection is not the only reason for re-emergence of TB in many areas, it is an important one – and one that has made control of TB much more difficult.
Data collected during the first half of the twentieth century on TB mortality showed notable increases in TB deaths during World War I and World War II, including in some countries that were not occupied (see Figure 4). This was an era before specific antituberculosis drugs were available. Increases in mortality were thought to relate to poor nutrition (especially limited protein supply), crowding in dark dwellings, and stress. Of note, Denmark was not occupied and did not take part in the armed conflict, yet TB mortality rose starting in 1915 (Figure 4). The rise in TB coincided with a drop in meat consumption, as meat was exported to England to support the war. Submarine attacks interrupted exportation from Denmark in 1917, making meat available for local consumption – and TB mortality fell (Figure 4).
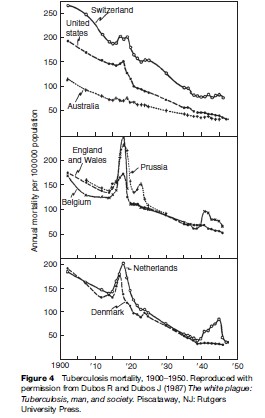
Even today with good drugs available for the treatment of TB, social, political, and economic factors can influence treatment and outcome. TB has been a problem in disadvantaged populations, including inmates in prisons and homeless populations. Gustafson and colleagues (2001) described events in Guinea-Bissau, West Africa, where armed conflict, starting in 1998, limited access to TB treatment. This was followed by an increase in the mortality rate ratio (MR) (3.21) among those being treated for TB and was most pronounced among those who were HIV infected (MR 8.19).
Contacts Between Host And Microbe
Many factors can lead to an increase in contact between host and microbe. The outbreaks of infectious diseases, such as shigellosis, cholera, and measles in refugee camps and in other displaced populations, indicate how rapidly infections can spread when basic supports, such as clean water, adequate sanitary facilities, and immunization, are unavailable (Goma Epidemiology Group, 1995).
Hepatitis C
Although a serologic test to identify persons infected with hepatitis C virus (HCV) was not developed until 1989, the infection had been described based on its clinical and epidemiologic features, and by the absence of antibodies to other common infections, including hepatitis A and hepatitis B. Before the wide use of tests to screen donated blood, hepatitis C was the most common blood-borne infection following blood transfusions. Although hepatitis C is found worldwide, prevalence varies widely. Seroprevalence in Egypt has been reported to be in the range of 20% in blood donors, one of the highest prevalence rates anywhere in the world. Prevalence varies by age and geographic region, but is higher in Egypt than in neighboring countries. The HCV subtypes in Egypt are mostly the same, suggesting epidemic spread throughout the country. Children have a low prevalence of infection and people living in desert areas have the lowest prevalence. Investigators have tried to find plausible explanations for these observations.
Schistosomiasis, a parasitic infection that requires snails as an intermediate host, is widespread in the Nile Delta and Nile Valley. After it was discovered in 1918 that injections with an antimony salt could kill schistosomal flukes residing in humans, use of parenteral antischistosomal therapy (PAT) became widespread. Because the drug could be administered only by injection and multiple injections (12–16) were required, mass treatment campaigns were organized to deliver the drug. Sterilization procedures of reusable injection equipment were likely to have been omitted in some instances or were inadequate. When effective oral therapies for schistosomiasis became available in the 1970s and 1980s, they replaced PAT. Investigators who have analyzed the prevalence of HCV antibodies by age and geographic region have found a significant association between exposure index for PAT and HCV prevalence rates. This suggests that the mass campaigns to treat schistosomiasis may have effectively spread HCV to a significant portion of the Egyptian population (Frank et al., 2000). Although initial infection with hepatitis C may be mild or asymptomatic, infection becomes persistent in 50–80% of those infected. The burden of disease related to infection, progression to cirrhosis or development of hepatocellular carcinoma, comes years or decades after infection was acquired. Unsafe injection equipment can be a source of infection in any country, but the prevalence of infection is less than 2–3% in most countries.
Although safer, more effective treatment for schistosomiasis is now available, schistosomiasis has increased in some communities in Egypt, Senegal, Cote d’Ivoire, and elsewhere following the building of dams (N’Goran et al., 1997). Dams can have potential economic and health benefits, but have also been associated with increases in vectorborne infections (see the following section).
Malaria
Malaria continues to kill a million or more, mostly young children in Africa, each year, and saps the strength and productivity of individuals in endemic areas by causing fatigue, episodic fevers, and anemia. Although in the overall area of the globe where malaria is endemic the disease has decreased by half in the last century, demographic changes have resulted in an increase of 2 billion people living in areas with malaria risk (Hay et al., 2004). The intensity of transmission has also increased in some regions. Some reasons for increase in malaria include insecticide resistance of the mosquito vector and breakdown in control programs, increase in resistance of the malaria parasite to the inexpensive, older antimalarial drugs (e.g., chloroquine), and population movements (both movement of nonimmune populations into malarious areas and also migration of malaria-infected persons into regions infested with competent malaria vectors and climatic conditions compatible with malaria transmission). Co-infection with malaria and HIV also appears to have adverse consequences, though not of the magnitude noted with HIV and TB. HIV infection is associated with an increased risk of parasitemia and clinical malaria in adults and increased risk of treatment failure. HIV-infected pregnant women have more frequent and higher-density malaria parasitemia than do uninfected women; infants born to women infected with both malaria and HIV have a threeto eightfold higher risk of postnatal death than infants born to mothers with either infection alone. Malaria in HIV-infected persons also leads to increased viral load, which returns to baseline with prompt treatment of malaria.
Because malaria is transmitted by a mosquito vector – environmental factors, including temperature, humidity, rainfall, and land use – can also influence abundance and location of mosquito vectors. A study in northwestern Ethiopia linked an increase in malaria transmission to intensified maize cultivation. Maize pollen, eaten by mosquito larvae, can influence the insect’s size and development. In many villages, maize was planted in fields less than 2 meters from houses. The predominant variety planted, chosen for its high yield, matured late, with pollen dispersal extending into the peak period of mosquito larval development (Kebede et al., 2005).
In the Peruvian Amazon in South America malaria prevalence increased sharply in the 1990s at a time of population migration and growth as well as extensive deforestation. Researchers gathered data from 56 different study sites that varied by type of vegetation and human population density. They captured mosquitoes that landed on human volunteers in the evening hours to assess whether deforestation was associated with increased biting rate of Anopheles darlingi, the primary vector of falciparum malaria in that region. They found that human-biting rates were consistently higher in deforested areas, with the A. darlingi biting rate more than 278 times higher in deforested areas than in areas that were predominantly forested (Vittor et al., 2006).
Dams built to provide irrigation for crops can also provide water for breeding of mosquito vectors. In a longitudinal study of communities living near dams and control communities at similar altitude but beyond the flight range of mosquitoes that might breed in standing water created by dams, researchers found that malaria was significantly more common in communities near the dams (14.0 episodes in 1000 child months vs. 1.9 in control villages) (Ghebreyesus et al., 1999).
Introduction of a mosquito species that is a more efficient vector for malaria transmission can also lead to an increase in transmission. An example of this occurred when Anopheles gambiae was introduced into Brazil, probably by way of boats that made mail runs between Dakar, Senegal and Natal, Brazil. A. gambiae larvae were first identified in March 1930 and subsequently spread along the coastal region and 200 miles inland into the Jaguaribe River valley. Although malaria was endemic in this area, the local mosquitoes were not efficient vectors, so the human burden from malaria was low. The establishment of the highly efficient vector, A. gambiae, was followed in the late 1930s by explosive malaria outbreaks with more than 100 000 cases and about 20 000 deaths in this population with low levels of immunity. A massive campaign to eradicate A. gambiae was successful, but the event illustrated the key role of the type of vector present in malaria transmission.
Movement Through Trade
Movement of microbes through travel and trade continues to lead to outbreaks in unexpected places and populations. In many instances, transmission cannot be sustained and the infection dies out. Infections that are transmitted from person to person can potentially be carried to any country, as has been observed with HIV. Influenza, though never absent, re-emerges every year in temperate climates during the colder months to cause epidemics. The severity of the epidemics depends on the extent of change in the virus (genetic drift or shift), and to some extent on use of vaccine in the population, and closeness of match between the vaccine virus and circulating virus. Traveling, infected humans introduce the virus into new geographic areas, where it can spread rapidly because it is highly transmissible. The H5N1 avian influenza that has caused massive die-offs in poultry and other birds, since it was first identified in 1997 in Hong Kong, can also be carried by migratory birds to new countries and continents (Rappole et al., 2000). Legal and illegal movement of poultry and trade involving pet birds and fighting cocks are other routes of spread.
Dengue And Mosquito Vectors
Humans are the main reservoir for dengue virus (primates are not significant in its epidemiology in most areas). They spread it into new geographic areas by traveling while infected (virus in bloodstream) and being bitten by mosquitoes competent to permit the replication of the dengue virus to levels in which it can be transmitted via mosquito saliva when it bites a nonimmune host many days later (typically a week or two for the extrinsic incubation period). Dengue has increased in geographic reach and severity and is now present in most tropical and subtropical areas of the world. Several factors are contributing to its re-emergence, with the increased travel of humans being an essential but insufficient reason. Much of global travel is by plane, allowing travelers to reach anywhere in the world within the incubation period of dengue fever, typically 4 –7 days (full range is 3–14 days). The most important dengue vector is Aedes aegypti, and it now infests most tropical and subtropical areas. It thrives in urban areas and is able to breed in discarded plastic cups, flowerpots, and used tires and enters homes and prefers a human host. The greatest volume of population growth today is occurring in urban areas in low latitude regions, and in areas infested with a competent vector and linked to the global community via travel and trade. More and more urban areas in tropical regions have reached the population size (estimated to be 150 000 to 1 million) needed to sustain the ongoing circulation of dengue virus.
Of note, presence of a competent vector and a climate that is warm enough to permit the virus to become infective in mosquitoes is not sufficient to lead to outbreaks of dengue fever. The mosquito vector must have access to the human population. Presence of screens and air conditioning can limit transmission as a study by Reiter and colleagues (2003) showed, which looked at two urban areas (Laredo, Texas and Nuevo Laredo in Mexico) with similar climate and separated only by the Rio Grande River. A. aegypti was present in both areas. In a serosurvey, residents in Mexico were significantly more likely to have antibodies indicating recent or remote dengue infection than were residents across the border in Texas. Absence of air conditioning was significantly associated with dengue antibodies. Poor housing is also associated with increased exposure to mosquitoes, and the absence of piped water can also increase risk of exposure to mosquitoes because water storage vessels kept in the household can serve as good breeding sites for mosquitoes.
Mosquitoes are also moved around the world on ships, airplanes, and other vehicles. In some instances, the species can become established in a new geographic area. Aedes albopictus, the Asian tiger mosquito, was introduced into the United States in 1985 in used tires imported from Asia and within 12 years had spread to at least 25 states. It has also recently been introduced into many areas in Latin America. It was the primary vector responsible for the outbreak of dengue fever that occurred in Hawaii in 2001–02 and is also competent to transmit West Nile and other viruses that can cause severe disease in humans.
Monkeypox
Monkeypox, a viral infection that resembles smallpox, was first recognized in captive primates in 1958 and first identified in humans in 1970 in the Democratic Republic of the Congo. Infections that occurred earlier were probably diagnosed as smallpox because of similar clinical findings. Only after smallpox had been eradicated from this area and surveillance for smallpox-like infections was instituted was this virus identified. Unlike smallpox, whose only host is the human, monkeypox is a zoonosis, though human-to-human spread can occur. It is not as easily transmitted as smallpox among humans, and transmission is usually not sustained beyond 2–3 generations. Although primates, including monkeys, can be infected, other animals, perhaps squirrels and other rodents, are thought to be the reservoir hosts for this virus. The virus is found in tropical rain forest areas of west and central Africa. Because vaccination with vaccinia virus gives partial protection against monkeypox, some investigators have speculated that monkeypox might increase after the eradication of smallpox and cessation of the vaccination.
No one expected monkeypox to appear in North America in 2003, though in retrospect public health officials should not have been surprised. Exotic animals, such as the Gambian giant rat, imported from Ghana had been housed by distributors with prairie dogs sold as pets in the United States. At least 37 human monkeypox infections were documented in pet dealers and owners and veterinarians. Humans became sick after contact with sick prairie dogs. No humans died; in African outbreaks mortality has ranged from 4 –22%, perhaps because of greater virulence of the virus subtype. There have been no additional cases since 2003 or any evidence that the virus has become established in prairie dogs or other animal populations in the United States.
Characteristics Of Infections Likely To Re-Emerge
Infections caused by microbes that have an animal reservoir (especially wild animal) or are found in the environment (soil, water, vegetation) are difficult to contain and to keep under containment with currently available tools. Even if an effective vaccine is available, as there are for the flaviviral infections, Japanese encephalitis and yellow fever, elimination of the agent is not feasible in most instances. Unless high levels of immunization are maintained or the vector (or contact with the vector) can be eliminated, risk of resurgence will remain. Even with vaccine-preventable diseases that do not have an animal or environmental reservoir, sustained, global control has been difficult to attain. Measles, for which a highly effective vaccine exists, still causes outbreaks, though endemic circulation has been eliminated in large areas of the world. As long as the infection persists anywhere and people travel, risk of reintroduction will persist.
The logical conclusion is that infections will continue to re-emerge in the foreseeable future because elimination almost never occurs and currently available tools (such as vaccines, vector control, education and change in behavior, screening blood and tissues, antimicrobial drugs, surveillance) are imperfect or incompletely or inconsistently applied. While efforts to control disease are taking place, microbes continue to evolve in ways to favor their continued existence in today’s world. The combination of population size, density, mobility, vulnerability (e.g., AIDS, aging, immunosuppressed populations), and location (increasingly in low latitude urban areas often without good infrastructure) provides the milieu in which continued episodes of re-emergence are likely.
Bibliography:
- Corso A, Severina EP, Petruk VF, Mauritz YR, and Tomaz A (1998) Molecular characterization of penicillin-resistant Streptococcus pneumoniae isolates causing respiratory disease in the United States. Microbiology and Drug Resistance 4: 325–337.
- Dittmann S, Wharton M, Vitek C, et al. (2000) Successful control of epidemic diphtheria in the states of the former Union of Soviet Socialist Republics: Lessons learned. Journal of Infectious Diseases 181(supplement 1): S10–S22.
- Dull PM, Abdelwahab J, and Sacchi CT (2005) Neisseria meningitidis serogroup W-135 carriage among U.S. travelers to the 2001 Hajj. Journal of Infectious Diseases 191: 33–39.
- Frank C, Mohamed MK, Strickland GT, et al. (2000) The role of parenteral antischistosomal therapy in the spread of hepatitis C in Egypt. The Lancet 355: 887–891.
- Furuya EY and Lowy FD (2006) Antimicrobial-resistant bacteria in the community setting. Nature Reviews Microbiology 4: 36–45.
- Ghebreyesus TA, Hile M, Witten KH, et al. (1999) Incidence of malaria among children living near dams in northern Ethiopia: Community based incidence survey. British Medical Journal 319: 663–666.
- Goma Epidemiology Group (1995) Public health impact of Rwandan refugee crisis: What happened in Goma, Zaire, in July, 1994? The Lancet 354: 339–344.
- Gustafson P, Gomes V, Vieira CS, et al. (2001) Tuberculosis mortality during a civil war in Guinea-Bissau. Journal of the American Medical Association 286(5): 599–603.
- Hay SI, Guerra CA, Tatem AJ, Noor AM, and Snow RW (2004) The global distribution and population at risk of malaria: Past, present, and future. Lancet Infectious Diseases 4: 327–336.
- Hjelle B and Glass GE (2000) Outbreak of hantavirus infection in the Four Corners region of the United States in the wake of the 1997–1998 El Nino-southern oscillation. Journal of Infectious Diseases 181: 1569–1573.
- Kebede A, McCann JC, Kiszewski AT, and Ye-Ebiyo Y (2005) New evidence of the effects of agro-ecologic change on malaria transmission. American Journal of Tropical Medicine and Hygiene 73(4): 676–680.
- Kew O, Morris-Glasgow V, Landaverde M, et al. (2002) Outbreak of poliomyelitis in Hispaniola associated with circulating type 1 vaccinederived poliovirus. Science 296: 356–359.
- Kirkland TN and Fierer J (1996) Coccidioidomycosis: A reemerging infectious disease. Emerging Infectious Diseases 3(2): 192–199.
- MacLennan C, Dunn G, Huissoon AP, et al. (2004) Failure to clear persistent vaccine-derived neurovirulent poliovirus infection in an immunodeficient man. The Lancet 363: 1509–1513.
- McDonald LC, Killgore GE, Thompson A, et al. (2005) An epidemic, toxin gene-variant strain of Clostridium difficile. New England Journal of Medicine 353(23): 2433–2441.
- N’Goran EK, Diabate S, Utzinger J, and Sellin B (1997) Changes in human schistosomiasis levels after the construction of two large hydroelectric dams in Central Cote d’Ivoire. Bulletin of the World Health Organization 75(6): 541–545.
- Rappole JH, Derrickson SR, and Hubalek Z (2000) Migratory birds and spread of West Nile virus in the Western Hemisphere. Emerging Infectious Disease 6(4): 319–328.
- Reiter P, Lathrop S, Bunning M, et al. (2003) Texas lifestyle limits transmission of dengue virus. Emerging Infectious Diseases 9(1): 86–89.
- Vittor AY, Gilman RH, Tielsch J, et al. (2006) The effect of deforestation on the human-biting rate of Anopheles darlingi, the primary vector of falciparum malaria in the Peruvian Amazon. American Journal of Tropical Medicine and Hygiene 74(1): 3–11.
- Connolly MA, Gayer M, Ryan MJ, et al. (2004) Communicable diseases in complex emergencies: Impact and challenges. The Lancet 364: 1974–1983.
- Drucker E, Alcabes PG, and Maarx PA (2001) The injection century: Massive unsterile injections and the emergence of human pathogens. The Lancet 358: 1989–1992.
- Gubler DJ (2002) Epidemic dengue/dengue hemorrhagic fever as a public health, social and economic problem in the 21st century. Trends in Microbiology 10(2): 100–102.
- Guernier V, Hockberg ME, and Guegan J-F (2004) Ecology drives the worldwide distribution of human diseases. Public Library of Science Biology 2(6): 740–746.
- Levy SB and Marshall B (2004) Antibacterial resistance worldwide: Causes, challenges and responses. Nature Medicine Supplement 10(12): S122–S129.
- Martens P and Hall L (2000) Malaria on the move: Human population movement and malaria transmission. Emerging Infectious Diseases 6(2): 103–109.
- Smolinski MS, Hamburg MA, and Lederberg J (eds.) (2003) Microbial Threats to Health: Emergence, Detection, and Response. Institute of Medicine of the National Academies. Washington, DC: The National Academies Press.
- Sutherst RW (2004) Global change and human vulnerability to vectorborne diseases. Clinical Microbiology Review 17(1): 136–173.
- Taubenberger JK, Reid AH, Lourens RM, et al. (2005) Characterization of the 1918 influenza virus polymerase genes. Nature 437: 889–893.
- Webby R, Hoffmann E, and Webster R (2004) Molecular constraints to interspecies transmission of viral pathogens. Nature Medicine Supplement 10(12): S77–S81.
- Wilson ME (1995) Travel and the emergence of infectious diseases. Emerging Infectious Diseases 1: 39–46.
- Wilson ME (1995) Infectious diseases: An ecological perspective. British Medical Journal 311: 1681–1684.
- Wilson ME (2003) The traveller and emerging infections: Sentinel, courier, transmitter. Journal of Applied Microbiology 94: S1–S11.
See also:
Free research papers are not written to satisfy your specific instructions. You can use our professional writing services to buy a custom research paper on any topic and get your high quality paper at affordable price.